Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.


66 Chapter 4: Models and Theories
as indicated in the figure. This gives the critical current in terms of the measured
magnetization through the high-field Bean model formula
Jc - 2(M+ -M_)/d- 1.59 x 106#oAM/d (A/m2), (49)
where current is measured in amperes, #oAM =/~o(M+ - M_) is in tesla, and d
is the diameter of the sample grains in meters. If CGS units are used Eq. (49)
becomes
Jc - 30(M+ -
M_)/d
(A/cm2),
(50)
where d is now in centimeters.
Hubbard Models and Band Structure
This section provides background information that is important for Hubbard-type
models (Lynn, 1990) and band structure calculations.
The electronic configurations of several atoms that occur commonly in
high-temperature superconductors are given in Table 4.1. The notation used is
nl N,
where n is the principal quantum number, the orbital quantum number
l -- 0 for an s state, l = 1 for a p state, l = 2 for a d state, and N is the number of
electrons in each/-state. A full/-state contains 2(21 + 1) electrons, correspond-
ing to 2, 6, and 10 for s, p, and d states, respectively. The Cu 2+ ion (3d 9) may be
looked upon as a filled d-shell (3d 1~ plus one 3d hole, and in the cuprates this
hole is a
dx2_y2
orbital in the CuO 2 plane.
The various s, p, and d wavefunctions called orbitals have the unnormalized
analytical forms given in Table 4.2, and the spacial electronic charge distribution
of the d orbitals is sketched in Fig. 4.5, the sign on each lobe being the sign of the
wavefunction. Figure 4.6 shows the sigma (a) bonding between oxygenpx andpy
orbitals and copper
dx2_y~
orbitals in a cuprate CuO2 plane.
The orbitals ~b(r- R) used in band structure calculations are normalized
J d p* (r - R)49(r - R)d 3
(51)
r
for an atom located at position R. The overlap integral
fl(R- R')
B(R - R') - 1 4~*(r - R)ck(r - R')d3r
(52)
is a measure of the extent to which the orbitals of atoms at positions R and R'
overlap. The Coulomb integral
U(R),
U(R) - J ck*(r - R)Vc(R)ck(r - R)d3r,
(53)
provides the Coulomb repulsion energy associated with orbital qS(r- R) on an
atom at position R.
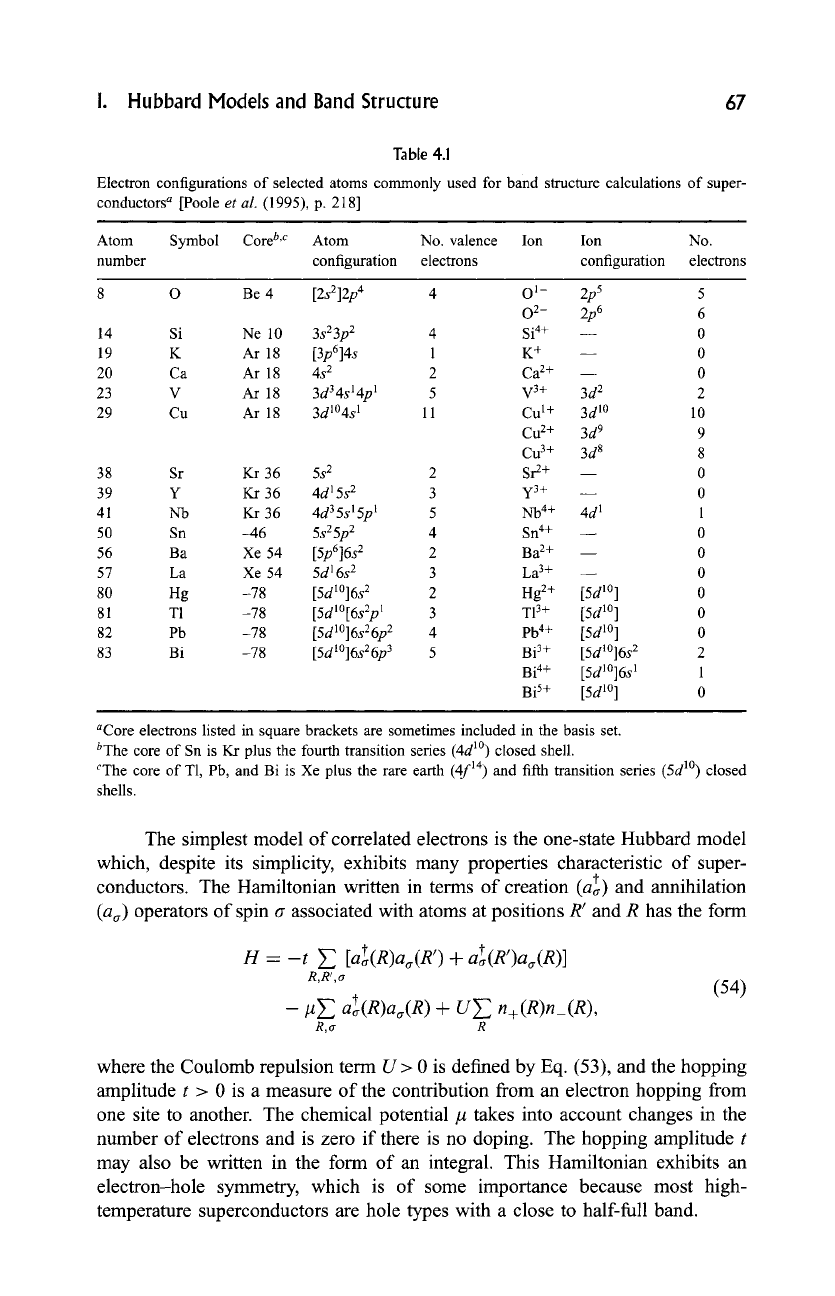
i. Hubbard Models and Band Structure 67
Table 4.1
Electron configurations of selected atoms commonly used for band structure calculations of
super-
conductors a
[Poole
et
al.
(1995), p. 218]
Atom
Symbol Core b'C Atom No.
valence Ion Ion
No.
number configuration electrons configuration electrons
8 O Be 4 [2s212p 4 4 O 1- 2p 5 5
02- 2p 6 6
14 Si Ne 10 3s 23p
2 4
Si 4+ 0
19 K Ar 18 [3p614s 1 K + 0
20 Ca Ar 18 4s 2 2 Ca 2+ 0
23 V Ar 18 3d 34s 1 @1 5 V 3+ 3d 2 2
29 Cu Ar 18 3d1~ 1 11 Cu 1+ 3d 1~ 10
Cu 2+ 3d 9 9
Cu 3+ 3d 8 8
38 Sr Kr 36 5s 2 2 Sr 2+ -- 0
39 Y Kr 36 4d 15s 2 3 y3+ 0
41 Nb Kr 36 4d 35s 15p 1 5 Nb 4+ 4d 1 1
50 Sn -46
5s25p 2
4 Sn 4+ -- 0
56 Ba Xe 54 [5p616s 2 2 Ba 2+ ~ 0
57 La Xe 54 5d 16s 2 3 La 3+ ~ 0
80 Hg -78 [5dl~ 2 2 Hg 2+ [5d 1~ 0
81 Yl -78
[5d1~ 1
3 Yl 3+ [5d l~ ] 0
82 Pb -78
[5dl~ 2
4 Pb 4+ [5d 10] 0
83 Bi -78
[5dl~ 3
5 Bi 3+ [5d1~ 2 2
Bi 4+ [5d1~ 1 1
Bi 5+ [5d 1~ 0
aCore electrons listed in square brackets are sometimes included in the
basis set.
byhe
core of
Sn is Kr plus
the fourth transition
series (4d 1~ closed shell.
CThe
core of
T1, Pb, and Bi is Xe plus
the rare earth (4f
TM)
and fifth transition
series (5d 1~
closed
shells.
The simplest model of correlated electrons is the one-state Hubbard model
which, despite its simplicity, exhibits many properties characteristic of super-
conductors. The Hamiltonian written in terms of creation (a~) and annihilation
(ao) operators of spin a associated with atoms at positions R' and R has the form
H - -t ~ [at~(R)a~(R ') + a;(R')a~(R)]
R,R',a
-lz~ a~(R)a~(R) + UY~ n+(R)n_(R),
R,a R
(54)
where the Coulomb repulsion term U > 0 is defined by Eq. (53), and the hopping
amplitude t > 0 is a measure of the contribution from an electron hopping from
one site to another. The chemical potential p takes into account changes in the
number of electrons and is zero if there is no doping. The hopping amplitude t
may also be written in the form of an integral. This Hamiltonian exhibits an
electron-hole symmetry, which is of some importance because most high-
temperature superconductors are hole types with a close to half-full band.
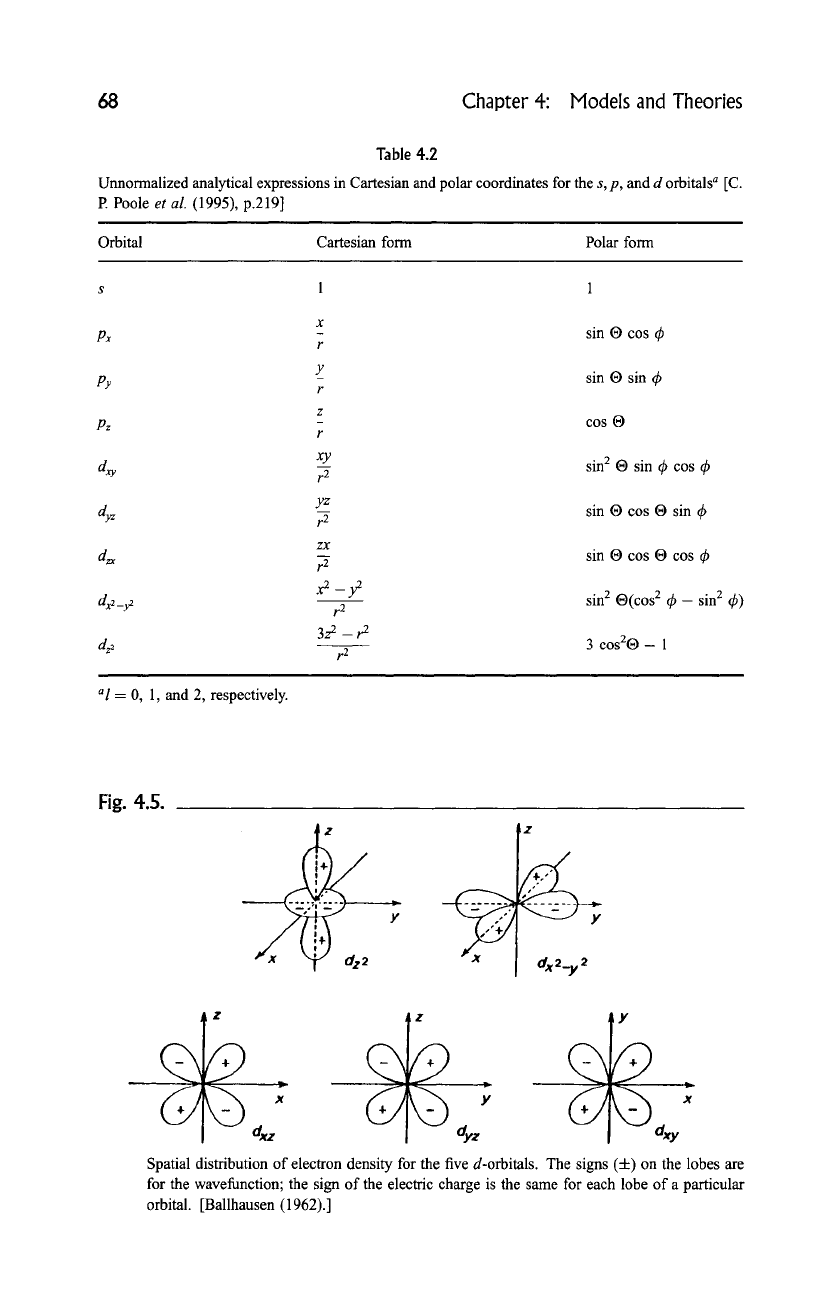
68 Chapter 4: Models and Theories
Table 4.2
Unnormalized analytical expressions in Cartesian and polar coordinates for the s, p, and d orbitals a [C.
P. Poole
et al.
(1995), p.219]
Orbital Cartesian form Polar form
s 1 1
X
Px
r
Y
PY
r
z
Pz
r
xy
dxy r--- ~
yz
dyz r~
zX
dzx r ~
d:_: x2
- y~
t.2
3z 2 _r 2
dg2
al
= 0, 1, and 2, respectively.
sin 19 cos ~b
sin 19 sin ~b
cos |
sin 2 19 sin ~b cos ~b
sin 19 cos 19 sin ~b
sin 19 cos 19 cos ~b
sin 2 | 2 tk- sin 2 ~b)
3 cos2| 1
Fig. 4.5.
~
..y,...Z.. Y
!
-'2
2'
~2~2
I z
" X X
Spatial distribution of electron density for the five d-orbitals. The signs (4-) on the lobes are
for the wavefunction; the sign of the electric charge is the same for each lobe of a particular
orbital. [Ballhausen (1962).]
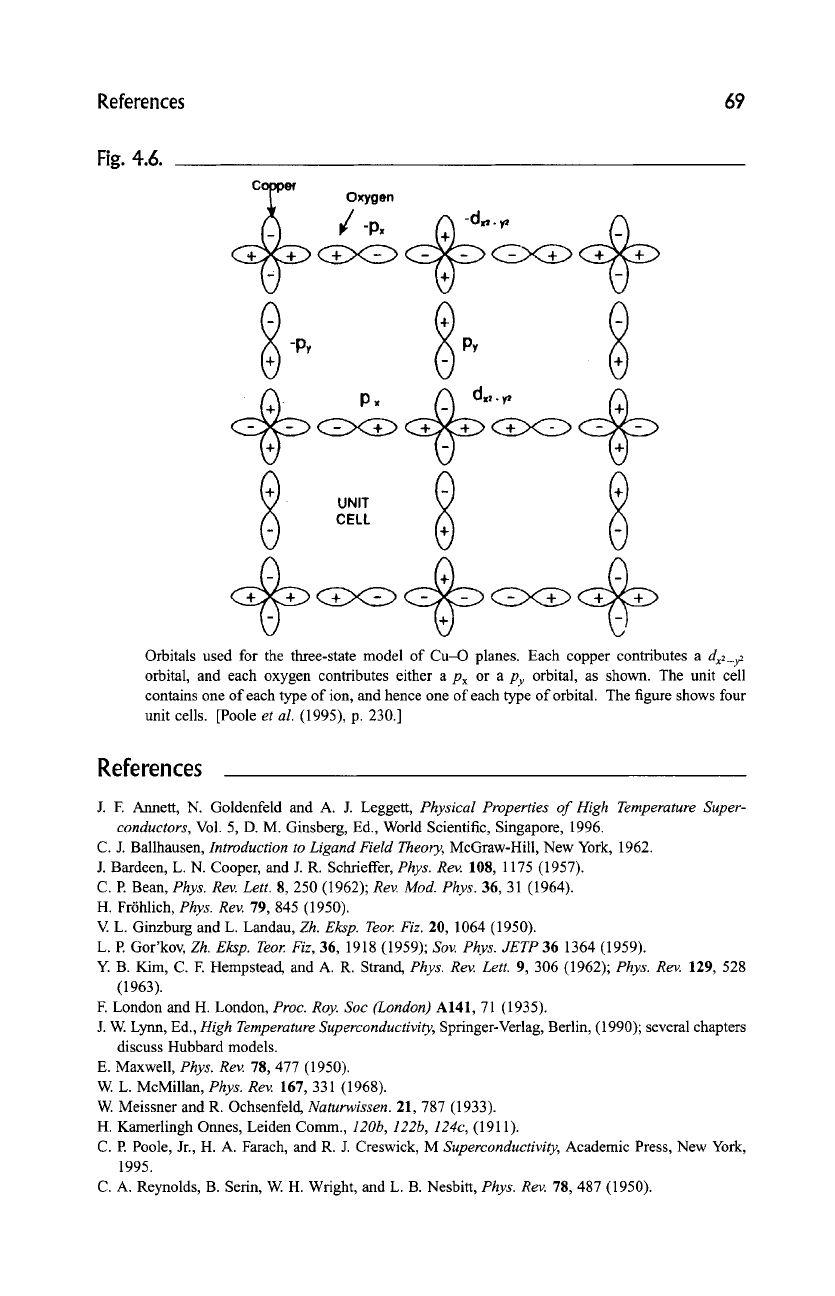
References 69
Fig. 4.6.
c
~
-p~
Oxygen
Orbitals used for the three-state model of Cu-O planes. Each copper contributes a
dx2_y2
orbital, and each oxygen contributes either a Px or a
py
orbital, as shown. The unit cell
contains one of each type of ion, and hence one of each type of orbital. The figure shows four
unit cells. [Poole
et al.
(1995), p. 230.]
References
J. E Annett, N. Goldenfeld and A. J. Leggett,
Physical Properties of High Temperature Super-
conductors,
Vol. 5, D. M. Ginsberg, Ed., World Scientific, Singapore, 1996.
C. J. Ballhausen,
Introduction to Ligand Field Theory,
McGraw-Hill, New York, 1962.
J. Bardeen, L. N. Cooper, and J. R. Schrieffer,
Phys. Rev.
108, 1175 (1957).
C. E Bean,
Phys. Rev. Lett.
8, 250 (1962);
Rev. Mod. Phys.
36, 31 (1964).
H. Fr6hlich,
Phys. Rev.
79, 845 (1950).
V. L. Ginzburg and L. Landau,
Zh. Eksp. Teor Fiz.
20, 1064 (1950).
L. E Gor'kov,
Zh. Eksp. Teor Fiz,
36, 1918 (1959);
Sov. Phys. JETP
36 1364 (1959).
Y. B. Kim, C. E Hempstead, and A. R. Strand,
Phys. Rev. Lett.
9, 306 (1962);
Phys. Rev.
129, 528
(1963).
E London and H. London,
Proc. Roy. Soc (London)
A141, 71 (1935).
J. W. Lynn, Ed.,
High Temperature Superconductivity,
Springer-Vedag, Berlin, (1990); several chapters
discuss Hubbard models.
E. Maxwell,
Phys. Rev.
78, 477 (1950).
W. L. McMillan,
Phys. Rev.
167, 331 (1968).
W. Meissner and R. Ochsenfeld,
Naturwissen.
21,787 (1933).
H. Kamerlingh Onnes, Leiden Comm.,
120b, 122b, 124c,
(1911).
C. P. Poole, Jr., H. A. Farach, and R. J. Creswick, M
Superconductivity,
Academic Press, New York,
1995.
C. A. Reynolds, B. Serin, W. H. Wright, and L. B. Nesbitt,
Phys. Rev.
78, 487 (1950).
This Page Intentionally Left Blank
Chapter S
Superconductor Types
Charles P. Poole, Jr.
Department of Physics and Institute of 5uperconductivit~
University of South Carolina, Columbia, South Carolina
Paul C. Canfield
Department of Physics and Ames Laboratory,
Iowa State University, Ames, Iowa
Arthur P. Ramirez
Lucent Technologies~ Bell Laboratories~ Murray Hill, New Jersey
A. Introduction 72
B. Elements and Alloys 72
C. Description of the Data Tables
D. Classical Compounds 80
E. Perovskites 85
E Heavy Electron Systems 87
G. Borocarbides 92
H. Fullerenes 96
I. Charge Transfer Organics
J. Crystal Chemistry 107
References 108
105
79
ISBN: 0-12-561460-8
$3O.OO
HANDBOOK OF SUPERCONDUCTIVITY
Copyright 9 2000 by Academic Press.
All rights of reproduction in any form reserved.
71

72 Chapter
5:
Superconductor Types
A
Introduction
Until the early 1980s, superconductivity studies were carried out with what are
called classical materials, consisting of elements, alloys, and compounds. Some
categories of compounds produced many superconductors, such as those with the
sodium chloride structure, Laves phases, Chevrel types, and A-15 compounds. In
addition, there are superconducting materials without isomorphous counterparts.
During the few years preceding the advent of superconductivity above 77 K, the
heavy electron and organic superconductors had been discovered and were widely
investigated. During this same period some work was carried out with noncubic
perovskites, precursors for the cuprates, and more recently superconductivity has
been found in cubic barium-potassium-bismuth perovskite. Two other compound
types, borocarbides and especially fullerenes, have been extensively investigated
in recent years.
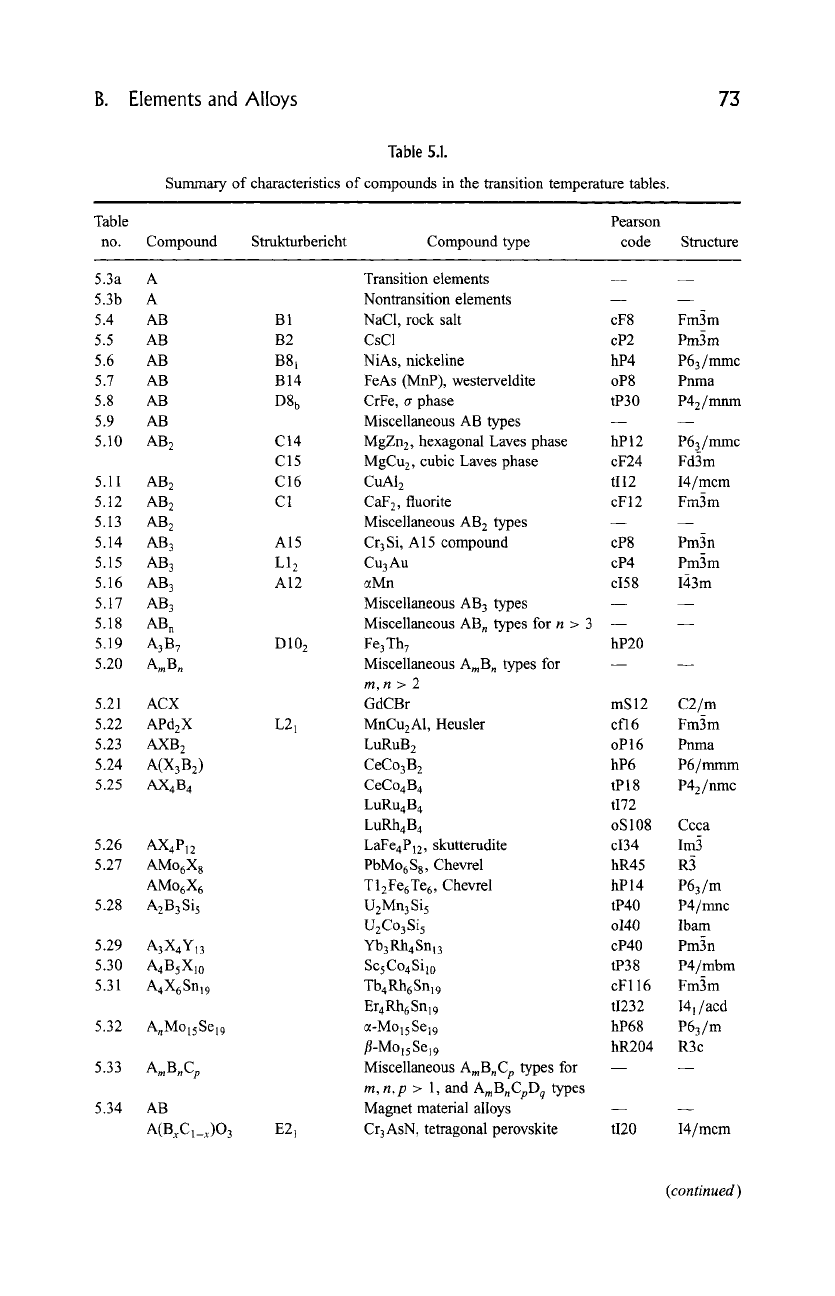
The present chapter comments on and provides 39 tabulations, summarized
in Table 5.1, with systematic listings of T c values for the main classes of
superconducting materials, and the next chapter discusses their structures. The
tabulated T c values were obtained, in almost all cases, from the data furnished by
Vonsovsky
et al.
(1982), Phillips (1989), and Chapter 6 of the present Handbook.
In some cases these sources quoted somewhat different values of T c, and when
this was the case either one specified value or an average was selected for
inclusion here. Our earlier work (Poole
et aL,
1995) may be consulted for more
details. Landolt-Bornstein is the most comprehensive source of T~ values, but
their tabulations are not yet completed. The main object in presenting T c values in
the present tabular form that had been adapted in our initial publication of the
data (Poole and Farach, 1999) is to make it easy to look them up in a context in
which they can be compared with values of related compounds.
Elements and Alloys
Superconductivity was discovered in 1911 when the element mercury exhibited
zero resistance at T c = 4.1 K, and it has been subsequently found in many
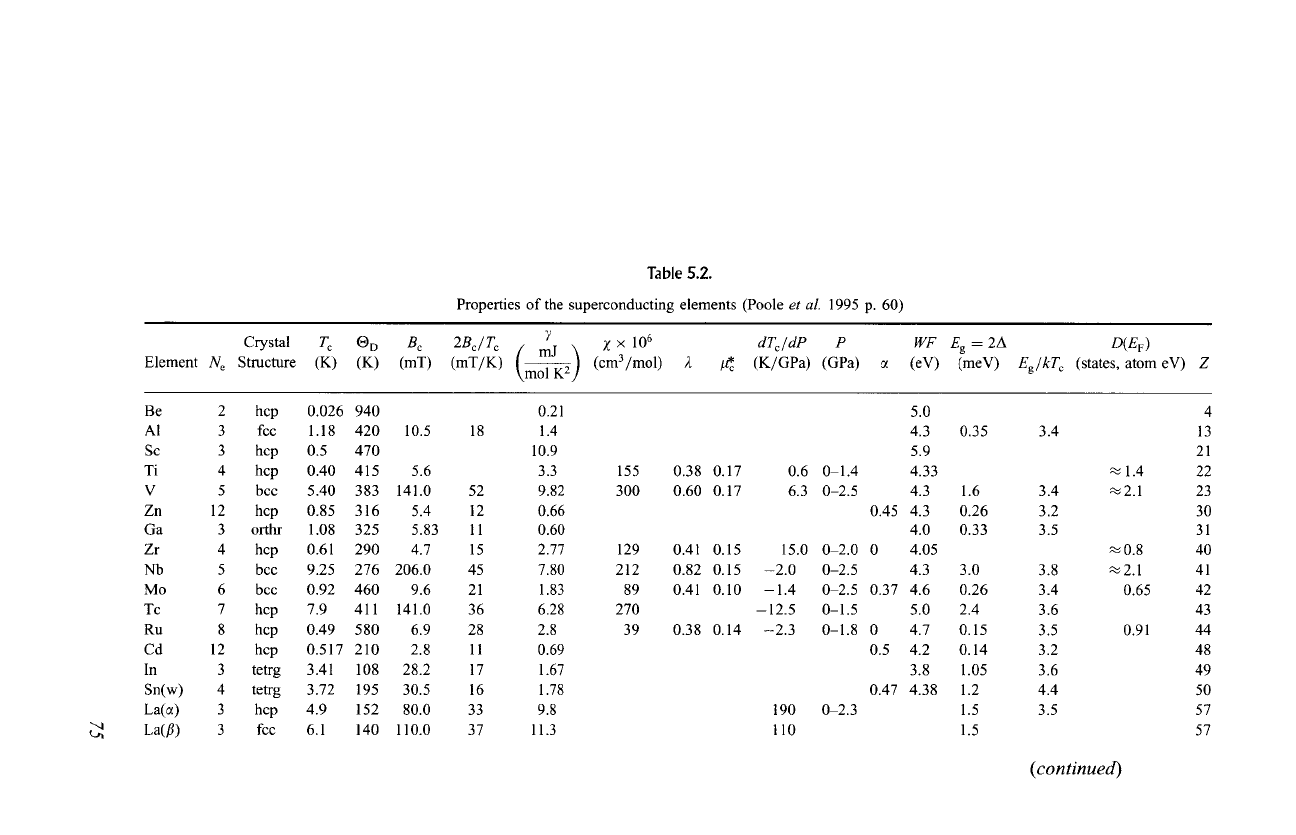
elements, alloys and compounds. Figure 5.1 shows how superconducting
elements cluster in two regions of the periodic table, with the transition metals
on the left and the nontransition metals on the fight. Some elements become
superconducting only as thin films, under pressure, or after irradiation, as
indicated. This figure gives the transition temperature To, the Debye temperature
0 D, the electronic specific heat constant 7, the dimensionless electron-phonon
coupling constant 2, and the density of states at the Fermi level D(EF) for the
B.
Elements and Alloys
73
Table
no.
Table 5.1.
Summary of characteristics of compounds in the transition temperature tables.
, ,,
Pearson
Compound Strukturbericht Compound type code
Structure
5.3a
5.3b
5.4
5.5
5.6
5.7
5.8
5.9
5.10
5.11
5.12
5.13
5.14
5.15
5.16
5.17
5.18
5.19
5.20
5.21
5.22
5.23
5.24
5.25
5.26
5.27
5.28
5.29
5.30
5.31
5.32
5.33
5.34
A Transition elements
A Nontransition elements
AB B 1 NaC1, rock salt cF8 Fm3m
AB B2 CsC1 cP2 Pm3m
AB B81 NiAs, nickeline hP4 P63/mmc
AB B 14 FeAs (MnP), westerveldite oP8 Pnma
AB D8 b CrFe, a phase tP30 P42/mnm
AB Miscellaneous AB types
AB2 C 14 MgZn 2, hexagonal Laves phase hP 12 P63/mmc
_
C 15 MgCu2, cubic Laves phase cF24 Fd3m
AB 2 C16 CuA12 tI12 I4/mcm
AB 2 C 1 CaF2, fluorite cF 12 Fm3m
AB 2 Miscellaneous AB 2 types
AB 3 A15 Cr3Si, A15 compound cP8 Pm3n
AB 3 L12 Cu3Au cP4 Pm3m
AB 3 A12 c~Mn ci58 I43m
AB 3 Miscellaneous AB 3 types --
AB n Miscellaneous AB n types for n > 3
A3B 7 D 102 Fe 3 Th 7 hP20
AraB n Miscellaneous AraB n types for
re, n>2
ACX GdCBr mS12 C2/m
APdEX L21 MnCuEA1, Heusler cfl6 Fm3m
AXB: LuRuB2 oP 16 Pnma
A(X3B2) CeCo3B 2 hP6 P6/mmm
AX4B 4 CeCo4B 4 tP 18 P42/nmc
LuRu4B 4 ti72
LuRh4 B 4 oS 108 Ccca
AX4P12 LaFe4PI2 , skutterudite ci34 Im3
AMo6X 8 PbMo6S8, Chevrel hR45 R3
AMo6X 6 T12Fe6Te6, Chevrel hP14 P63/m
AzB3Si 5 UzMn3Si 5 tP40 P4/nmc
UzCo3Si 5 oi40 Ibam
A3X4Y13 Wb3Rh4Snl3 cP40 Pm3n
A4BsXlo Sc5Co4Silo tP38 P4/mbm
A4X6Snl9 Tb4Rh6Snl9 cF116 Fm3m
Er4Rh6Snl9 ti232 I41/acd
AnMotsSe19 ~-Mo15Se19 hP68 P63/m
fl-MolsSe19 hR204 R3c
AmBnC p Miscellaneous AmBnC p types for
m, n, p > 1, and AmBnCpD q types
AB Magnet material alloys
A(BxCI_z)O 3 E21 Cr3AsN, tetragonal perovskite tI20 I4/mcm
(continued)
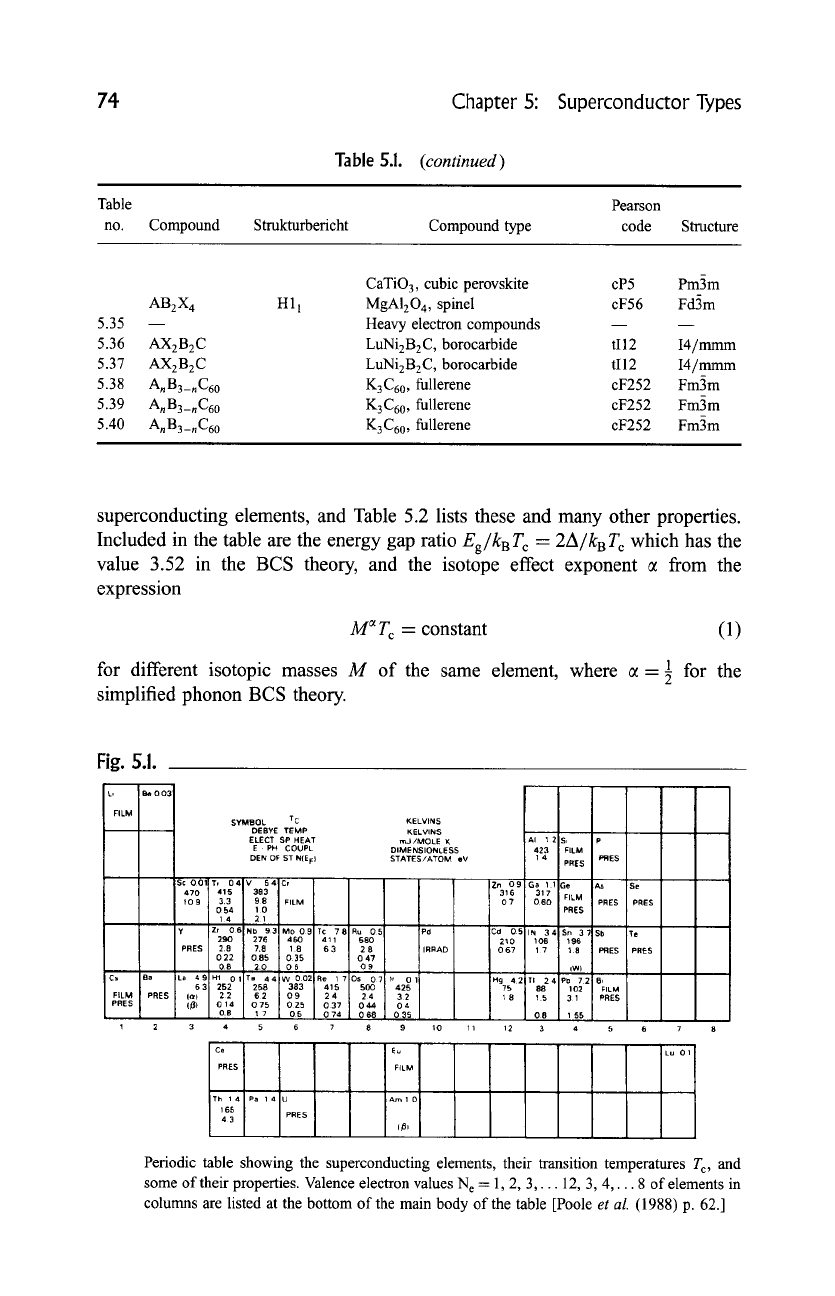
74
Chapter 5: Superconductor Types
Table 5.1.
(continued)
Table Pearson
no. Compound Strukturbericht Compound type code Structure
CaTiO 3, cubic perovskite cP5 Pm3m
AB2X 4 H11 MgA120 4, spinel cF56 Fd3m
5.35 Heavy electron compounds
5.36 AX2B2C LuNi2B2C, borocarbide tI12 I4/mmm
5.37 AX2B2C LuNi2B2C, borocarbide tI12 I4/mmm
5.38 AnB3_nC60 K3C60 , fullerene cF252 Fm3m
5.39 AnB3_nC60 K3C60 , fullerene cF252 Fm3m
5.40 AnB3_nC60 K3C60 , fullerene cF252 Fm3m
superconducting elements, and Table 5.2 lists these and many other properties.
Included in the table are the energy gap ratio
Eg/k B T c - 2A/k B T c
which has the
value 3.52 in the BCS theory, and the isotope effect exponent c~ from the
expression
M" T c - constant (1)
for different isotopic masses M of the same element, where a-89 for the
simplified phonon BCS theory.
Fig. 5.1.
Li
FILM
o.o31
SYMBOL TC KELVINS
DEBYE TEMP KELVINS
ELECT SP HEAT n'~/MOLE K
E - PH COUPL DIMENSIONLESS
DEN OF ST N{EF) STATES/ATOM eV
ISc 0.01 T~ 0 4 V 5.4 Cr ....
470 415 383
10.9 3.3 98 FILM
054 1.0
1.4 2.1
y Zr 0.6; Nb 9.3 MO 0.9 Tc 7.8 Ru 0,5
290 276 460 41 1 580
PRES 2.8 7.8 1.8 63 2.8
0.22 0.85 0.35 0.47
o.e 3.9 06 o
9
Cs Ba La 4.9 HI 0 1 Ta 4 4 W 0.02 Re 1 70s 0.7 tr 0 11
6 3 252 258 383 415 50(3 425
FILM PRES Ia'l 2.2 6.2 0.9 2 4 2.4 3.2
PRES (,~l 014 0 75 0.25 037 0 44 0,
.. 0.8 1 7 0.5 0 74 0 68 0,35
1 2 3 4 5 6 7 8 9
Ce Eu
PRES FILM
Pd
IRRAD
10 11
]
Th 1 4 Pa I 4 I..J Am 1 o
165 PRES
4.3
0.7
Cd 0.5
210
0 67
IHg 4.2
75
18
AI 1.2 St P
423 FILM
1 4 PRES
PRES
Ga 1.1 Ge As Se
317 FILM
0.60 PRES PRES
PRES
IN103 4 S~963.7 Sb Te
1.7 1.8 PLIES PRES
/
TI 2 4 Pio 7.2 6t
88 102 FILM
9 3.1 PRES
0.8 1.55
3 4 5 6 12 7 6
Lu O1
Periodic table showing the superconducting elements, their transition temperatures To, and
some of their properties. Valence electron values N e -- 1, 2, 3 .... 12, 3, 4 .... 8 of elements in
columns are listed at the bottom of the main body of the table [Poole
et aL
(1988) p. 62.]
~
c~
Lr~ ~ t~
l I ~T I
~-~ ~ ~- 00 ~ = ~-~
75
