Odekon M. Encyclopedia of paleoclimatology and ancient environments
Подождите немного. Документ загружается.

Sun prior to 3 billion years was offset by higher levels of
greenhouse gases such as carbon dioxide or methane (see Faint
Young Sun Paradox). The lack of siderite in paleosols older
than ~2 billion years has been interpreted to indicate an insuf-
ficient amount of atmospheric CO
2
to compensate for the faint
young Sun, and that therefore the major greenhouse gas was
CH
4
. However, Ohmoto et al. (2004) use thermodynamic argu-
ments to conclude that massive Archean siderite deposits were
laid down under a CO
2
-rich atmosphere. The formation of iron
(II)-rich carbonate (Fe
0.53
Mg
0.44
CO
3
) weathering rinds on con-
glomerate pebbles from the 3.2 billion year old Barberton
Greenstone Belt, South Africa also hints at higher paleo-
atmospheric CO
2
levels than present (Hessler et al., 2004).
Sulfates
Gypsum and anhydrite
Gypsum (CaSO
4
·2H
2
O) and anhydrite (CaSO
4
) are among
the most abundant evaporite minerals. Gypsum also crys-
tallizes within muds in desert playas or coastal sabkhas
(Figure M33a–c). Gypsum nodules grow directly by displacing
the enclosing mud sediment, coalescing into nodular layers.
Gypsum dehydrates to anhydrite at the higher temperatures and
pressures caused by burial to depths of several hundred meters
(McLane, 1995). Anhydrite precipitates directly from highly
saturated brines at temperatures above 22
C (McLane, 1995).
Mirabilite
Mirabilite (Na
2
SO
4
·10H
2
O) is a soluble evaporite mineral
that precipitates under conditions of high salinity and at low
temperatures.
Barite
Barite (BaSO
4
) forms in hydrothermal and in sedimentary envir-
onments. It also precipitates from seawater, accumulating on the
ocean floor, often in association with plankton and organic matter
(Derry and Murray, 2004; Kastner, 1999). Furthermore, its insolu-
bility and resistance to diagenetic alteration under oxic conditions
help preserve it in marine sediments. These features make
barite accumulation rates a useful marker for ocean paleoproduc-
tivity (Parrish, 1998; Paytan et al., 1996)(seeOcean paleopro-
ductivity). Oxygen isotope ratios in marine barite record changes
in exposure to sulfur oxidation on continental shelves during
glacial-interglacial cycles and thus indirectly record fluctuations
in sea level (Turchyn and Schrag, 2004).
Phosphates
Francolite, or carbonate-fluorapatite (Ca
5
(PO
4
,CO
3
)
3
(F,OH), is
the chief mineral in phosphorite, a sedimentary rock rich in
phosphates (see Phosphorite). The chemical composition
of francolite is variable, with differing amounts of Na, Sr,
or Mg replacing Ca, and SO
4
substituting for PO
4
. Francolite
occurs as pellets, nodules, crusts, or laminations. Collophane
is an amorphous or cryptocrystalline form of sedimentary
phosphate.
Phosphorites accumulate on continental margins, epeiric
seas, and to a lesser extent on seamounts or ocean islands. They
are generally formed in zones of marine upwelling and high
biologic productivity (Parrish, 1998). The main source of phos-
phorus comes from vertebrate skeletal remains, or breakdown
of organic matter. Fish scales or bones frequently act as nuclei
for the buildup of francolite pellets or nodules.
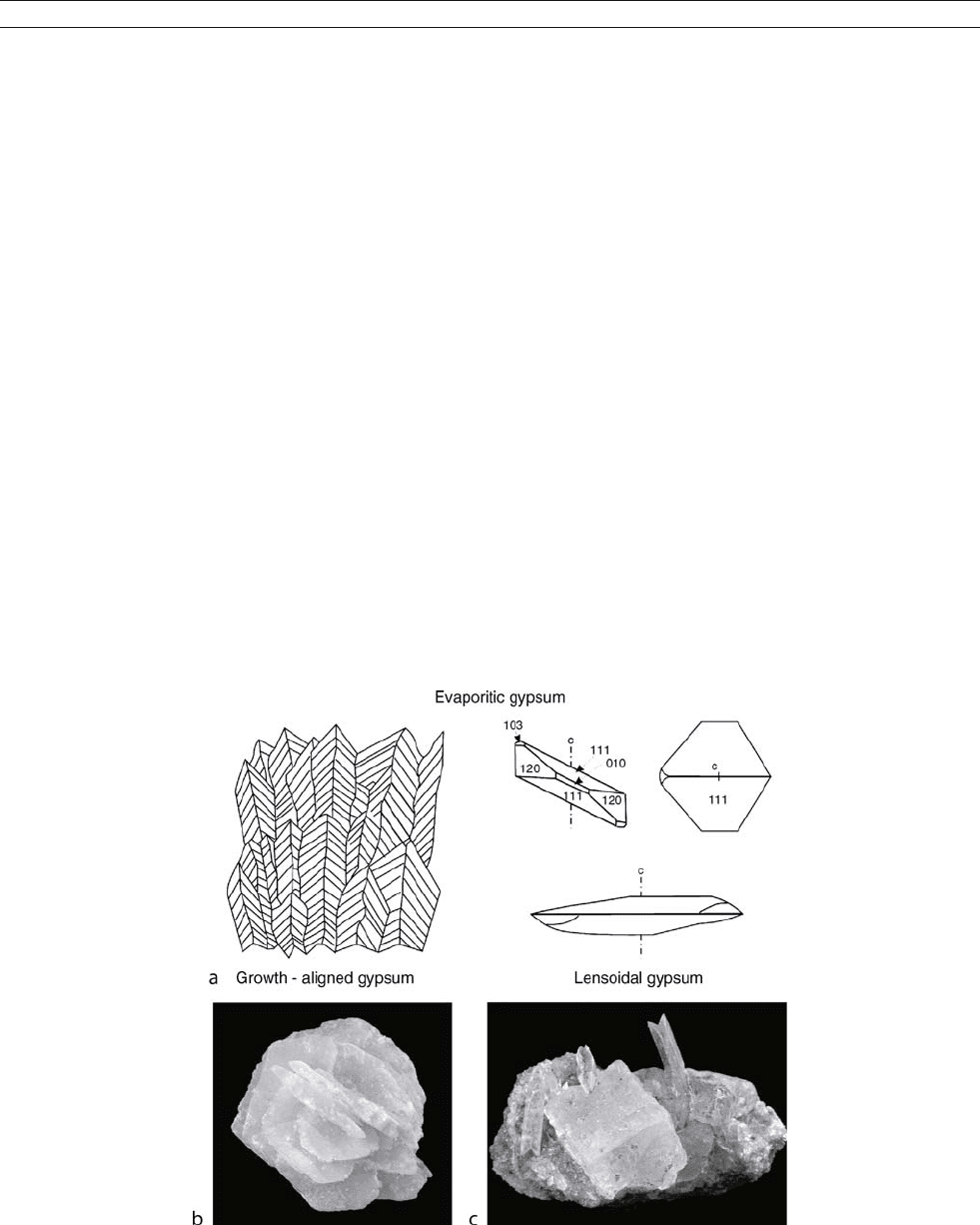
Figure M33 (a) Gypsum textures typical of evaporitic environments – growth aligned gypsum (left), lensoidal gypsum (right) (after Warren, 1999).
(b) Gypsum rose, Chihuahua, Mexico (4.4 cm 3.2 cm, specimen, V. Gornitz; photograph, John Betts). (c) Gypsum (prismatic, twinned crystals)
and halite (cube) on fine-grained halite, salinas de otomo, Pisco Province, Ica Pagion, Peru (6.4 cm 2.5 cm; specimen, V. Gornitz; photograph,
John Betts).
MINERAL INDICATORS OF PAST CLIMATES 579
Silica minerals
The most common varieties of silica found near the earth’s
surface include quartz, chalcedony, chert, and opal.
Quartz
Low-quartz (a-SiO
2
) is the stable form of crystalline silica at
the earth’ s surface and one of the most abundant minerals in
crustal rocks. Quartz is a hard (H = 7 Mohs scale), chemically
resistant mineral that survives the rock weathering cycle in
nearly all climates, except for hot and humid tropical climates
(e.g., see Bauxite, above). It is a major constituent of eolian
desert deposits, sandstones, and many littoral beach sands.
In deserts, winds concentrate quartz grains into dunes and
huge sand seas (ergs). Fossil sand dunes show characteris-
tic cross-bedding and wind-ripple forms. The orientation of
cross-bedding and other sedimentological features enable the
reconstruction of paleowind directions (Parrish, 1998). Examples
of quartz-rich eolian sandstones include the Jurassic Navajo
and Kayenta Sandstones of Utah and Colorado, the Permian
Coconino Sandstone of the Grand Canyon region, New Red
Sandstone (Permo-Triassic, England) and the Yellow Sands –
Permian, England (Parrish, 1998). Quartz is also a significant
component of eolian dust in marine sediments and in ice cores.
Variations in concentrations and grain sizes of eolian dust grains
provide information on changes in glacial to interglacial wind
directions and intensities (Eolian dust, marine sediments).
Opal-A, opal-CT, chert, chalcedony
Opal-A is an amorphous, structurally disordered form of silica
which contains several percent of water. Widespread in deep
sea sediments (e.g., in siliceous oozes), it is largely derived
from the remains of marine micro-organisms, such as diatoms,
radiolarians, silicoflagellates, and siliceous sponge spicules
(Parrish, 1998). (However, some marine opal may be of volca-
nic origin). Over time, opal-A gradually transforms to opal-CT,
which shows domains of short-range order similar to the
atomic arrangement in cristobalite and tridymite. Further diag-
enesis leads to opal-C and chert.
Chert is a sedimentary rock consisting of opal, crypto- to
microcrystalline quartz, and chalcedony (a fibrous variety of
quartz). Extensive bedded chert deposits are mostly products
of former marine biogenic sedimentation. Bedded chert is often
associated with phosphorites and black shales (see Phosphor-
ite; McLane, 1995). Such cherts, or siliceous oozes, have
deposited in zones of marine upwelling that are diagnostic of
high organic primary productivity (Kastner, 1999; Parrish,
1998). Tracking shifts in the distribution of upwelling zones
over time may also reveal information about changes in
paleo-ocean circulation.
Clay minerals
Clay minerals are phyllosilicates, or sheet silicates, consisting
of layers of silica tetrahedra attached to layers of aluminum
or magnesium atoms, each of which is surrounded by six oxy-
gen atoms or hydroxyl ions in octahedral layers. Other cations,
such as iron, potassium, sodium, or calcium, can also be pre-
sent. The clays differ in the nature of the cations within and
between sheets and in their stacking arrangements. Important
clay minerals and their ideal chemical formulas are listed
in Table M2.
Clay minerals are produced by the chemical weathering
of rocks near the earth’s surface (Figure M34). The clay
detritus is removed by water erosion and accumulates in lacus-
trine, estuarine, and marine sediments. Clays are also major
constituents of terrestrial soils and of airborne dust. The rela-
tive abundance of clays is closely related to climate or to
environmental setting, although source rock mineralogy also
influences their development. Kaolinite (Figure M34a), for
example, is created by intense chemical weathering of warm,
humid climates, in which silica is leached out, leaving soils
enriched in alumina (Chamley, 1989). Chlorite (Figure M34b)
and illite (Figure M34c) tend to form in soils dominated by
physical weathering – both in colder, often formerly glaciated
regions, and hot, dry climates (Chamley, 1989). Consequently,
these clays generally reflect the composition of their parent
rocks. Smectite (Figure M34d), illite, chlorite, and mixed-
layer clays are more prevalent in temperate-cool, moist cli-
mates (Chamley, 1989). Well-crystallized iron-rich smectites
(e.g., nontronite) occur in sub-arid climates (Chamley, 1989).
Montmorillonite – a smectite – also forms by weathering
of volcanic ash. Sepiolite, palygorskite, and attapulgite are
three rather rare clay minerals found in calcrete soils, and in
warm, semi-arid to arid climates (Chamley, 1989; Parrish,
1998; Watson, 1992).
Glauconite, (K,Na,Ca)(Fe
3+
,Fe
2+
,Mg)(Si,Al)
4
O
10
(OH)
2
), is a
dark-green to black mineral, related to mica and mixed-layer
clays, usually found as tiny rounded grains that most likely
formed as fecal pellets. It occurs in weakly reducing environ-
ments, near upwelling zones on continental shelves and mar-
gins between depths of 100–250 m (Chamley, 1989),
characterized by relatively slow sedimentation rates (Kastner,
1999; Parrish, 1998; Velde, 2003), and is often associated
with phosphorites (McLane, 1995; Velde, 2003). The presence
of potassium in glauconite allows dating of sea level cycles
in marine sediments, via the potassium-argon or rubidium-
strontium methods (Velde, 2003).
Berthierine, (Fe
5
,Al)
3
(Si,Al)
2
O
5
(OH)
4
, a dark-greenish phy-
llosilicate closely related to kaolinite and serpentine, occurs in
near-shore or deltaic sediments (Velde, 2003) from chemically
reducing environments, but more rarely in terrigenous soils. Its
presence in early Triassic continental paleosols may indicate low
atmospheric oxygen levels, possibly resulting from catastrophic
methane release, mass mortality, and rise in CO
2
at the Permian-
Triassic boundary (Sheldon and Retallack, 2002).
Inasmuch as non-biogenic marine sediments are largely
derived from continental sources, the distribution of clay minerals
in the oceans closely corresponds to that of adjacent landmasses.
Thus, marine clays display a latitudinal zonation broadly parallel-
ing the major climate zones (Chamley, 1989;Evans,1992;Parrish,
1998). Kaolinite is abundant in equatorial waters, whereas chlorite
Table M2 Common clay minerals
Kaolinite Al
2
Si
2
O
5
(OH)
4
Smectite group (expanding clays)
Montmorillonite (Na,Ca)
0.3
(Al,Mg)
2
Si
4
O
10
(OH)
2
·nH
2
O
Beidellite (Na,Ca)
0.3
Al
2
(Si,Al)
4
O
10
(OH)
2
·nH
2
O
Nontronite Na
0.3
Fe
2
(Si,Al)
4
O
10
(OH)
2
·nH
2
O
Saponite (Ca,Na)
0.3
(Mg,Fe)
3
(Si,Al)
4
O
10
(OH)
2
·nH
2
O
Illite KAl
2
(Si
3
Al)O
10
(OH)
2
Glauconite (K,Na,Ca)(Fe
3+
,Al,Fe
2+
,Mg)(Si,Al)
4
O
10
(OH)
2
Vermiculite (Mg
2.7
Fe
0.3
)(Si
3
Al)O
10
(OH)
2
·nH
2
O
Chlorite group (Mg
2.6
Fe
0.4
)Si
2.5
(Al,Fe)
1.5
O
10
(OH)
8
Berthierine (Fe
5
,Al)
3
(Si,Al)
2
O
5
(OH)
4
Sepiolite Mg
4
Si
6
O
15
(OH)
2
·6H
2
O
Palygorskite (Mg,Al)
2
Si
4
O
10
(OH)
2
·4H
2
O
580 MINERAL INDICATORS OF PAST CLIMATES
and illite concentrations increase toward higher latitudes. The dis-
tribution of smectites, on the other hand, is not quite as strongly
zoned, indicating the influence of other, non-climate factors.
Temporal changes in clay mineralogy record important paleo-
climate events. For example, Kennedy et al., (2006)associateda
significant increase in clay minerals relative to quartz during the
late Neoproterozoic (800–550 million years ago) with a growing
degree of terrigenous chemical weathering, clay-organic matter
burial, and increasing oxygen levels. Kaolinite and expandable
clays (i.e., smectites) became more abundant at the expense of
illite, chlorite, and mica during this period, consistent with devel-
opment of soils, possibly promoted by biotic activity. Variations
in relative abundances of kaolinite, smectite and mixed-layer
illite/smectite have been used as a paleoclimate proxy for wet ver-
sus dry climates during the Mesozoic of northwestern Europe
(Ruffell et al., 2002), with high kaolinite indicating humid periods.
Elsewhere, increases in smectite and kaolinite relative to illite
in marine sediments off Antarctica at around 33.5 Ma have been
correlated with a marked positive dO
18
shift and onset of signifi-
cant Antarctic glaciation (Robert and Kennett, 1997). Decreasing
kaolinite/illite, smectite/illite ratios after 33.4 Ma signal enhanced
physical weathering under cooler, drier climates. A similar pattern
of higher kaolinite/illite and smectite/illite ratios at ~33.5 and
33.4 Ma, succeeded by increased illite content is observed in cores
from the Great Australian Bight (Mallinson et al., 2003). These
cores also show 22-Kyr cyclicity in kaolinite/smectite ratios,
linked to precessional changes in precipitation, wind patterns
and runoff that affected the mineralogy and transport of clays
to the Bight.
Enhanced chemical weathering during warmer interglacials
generated higher concentrations of interlayered illite-smectite
in Lake Baikal sediments, Russia, whereas greater physical
weathering during colder, glacial periods resulted in chlorite
and illite enrichment (Yuretich et al., 1999). Changes in illite/
chlorite ratios in Chinese loess sections have been used to
reconstruct monsoonal activity during the late Pleistocene
(Zhao et al., 2005). In the southern Loess Plateau, the illite/
chlorite ratio and magnetic susceptibility are higher in inter-
glacial paleosols and lower in glacial loess. More intense
weathering of chlorite, leading to higher illite/chlorite ratios,
indicates a stronger monsoonal regime operating during inter-
glacial periods.
The distinctive mineral assemblage of high illite, low
smectite, and low kaolinite/chlorite ratio in the dust from the
GISP2 (Greenland) ice core pinpoints a likely source of
the clays in deserts of eastern Asia. Although dust composi-
tion (and sources) remained fairly constant during the last gla-
ciation, changes in total concentrations and grain sizes reveal
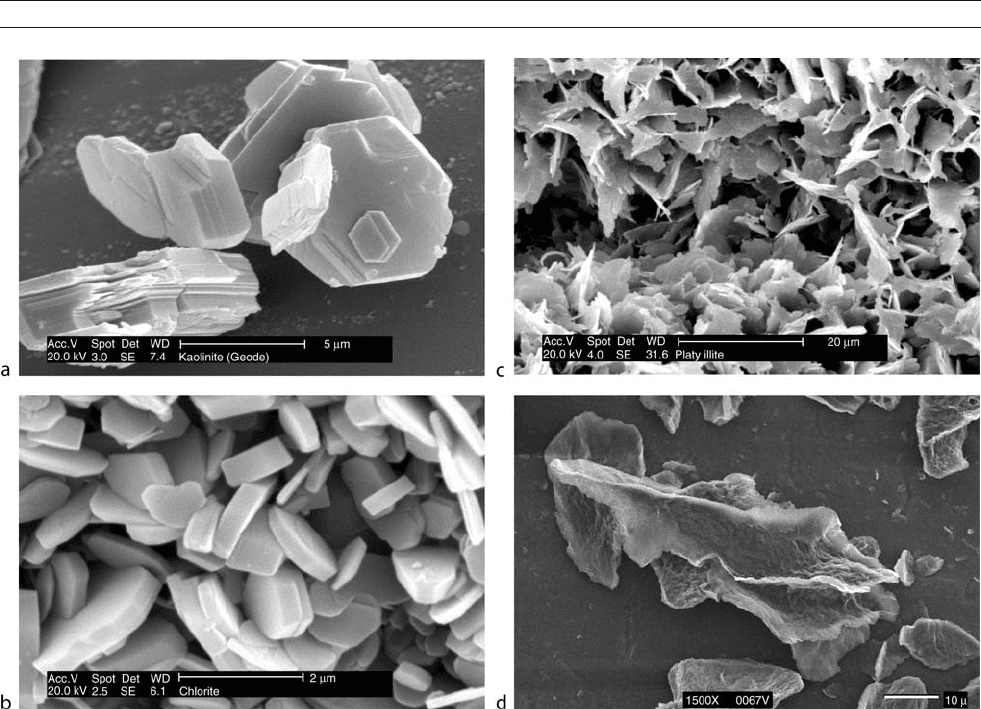
Figure M34 (a) Kaolinite, Keokuk geode, USA. (b) Iron-rich chlorite, Spiro Sandstone, Arkoma Basin, Oklahoma, USA. (c) Illite, Rotliegend Formation,
Northern Germany. (d) Smectite, Ap horizon, Webster soil, University of Minnesota Southern Agricultural Station, Waseca, Minnesota.
Reproduced with permission from the “Images of Clay” gallery published by the Clay Minerals Group of the Mineralogical Society and the Clay
Minerals Society (www.minersoc.org/ pages/ gallery/ claypix).
MINERAL INDICATORS OF PAST CLIMATES 581
significant variations in wind speed and strength during stadials
and interstadials (Biscaye et al., 1997).
Zeolites
Zeolites (Table M3) are a group of hydrated aluminosilicate
minerals with an open framework structure, into which various
large ions and water molecules can fit and which possess a cer-
tain degree of mobility. They are widespread in cavities in
basaltic lavas, as authigenic or diagenetic minerals in marine
sediments, and in low-grade metamorphic rocks. Phillipsite is
an authigenic zeolite that forms on the seafloor or at shallow
depths (Kastner, 1999) Clinoptilolite forms in silica-rich marine
sediments, derived from opal-A. Zeolites also form from the
alteration of volcanic glass, tuff, or lavas in high pH, alkali-
rich, semi-arid to arid environments. Analcime is common in
sodium-rich alkaline desert soils (Watson, 1992). Zeolites from
saline, alkaline lakes include analcime, chabazite, clinoptilolite,
erionite, mordenite, and phillipsite. These zeolites are often
associated with evaporites (Parrish, 1998). Zeolites in lacus-
trine deposits have been used as markers for dry, alkaline
environments, as for example at Lake Naivasha, Kenya, where
low lake levels are represented by the presence of chabazite
and phillipsite, with even greater aridity indicated by clinoptilo-
lite and analcime (Trauth et al., 2001). The East African lake
level fluctuations have been linked to precessional cycles in
springtime insolation and monsoonal precipitation.
Summary
Minerals that form at or near the earth’s surface in contact with
the atmosphere and hydrosphere can provide important infor-
mation about former climates. They have been used to recon-
struct past climates and changes over time, deduce changes in
atmospheric composition, trace eolian and precipitation pat-
terns, and serve as “hosts” for climate-sensitive stable isotopes
and trace elements.
The most useful mineral indicators or proxies are those that
are generated under relatively narrow climatic ranges or within
restricted environmental settings. Examples include evaporites,
low temperature minerals (e.g., ikaite and hydrohalite), residual
soils (e.g., iron and aluminum oxyhydroxides, kaolinite), and
certain authigenic minerals (that form in situ, e.g., glauconite,
bertherine, phillipsite, barite). Minerals that are sensitive to oxi-
dation (e.g., pyrite and uraninite) reflect past atmospheric oxy-
gen levels. Distinctive detrital minerals in eolian deposits act as
markers of wind direction and intensity. Similarly, minerals in
deltaic sediments point not only to variations in the relative
contributions from source tributaries, but can also indicate pre-
cipitation variability in source areas. Trace elements and stable
isotope ratios contained within minerals such as calcite (both
biogenic and non-biogenic) have provided a wealth of paleocli-
matological information.
Vivien Gornitz
Bibliography
Benison, K.C., and Goldstein, R.H., 1999. Permian paleoclimate data from
fluid inclusions in halite. Chem. Geol., 154,113–132.
Biscaye, P.E., Grousset, F.E., Revel, M., Van der Gaast, Zielinski, G.A.,
Vaars, A., and Kukla, G., 1997. Asian provenance of glacial dust (stage 2)
in the Greenland Ice Sheet Project 2 ice core, Summit, Greenland.
J. Geophys. Res., 10226,765–26, 781.
Bond, G., Showers, W., Cheseby, M., Lotti, R., Almasi, P., deMenocal, P.,
Priore, P., Cullen, H., Hajdas, I., and Bonani, G., 1997. Massive millen-
nial-scale cycle in North Atlantic Holocene and Glacial climates.
Science, 278, 1257–1266.
Broecker, W.S., and Liu, T., 2001. Rock varnish: recorder of desert
wetness? GSA Today, 11(8), 10.
Chamley, H., 1989. Clay Sedimentology. Berlin: Springer, 623p.
De Lurio, J.l., and Frakes, L.A., 1999. Glendonites as a paleoenvironmental
tool: implications for early Cretaceous high latitude climates in
Australia. Geochim. et Cosmochim. Acta, 63, 1039–1048.
Derry, L.A., and Murray, R.W., 2004. Continental margins and the
sulfur cycle. Science, 303, 1981–1982.
England, G.L., Rasmussen, B., Krapez, B., and Groves, D.I., 2002.
Palaeoenvironmental significance of rounded pyrite in siliciclastic
sequences of the Late Archean Witwatersrand Basin: Oxygen-
deficient atmosphere or hydrothermal alteration? Sedimentology, 49
(6), 1133–1156.
Evans, L.J., 1992. Alteration products at the Earth’s surface–the clay
minerals. In Martini, I.P., and Chesworth, W. (eds.), Weathering, Soils,
and Paleosols, Amsterdam: Elsevier, Chap. 5, pp. 107–125.
Fleet, M.E., 1998. Detrital pyrite in Witwatersrand gold reefs: X-ray
diffraction evidence and implications for atmospheric evolution. Terra
Nova, 10(6), 302–306.
Foucault, A., and Stanley, D.J., 1989. Late Quaternary palaeoclimatic
oscillations in East Africa recorded by heavy minerals in the Nile delta.
Nature, 339,44–46.
Genty, D., Blamart, D., Ouahdi, R., Gilmour, M., Baker, A., Jouzel, J., and
Van-Exter, S., 2003. Precise dating of Dansgaard-Oeschger climate
oscillations in western Europe from stalagmite data. Nature, 421,
833–837.
Gornitz, V.M., and Schreiber, B.C., 1981. Displacive halite hoppers
from the Dead Sea: implications for ancient evaporite deposits. J. Sed.
Petrol., 51, 787–794.
Hessler, A.M., Lowe, D.R., Jones, R.L., and Bird, D.K., 2004. A
lower limit for atmospheric carbon dioxide levels 3.2 billion years
ago. Nature, 428, 736–738.
Kastner, M., 1999. Oceanic minerals; their origin, nature of their environ-
ment, and significance. Proc. Natl. Acad. Sci., 96, 3380–3387.
Kennedy, M., Droser, M, Mayer, L.M., Pevear, D., and Mrofka, D., 2006.
Late Precambrian oxygenation; inception of the clay mineral factory.
Science, 311, 1446–1449.
Kirk, J., Ruiz, J., Chesley, J., and Titley, S., 2003. The origin of gold in
South Africa. Am. Scientist, 91, 536–541.
Li, Y.H., and Schoonmaker, J.E., 2003. Chemical composition and
mineralogy of marine sediments. In: Mackenzie F.T. (ed.), Sediments,
Diagenesis, and Sedimentary Rocks (vol. 7), Treatise of Geochemistry,
Holland, H.D., and Turekian, K.K. (eds.). Amsterdam: Elsevier,
pp. 1–35.
Lowenstein, T.K., Timofeeff, M.N., Brennan, S.T., Hardie, L.A., and
Demicco, R.V., 2001. Oscillations in Phanerozoic seawater chemistry:
Evidence from fluid inclusions. Science, 294, 1086–1088.
Ludvigson, G.A., Gonzalez, L.A., Metzger, R.A., Witzke, B.J., Brenner, R.L.,
Murillo, A.P., and White, T.S., 1998. Meteoric sphaerosiderite lines and
their use for paleohydrology and paleoclimatology. Geology, 26,
1039–1042.
Ludvigson, G.A., Ufnar, D.F., Gonzalez, L.A., White, T.S., Phillips, P.L.,
Witzke, B.J., and Brenner, R.L., 2000. When good sphaeorosiderites
go bad: Caveats in the application of a paleoclimate proxy. Geol. Soc.
Am. Abstr. Prog., 32(7), pp. A–524.
Maher, B.A., 1998. Magnetic properties of modern soils and Quaternary
loessic paleosols: paleoclimatic implications. Palaeogeog. Palaeoclim.
Palaeoecol., 137,25– 54.
Mallinson, D.J., Flower, B., Hine, A., Brooks, G., and Garza, R.M., 2003.
Paleoclimate implications of high latitude precession-scale mineralogic
fluctuations during early Oligocene Antarctic glaciation: The Great
Australian Bight record. Glob. Planet. Change, 39, 257–269.
Table M3 Common zeolite minerals
Analcime NaAlSi
2
O
6
·H
2
O
Chabazite Ca
2
(Al
4
Si
8
O
24
)·12H
2
O
Clinoptilolite (Na,K,Ca
0.5
)
6
(Al
6
Si
30
O
72
)·24H
2
O
Erionite (Na,K
2
,Mg,Ca
1.5
)(Al
8
Si
28
O
72
)·28H
2
O
Mordenite Na
3
KCa
2
(Al
8
Si
40
O
96
)·28H
2
O
Phillipsite (K,Ca
0.5
,Na)
4
(Al
6
Si
10
O
32
)·12H
2
O
582 MINERAL INDICATORS OF PAST CLIMATES

McKeown, D.A.M., and Post, J.E., 2001. Characterization of manganese
oxide mineralogy in rock varnish and dendrites using X-ray absorption
spectroscopy. Am. Min., 86, 701–713.
McLane, M., 1995. Sedimentology, New York: Oxford University
Press, 432p.
Milnes, A.E., 1992. Calcrete. In Martini, I.P., and Chesworth, W. (eds.),
Weathering Soils Paleosols. Amsterdam: Elsevier, Chap. 13, pp. 309–347.
Montañez, I.P., 2002. Biological skeletal carbonate records changes
in major-ion chemistry of paleo-oceans. Proc. Natl. Acad. Sci., 99
(25), 15852–15854.
Morse, J.W., 2003. Formation and diagenesis of carbonate sediments. In
Mackenzie, F.T. (ed.). Sediments, Diagenesis, and Sedimentary Rocks
(vol. 7), Treatise of Geochemistry, Holland, H.D. and Turekian, K.K.
(eds.). Amsterdam: Elsevier, pp. 67–85.
Ohmoto, H., Watanabe, Y., and Kumazawa, K., 2004. Evidence from
massive siderite beds for a CO
2
-rich atmosphere before 1.8 billion
years ago. Nature, 429, 395–399.
Parrish, J.T., 1998. Interpreting pre-Quaternary Climate from the Geologic
Record, New York: Columbia University Press, 338p.
Paytan, A., Kastner, M., and Chavez, F.P., 1996. Glacial to interglacial
fluctuations in productivity in the equatorial Pacific as indicated by
marine barite. Science, 274, 1355–1357.
Raiswell, R., Buckley, F., Berner, R.A., and Anderson, T.F., 1988. Degree
of pyritization of iron as a paleoenvironmental indicator of bottom-
water oxygenation. J. Sed. Petrol., 58(5), 812–819.
Reynolds, R.L., and King, J.W., 1995. Magnetic records of climate
change. US National Report to IUGG, 1991–1994, Rev. Geophys., 33
(Suppl. 95), RG00354.
Robert, C., and Kennett, J.P., 1997. Antarctic continental weathering
changes during Eocene-Oligocene cryosphere expansion: clay mineral
and oxygen isotope evidence. Geology, 25, 587–590.
Roberts, S., Spencer, M., Yang, R.J., and Krouse, H.R., 1997. Deciphering
some unique paleotemperature indicators in halite-bearing saline
lake deposits from Death Valley, California, USA. J. Paleolimnol., 17,
101–130.
Roychoudhury, A.N., Kostka, J.E., and Van Cappellen, 2003. Pyritization:
A palaeoenvironmental and redox proxy reevaluated. Estuar. Coast.
Shelf Sci., 57,1–11.
Ruffell, A., McKinley, J.M., and Worden, R.H., 2002. Comparison of
clay mineral stratigraphy to other proxy palaeoclimate indicators in
the Mesozoic of NW Europe. Phil. Trans. Royal Soc. Lond. A, 360,
675–693.
Sheldon, N.D., and Retallack, G.J., 2002. Low oxygen levels in earliest
Triassic soils. Geology, 30, 919–922.
Stiles, C.A., Mora, C.I., and Driese, S.G., 2001. Pedogenic iron-manganese
nodules in Vertisols: A new proxy for paleoprecipitation? Geology, 29,
943–946.
Swainson I.P., and Hammond, R.P., 2001. Ikaite, CaCO
3
.6H
2
O: cold
comfort for glendonites as paleothermometers. Am. Mineral., 86,
1530–1533.
Tardy, Y., 1992. Diversity and terminology of lateritic profiles. In Martini,
I.P., and Chesworth, W. (eds.), Weathering, Soils & Paleosols.
Amsterdam: Elsevier, Chap. 15, pp. 379–405.
Tardy, Y., and Roquin, C., 1992. Geochemistry and evolution of lateritic
landscapes. In Martini, I.P., and Chesworth, W. (eds.), Weathering,
Soils & Paleosols, Amsterdam: Elsevier, Chap. 16, pp. 407–443.
Torii, T., and Ossaka, J., 1965. Antarcticite: a new mineral, calcium
chloride hexahydrate, discovered in Antarctica. Science, 149, 975–977.
Trauth, M.H., Deino, A., and Strecker, M.R., 2001. Response of the
East African climate to orbital forcing during the last interglacial
(130–117 ka) and the early last glacial (117–60 ka). Geology, 29,
499–502.
Turchyn, A.V., and Schrag, D.P., 2004. Oxygen isotope constraints on
the sulfur cycle over the past 10 million years. Science, 303,
2004–2007.
Velde, B., 2003. Green clay minerals. In Mackenzie, F.T. (ed.), Sediments,
Diagenesis, and Sedimentary Rocks (vol. 7), Treatise of Geochemistry,
Holland, H.D. and Turekian, K.K. (eds.). Amsterdam: Elsevier,
pp. 309–324.
Warren, J., 1999. Evaporites: Their Evolution and Economics. Oxford, UK:
Blackwell Science, Chap. 1, 19. p. 13, 19.
Watson, A., 1992. Desert soils. In Martini, I.P., and Chesworth, W. (eds.),
Weathering, Soils & Paleosols. Amsterdam: Elsevier, Chap.10,
pp. 225–260.
Yuretich, R., Melles, M., Sarata, B., and Grobe, H., 1999. Clay minerals
in the sediments of Lake Baikal: A useful climate proxy. J. Sed. Res.,
69, 588–596.
Zhao, L., Ji, J., Chen, J., Liu, L., Chen, Y., and Balsam, W., 2005.
Variations of illite/ chlorite ratio in Chinese loess sections during
the last glacial and interglacial cycle: Implications for monsoon
reconstruction. Geophys. Res. Lett., 32, L20718. doi:10.1029/
2005GL024145.
Cross-references
Atmospheric Evolution, Earth
Banded Iron Formations and the Early Atmosphere
Beachrock
Carbonates, Cool Water
Carbonates, Warm Water
Cryosphere
Dating, Magnetostratigraphy
Desert Varnish as Climate Proxy
Duricrusts
Eolian Dust, Marine Sediments
Eolian Sediments and Processes
Eolianite
Evaporites
Faint Young Sun Paradox
Glacial Geomorphology
Glendonite/ Ikaite
Ice Cores, Antarctica and Greenland
Ice Cores, Mountain Glaciers
Ice-Rafted Debris (IRD)
Laterite
Marine Biogenic Sediments
Marine Clay Minerals
Ocean Paleoproductivity
Paleosols, Pre-Quaternary
Paleosols–Quaternary
Phosphorite
Red Beds
Sedimentary Indicators of Climate Change
Speleothems
Stable Isotope Analysis
MONSOONS: PRE-QUATERNARY
Monsoon circulation has existed throughout geological history
whenever the tropics were occupied by both land and sea. Pre-
Quaternary monsoons, however, are poorly understood because
paleo-monsoon studies have been heavily biased toward varia-
tions of the Quaternary monsoon on the orbital and suborbital
time scales. Pre-Quaternary monsoon systems are often consid-
ered over a much longer time span, so they show more signifi-
cant variations in responding to tectonically-induced geographic
and topographic changes. Pre-Quaternary monsoons have so far
only sporadically been mentioned in the literature for most of the
geological periods, except for two better studied intervals: the
Permian and Triassic where a “megamonsoon” developed on
the megacontinent Pangaea, and the late Cenozoic, which led to
the establishment of the modern monsoon system.
Megamonsoon of the megacontinent
As monsoons are caused by land-sea contrast in the heating
rate, the ideal conditions for a monsoon system to develop exist
when only one major continent and one complementary ocean
exist on Earth. This was the real case around 250–200 Myr
ago, during the late Permian and Triassic, when all continents
MONSOONS: PRE-QUATERNARY 583
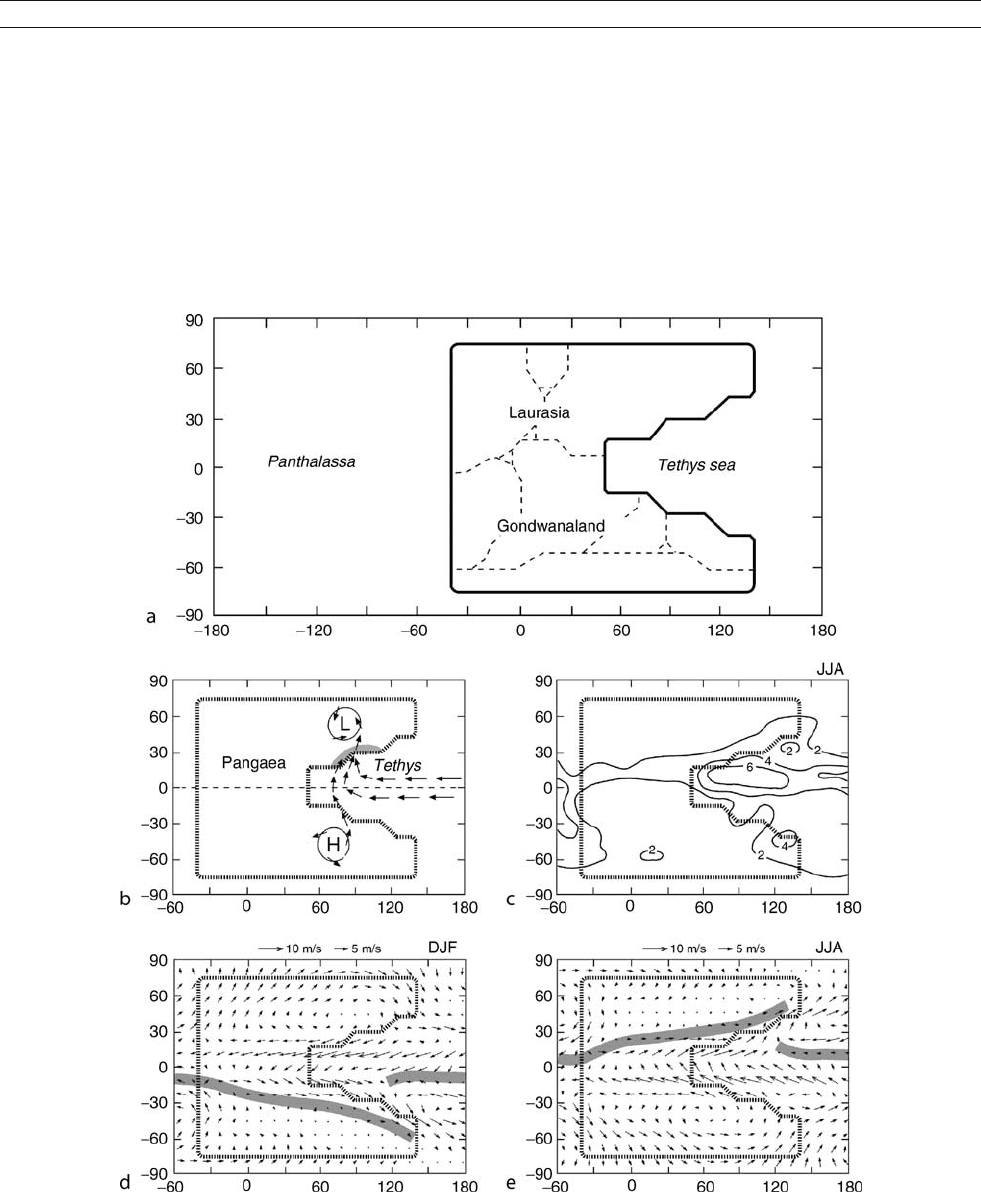
assembled into two major landmasses, Laurasia and Gondwa-
naland, that joined near the equator to form the supercontinent
Pangaea and the super-ocean Panthalassa (Figure M35a). The
extensive distribution of evaporites and many biogeographic
features indicate a maximum of continentality, leading to spec-
ulation about the association of monsoon-type seasonal rains
with large landmasses at that time (Robinson, 1973). Parrish
et al. (1982) used the basic principles of atmospheric and ocea-
nic circulation to reconstruct global paleo-precipitation maps
for seven time intervals of the Mesozoic and Cenozoic, and
found patterns of a strong summer monsoon low over Laurasia
and a winter monsoon high over Gondwanaland in the Triassic
and reversed monsoon features for opposite seasons. According
to these authors, the Triassic was distinguished by maximal arid-
ity with precipitation on the supercontinent provided only by
monsoons (Figure M35b). Kutzbach and Gallimore (1989)were
the first to use numerical modeling to explore the “megamonsoon
of the megacontinent.” Applying a low-resolution general cir-
culation model to the idealized Pangaea (Figure M35a), they
found extreme continentality with a hot summer and cold win-
ter, and large-scale summer and winter monsoon circulations
(Figure M35c,d. At the same time, Crowley et al. (1989) used
Figure M35 Megamonsoon of the Pangaea. (a) The idealized Pangaean continent. Fine dashed lines indicate the approximate outlines of modern
landmasses (after Kutzbach and Gallimore, 1989). (b) Schematic diagram illustrating monsoonal circulation in northern summer. Arrows show
surface winds, stippling indicates heavy seasonal rains (modified from Parrish and Peterson, 1988). (c) Modeled precipitation rate (mm d
1
)on
Pangaea for summer. (d), (e) Modeled surface winds on Pangaea for winter (d) and summer (e), note the seasonal reversal of the wind direction.
The gray bar shows the poleward limit of summer monsoon over land and the Intertropical Convergence Zone over ocean (modified from
Kutzbach and Gallimore, 1989).
584 MONSOONS: PRE-QUATERNARY
energy balance modeling (EBM) to study the late Permian cli-
mate. Both modeling results show an extremely wide annual
range of temperature (50
C) for hinterland and strong mon-
soon circulation over Pangaea.
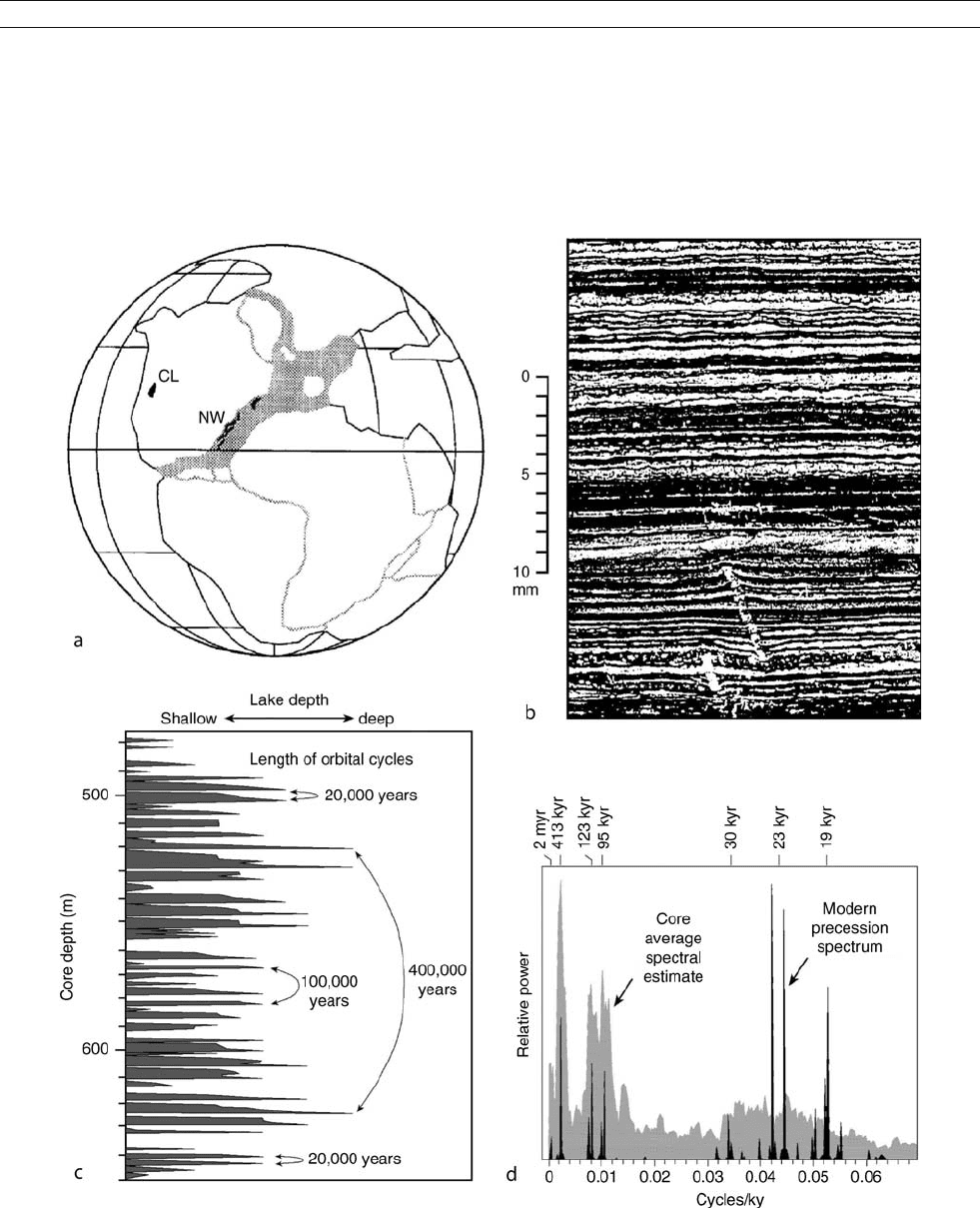
The geological record of the “megamonsoon” was found
from the upper Triassic of North America: the Newark and
related basins in the east and the Colorado Plateau in the west
(Figure M36a). Late Triassic lacustrine deposits from the New-
ark Basin, New Jersey, which were about 10
N in the mon-
soon-prevalent tropics at that time, display clear evidence of
monsoon-driven seasonality and lake level fluctuations (Olsen,
1986). Part of the sequence consists of micro-laminated mud-
stones (varves) with 0.2–03 mm thin couplets of alternating
light and dark layers, implying significant seasonal contrast
Figure M36 Late Triassic monsoon records from North America. (a) Locations of the Colorado Plateau with the Chinle Formation (CL)
and, to the east, a chain of rifted basins containing the Newark Supergroup (NW). (b) Photograph of microlaminated mudstone showing
organic-rich/ carbonate-rich couplets as annual varves. (c) Lake-level fluctuations revealed in a section of the Newark lake sediments, showing
20-kyr, 100-kyr, and 400-kyr cycles. (d) Average spectral estimates of sediment cycles in the Newark Basin against the modern precession spectrum.
(Modified from Olsen and Kent, 1996 and Ruddiman, 2001).
MONSOONS: PRE-QUATERNARY 585
(Figure M36b). Detailed studies including spectral analyses on
the nearly 7,000 m long section reveal a full range of
precession-related periods of lake level change: the 20-kyr pre-
cession cycles, and 100-kyr and 400-kyr eccentricity cycles
which modulate the amplitude of precessional cyclicity
(Figure M36c,d). All these cycles are characteristic of the tropi-
cal response to orbital forcing and support a monsoon-climate
origin of lake level change, whereas the absence of obliquity
cycles preclude the possibility of direct linkages to high-
latitude climate systems (Olsen and Kent, 1996). Similar
records of the Pangaean “megamonsoon” were also found in
the Chinle Formation, Colorado Plateau, on the tropical wes-
tern margin of the supercontinent (Figure M36a; Dubial et al.,
1991). During the Pangaean interval, eolian, playa deposits
and evaporates were widespread on the Colorado Plateau, but
the Chinle formation represents an unusually wet episode with
well-developed fluvial and lacustrine deposits and paleosol
sequences, indicating abundant moisture brought about by
enhanced monsoon circulation and strong seasonality.
Establishment of the modern monsoon system
The modern Asian-Australian and African monsoons cover
most of the Eastern Hemisphere, and fundamental questions
in Cenozoic paleoclimatology ask when the modern monsoon
system was established and how it has evolved since then.
Three tectonic factors have been proposed to exert a control
over the evolution of Asian monsoon circulation: plateau uplift,
sea-land distribution, and closing of oceanic gateways. Contin-
uous records of the monsoon history provided by Deep Sea
Drilling Project/Ocean Drilling Program (DSDP/ODP) cruises
to the Arabian, Mediterranean and South China Seas, as well
as from the Loess plateau in central China, have been used to
verify the various tectonic hypotheses (Sun and Wang, 2005).
Tectonic forcing and numerical modeling
1. Plateau uplift. GCM experiments on the modern land-sea dis-
tribution indicate that strong monsoons can be induced by solar
forcing only when the elevation of Tibet-Himalaya has reached
at least half that of today (Prell and Kutzbach, 1992). A number
of studies in the late 1980s investigated the climatic conse-
quences of uplift (e.g., Ruddiman et al., 1989) and found that
uplift may have been responsible for both the global cooling
and significant strengthening of the Asian monsoon system in
the late Cenozoic. According to the prevalent hypothesis, uplift
of the Tibetan Plateau intensified around 8 Ma and caused
enhanced aridity over the Asian interior and the onset of the
Indian and east Asian monsoons (Figure M37a, Prell and
Kutzbach, 1997;Anetal.,2001). However, this cognition is
challenged by the new discovery of Miocene loess (Guo et al.,
2002) and other evidence that indicates an older age (see
below).
2. Sea-land distribution. The results of Atmospheric General
Circulation Model (AGCM) simulation by Ramstein et al.
(1997) indicate that the Paratethys, an epicontinental sea
stretching over Eurasia 30 Ma ago, had progressively
receded during the Miocene, resulting in major continentali-
zation of the Asian interior and enhancement of monsoon
circulation. They consider the retreat of the Paratethys Sea
as important as the uplift of the Himalayan/Tibetan Plateau
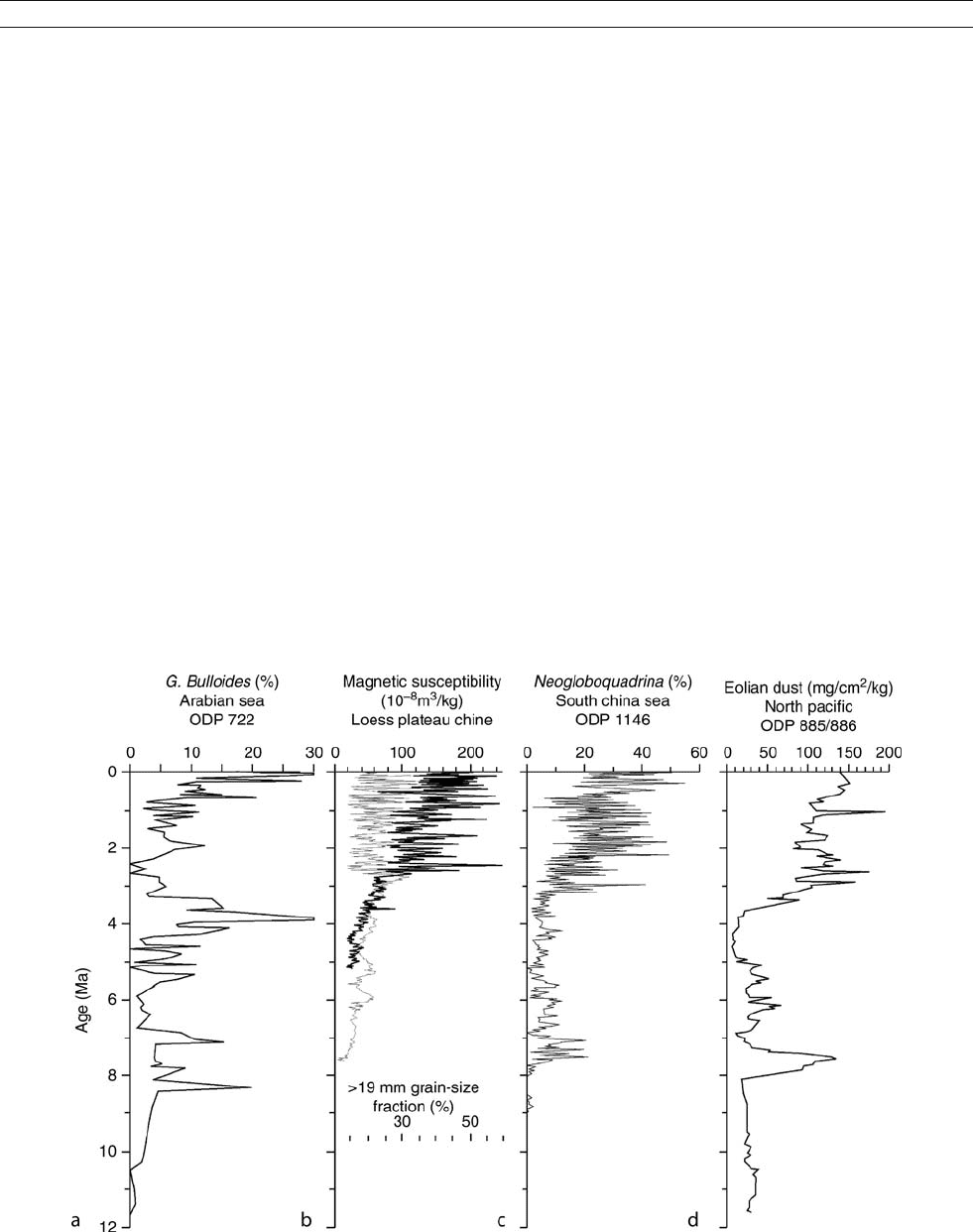
Figure M37 Records of Asian monsoon and aridity evolution over the past 12 Myr. (a) Globigerina bulloides %(>150 mm), ODP Site 722,
Arabian Sea; (b) Magnetic susceptibility (thin line) and grain size fraction (thick line) from the Loess Plateau (An et al., 2001); (c) Neogloboquadrina
dutertrei %, ODP Site 1146, South China Sea (Wang et al., 2003); (d) Dust flux (mg cm
2
kyr
1
), ODP sites 885 /886, north Pacific (Rea et al., 1998).
586 MONSOONS: PRE-QUATERNARY
for development of the Asian monsoon. As the shrinkage of
the Paratethys Sea occurred during the Oligocene-late Mio-
cene, its effects on the monsoon may have lasted from 30 to
10 Ma, significantly earlier than the 8-Ma date implied by
the uplift hypothesis.
3. Oceanic gateways. An oceanic circulation model reveals
that the “closure” of the Indonesian seaway 3–4 Ma ago could
be responsible for east African aridification (Cane and Molnar,
2001). The northward drift of the Australian Plate may have
switched the source of the Indonesian Throughflow current
fromthe warm South Pacific to the relatively cold North Pacific
waters. This would have decreased Sea Surface Temperatures
(SSTs) in the Indian Ocean and would have subsequently
reduced precipitation over east Africa, as well as the
overall strength of the Indian summer monsoon as recorded
in marine deposits.
All of these three factors are believed to be significant in the
development of the modern monsoon, but their relative roles
remain unclear. To single out the role played by each of the fac-
tors, many more long-term sequences and better constraints on
the timing of the tectonic and climate events are needed.
Geological records
Studies on the long-term evolution of the Asian monsoon started
with ODP Leg 117 to the Arabian Sea in 1987. A sudden increase
in the cool-water planktonic foraminifer Globigerina bulloides in
sediment cores of Leg 117 around 8.5 Ma ago was considered
by Kroon et al. (1991) as indicating the onset of monsoon-related
upwelling (Figure M37a). This date is very close to the rapid eco-
logical transition from C3-dominated to C4-dominated vegetation
around 7.4–7.0 Ma, as revealed by the d
13
C data of pedogenic car-
bonates from northern Pakistan in the Himalayan foreland. These
were interpreted as evidence for the origin or intensification of
the Asian monsoon system (Quade et al., 1989). In addition, the
dating of the extensive faults on the Tibetan Plateau suggests a sig-
nificant uplift/extension period at about 8 Ma. On the basis
of these findings and GCM simulations, Prell and Kutzbach
(1992) hypothesized that uplift of the Tibetan Plateau to at least
half of its present height at ~8 Ma caused the intensification of
the Asian monsoon.
In the Chinese Loess Plateau, the base of the loess-paleosol
sequences dated to about 2.6 Ma was previously taken as indicat-
ing the initiation of the East Asian monsoon (Liu and Ding, 1993).
Later, Chinese scientists found that the Red Clay underlying the
loess sequence was also of wind-blown origin and indicative
of monsoon transport (Table M4), so the history of eolian deposits
of the Loess Plateau should be extended to 7–8Ma(Figure M37b;
An et al., 2001). Since the uplift of the Tibetan Plateau can lead
to enhanced aridity in the Asian interior and to intensification
of the Asian monsoon system (Kutzbach et al., 1993), a nearly
concurrent beginning of both the Indian Ocean upwelling and dust
accumulation in central China about 8 Ma ago has been interpreted
as marking the onset of the Indian and East Asian monsoons,
which in turn implies a significant increase in the altitude of the
Plateau (An et al., 2001). However, the recent discovery by Guo
et al. (2002) of a Miocene loess sequence from Qinan, western
Loess Plateau, has further extended the Chinese dust history. Like
the Pleistocene loess, the Miocene loess evinces enhanced aridity
in the dust source areas and energetic winter monsoon winds
required for dust transport, whereas paleosols point to increased
moisture supply by summer monsoon winds. A total of 231 inter-
bedded loess-paleosol layers, representing a nearly continuous his-
tory of eolian dust accumulation from 22 to 6.2 Ma, indicates that
large source areas of eolian dust and energetic winter monsoon
winds existed since early Miocene, at least 14 Ma earlier than pre-
viously thought (Table M4).
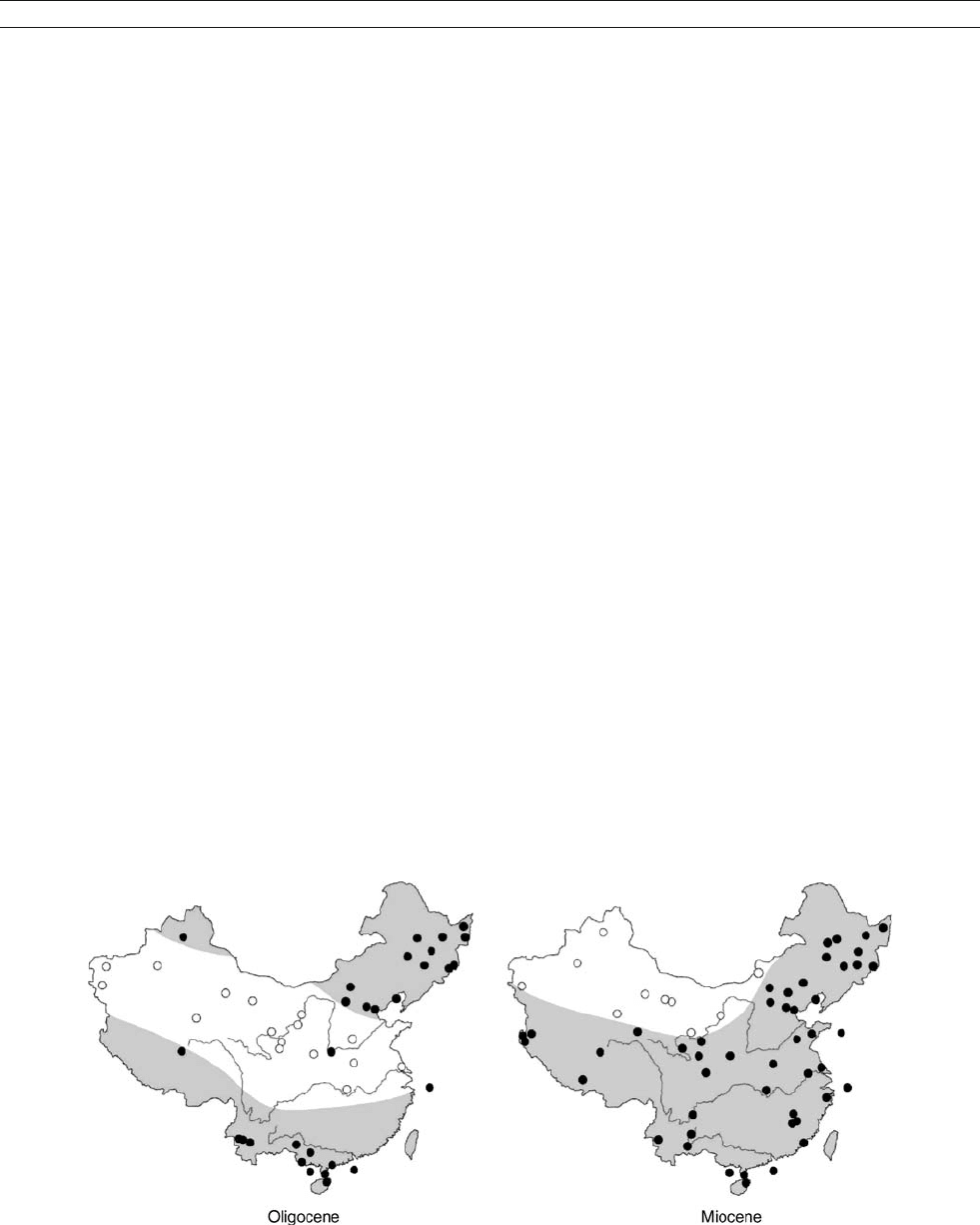
Noticeable climate change from arid to humid climatic condi-
tions in East China occurred as early as around the Oligocene/
Miocene boundary. The synthesized data from oil exploration
and stratigraphic studies indicate that a broad aridity belt stretched
across China from west to east during the Paleogene, particularly
in the Paleocene, before it contracted to Northwest China in the
Neogene (Figure M37), suggesting a transition from a planetary
to monsoonal system in atmospheric circulation over the region.
This climate transition, now confirmed by abundant paleobotani-
cal/palynological and lithostratigraphic data, may further imply
that monsoonal moisture brought westward from the ocean to East
Figure M38 Distribution of arid climate zones in China based on paleobotanical and palynological data: (a) Oligocene; (b) Miocene. Open dots
denote humid vegetation, filled dots denote arid vegetation. The Paleocene and Eocene patterns are similar to those of the Oligocene, while
Pliocene and Pleistocene patterns are close to those of the Miocene. The drastic change between the Oligocene and Miocene implies the
beginning of the modern East Asian monsoon system (Sun and Wang, 2005).
MONSOONS: PRE-QUATERNARY 587
China as a response to the reorganization of the climate system
about 24 Ma ago was probably caused by an enhancement, if
not the first establishment, of the East Asian summer monsoon
(Sun and Wang, 2005). The loess-paleosol sequence at Qinan sup-
ports the Oligocene/Miocene climate transition. The existence of
the Asian monsoon at about 16–14 Ma has been reported from
northern Thailand, where middle Miocene mammalian faunas
were found to have adopted to a monsoon-styled wet climate.
Sedimentological data from ODP Leg 116, Bay of Bengal
also imply the intensification of uplift-induced monsoon in
the early Miocene. Although the dramatic d
13
C increase of total
organic carbon in Bengal Fan sediments at ca. 7 Ma supports
the development of the monsoon in the Himalayan foreland at
this time, the sediment accumulation records are in conflict
with the 8 Ma uplift model. Accumulation rates at several
DSDP/ODP Sites were high for the 17–7 Ma old Bengal
Fan, but decreased from 7 to 1 Ma with the clay mineral assem-
blages indicating reduced physical erosion and strengthened
chemical weathering (Derry and France-Lanord, 1997). No sig-
nificant change in sediment accumulation around 8 Ma has
been found at any ODP Leg 184 sites in the South China Sea
(Wang et al., 2000). On the other hand, if using the planktonic
foraminifer Neogloboquadrina dutertrei as an indicator of the
East Asian monsoon and enhanced productivity in the South
China Sea, its abundance peaks at 7.6 Ma and 3.2 Ma at Site
1146 on the northern slope correspond well to the Indian mon-
soon records (Figure M37c; Wang et al., 2003).
The dust record also indicates that the Asian monsoon
system has a longer history and greater variability both in space
and in time than previously thought. In the Miocene sequence
of Qinan, two intervals are distinguished by higher dust accu-
mulation: 15–13 Ma and 8–7 Ma (Guo et al., 2002). These
might represent periods of enhanced aridity in the source areas,
an interpretation supported by pollen data from Yumen, north-
east of Tibet. Increased aridity over Asia around 8–7 Ma also
explains a peak in dust accumulation rate in the North Pacific
(Figure M37d; Rea et al., 1998).
Monsoon evolution in geological history
The number and geographic coverage of the monsoon records
decrease with increasing age, resulting in relatively deficient
knowledge of the pre-Quaternary monsoon history. Although
it may be premature to discuss the evolution of the monsoon
system through the entire geological history, the examples of
the Pangaean and late Cenozoic times provide convincing
evidence that tectonically-induced changes in sea-land distribu-
tion and in topography have played the primary role in control-
ling the monsoon system over the 10
6
–10
7
year and longer
timescales. Tracing back the entire Phanerozoic history, the
monsoon system strengthened with increased size of continents
and increased altitude of mountains. With the collapse of the
megacontinent, for example, the “megamonsoon” circulation
over Pangaea was subsequently replaced by a basically zonal
circulation (Parrish et al., 1982). The late paleozoic American
Appalachians (estimated average altitude of 4,500 m) and the
European Variscan (2,000–3,000 m) in Pangaea might have
played a role similar to the late Cenozoic Himalaya-Tibet in
the intensification of the concomitant monsoon circulation
(Fluteau et al., 2001).
The development of polar ice-sheets is another major con-
trol of the monsoon system. Clemens et al. (1996) found a non-
stationarity in the phase of the summer monsoon system
relative to a growing Northern Hemisphere ice volume over
the past 3.5 Myr. During the initiation and the growth of the
Northern Hemisphere ice sheets, the phase of strong monsoons
moved away from the phase of maximum ice volume and sys-
tematically drifted in a similar pattern over the past 2.6 Myr.
An intensified winter monsoon resulting from the growth of
the boreal ice sheet was also reported from the Chinese loess
records (Liu and Ding, 1993). Accordingly, the nature of the
monsoon system in the ice-house vs. hot-house Earth must
have been different, and the transition between hot- and ice-
house regimes can be of paramount importance for reconstruct-
ing the monsoon history.
A trustworthy reconstruction of the monsoon history depends
on the proxies adopted. Care is necessary to evaluate how the mon-
soon proxies that were developed for the Quaternary period can be
applied to the earlier geological past. It is equally crucial to distin-
guish monsoon proxies from those driven by other factors. An
example is the late Miocene development of C4 vegetation, exhib-
ited by a d
13
C shift in pedogenic carbonates, which was considered
by Quade et al., (1989) as a signal of the onset of the Indian mon-
soon when first found in Pakistan. Since many subsequent find-
ings have occurred in various continents, this d
13
C shift has been
re-interpreted as representing a large-scale vegetation change
caused by decreased CO
2
concentration on the globe (Cerling
et al., 1997).
Regardless of all the complexity, paleo-monsoons are
increasingly important in pre-Quaternary studies. Climate var-
iations throughout geological history have been driven not only
by higher latitudes where ice-sheets develop but also by lower
latitude factors, especially by the monsoons.
Pinxian Wang and Qianyu Li
Bibliography
An, Z., Kutzbach, J.E., Prell, W.L., and Porter, S.C., 2001. Evolution
of Asian monsoons and phased uplift of the Himalaya-Tibetan plateau
since Late Miocene times. Nature, 411,62–66.
Cane, M.A., and Molnar, P., 2001. Closing of the Indonesian seaway as
a precursor to east African aridification around 304 million years ago.
Nature, 411, 157–162.
Cerling, T.E., Harris, J.M., MacFadden, B.J., Leakey, M.G., Quade, J.,
Eisenmann, V., and Ehleringer, J.R., 1997. Global vegetation change
through the Miocene/ Pliocene boundary. Nature, 389, 153–158.
Clemens, S., Murray, D., and Prell, W., 1996. Nonstationary phase of
the Plio-Pleistocene Asian monsoon. Science, 274, 943–948.
Crowley, T.J., Hyde, W.T., and Short, D.A., 1989. Seasonal cycle variations
on the supercontinent of Pangaea. Geology, 17, 457–460.
Derry, L.A., and France-Lanord, C., 1997. Himalayan weathering and
erosion fluxes: Climate and tectonic controls. In Ruddiman, W.F.
(ed.), Tectonic Uplift and Climate Change. New York: Plenum Press,
pp. 290–312.
Dubial, R.F., Parrish, J.T., Parrish, J.M., and Good, S.C., 1991. The
Pangaean megamonsoon – Evidence from the Upper Triassic Chinle
Formation, Colorado Plateau. Palaios, 6, 347–370
Fluteau, F., Besse, J., Broutin, J., and Ramstein, G., 2001. The Late Permian
climate. What can be inferred from climate modeling concerning
Pangea scenarious and Hercynian range altitude? Palaeogeogr. Palaeo-
climatol. Palaeoecol., 167,39–71.
Table M4 Development of the dust history in the Loess Plateau, China
Deposits Age Reference
Loess-paleosol sequence 0–2.6 Ma Liu and Ding, 1993
Red clay 2.6–8 Ma An et al., 2001
Qinan loess sequence 6.2–22 Ma Guo et al., 2002
588 MONSOONS: PRE-QUATERNARY
