Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

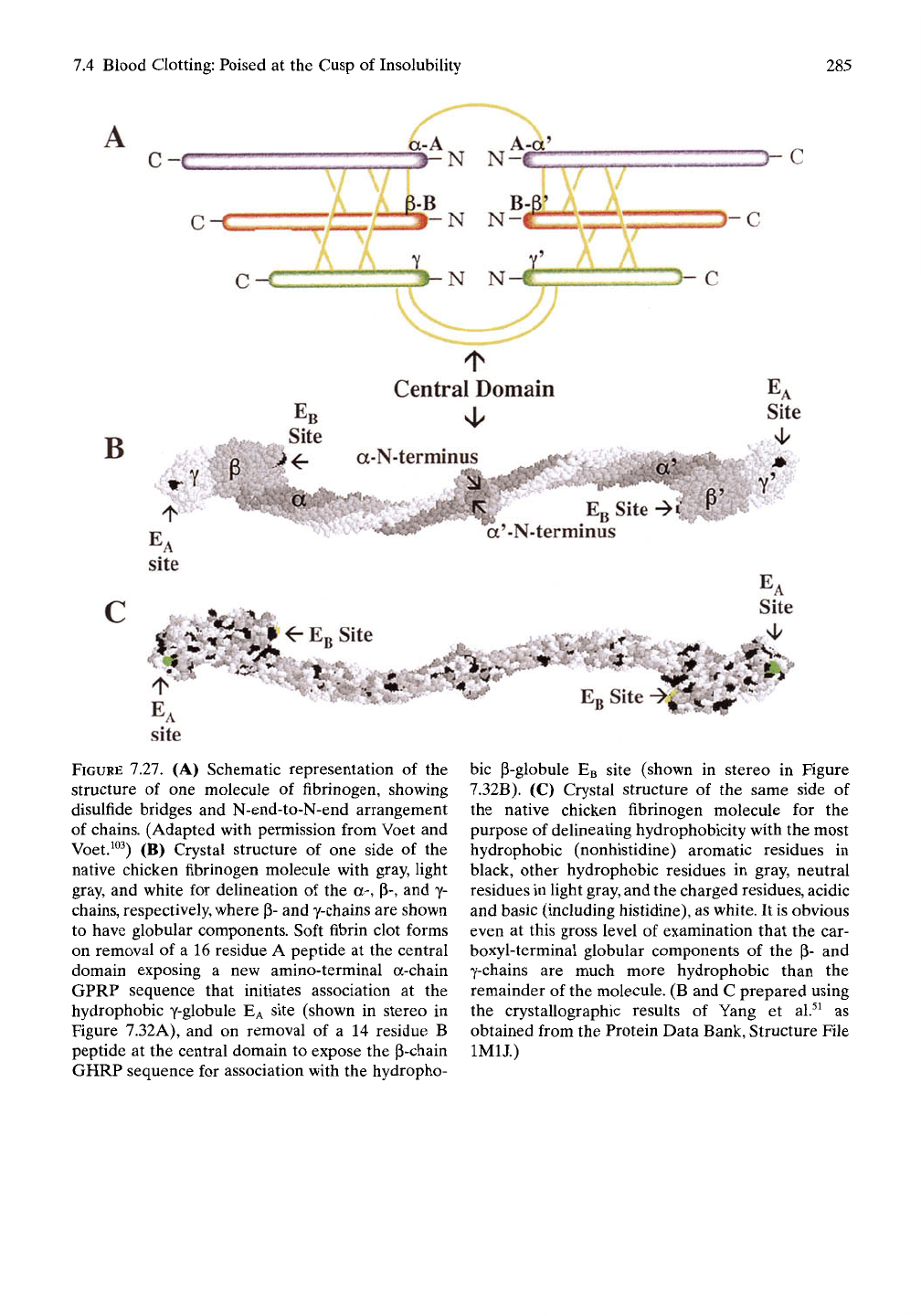
7.4 Blood Clotting: Poised at the Cusp of Insolubility
285
B
c-c
Central Domain
si/
FIGURE
7.27. (A) Schematic representation of the
structure of one molecule of fibrinogen, showing
disulfide bridges and N-end-to-N-end arrangement
of chains. (Adapted with permission from Voet and
Voet.^^^) (B) Crystal structure of one side of the
native chicken fibrinogen molecule with gray, light
gray, and white for dehneation of the a-, (3-, and y-
chains, respectively, where p- and y-chains are shown
to have globular components. Soft fibrin clot forms
on removal of a 16 residue A peptide at the central
domain exposing a new amino-terminal a-chain
GPRP sequence that initiates association at the
hydrophobic y-globule EA site (shown in stereo in
Figure 7.32A), and on removal of a 14 residue B
peptide at the central domain to expose the p-chain
GHRP sequence for association with the hydropho-
bic P-globule EB site (shown in stereo in Figure
7.32B). (C) Crystal structure of the same side of
the native chicken fibrinogen molecule for the
purpose of delineating hydrophobicity with the most
hydrophobic (nonhistidine) aromatic residues in
black, other hydrophobic residues in gray, neutral
residues in fight gray, and the charged residues, acidic
and basic (including histidine), as white. It is obvious
even at this gross level of examination that the car-
boxyl-terminal globular components of the p- and
y-chains are much more hydrophobic than the
remainder of the molecule. (B and C prepared using
the crystaUographic results of Yang et al.^^ as
obtained from the Protein Data Bank, Structure File
IMIJ.)
286
7.
Biology Thrives Near a Movable Cusp of Insolubility
xi
o
^-
a
cd
<
X3
O
a
cd
g
3
X
O Q ^
•-^ ^ ^ H
^ Q O
gn
c^
^ S
5:^
S £
pq
t2j
Q P P
C/5 C/5 5 ^ O
e Q w B 5
M
fc^
OH
CD
Q
<
H Q W W
I P^ ri O
: u o
on
c/5 $5 S 5 p^
o ^ 2 '^ '^
w w :^ o
W O ;z; c/5 tH
^ ^ o u p ^
o o 00 00 E S
.
PIH
H ffi O
00
PLH
C/3 iS ^
O ^ ^ ffi So
H O c^ 5 P^
00 00 H K PH
d ^ ^
o ffi o
^
c/5
1^4
P o <l
< O
C/3
P ^ < ffi
s ^ > 3 P S ^
a
o
U
asgs
r\ "^ PM
J ^ > X^ PMI
w ^ 9
^ C/5 O bi^
>-H LL r>^
Bmm
g
w
3 ^ o o
w
o
E
w Q
ol
^^
hJ HH LJ
i-H fV5 ri
• « rr' < O
5 ^ o S S ^
w Q
$i3
H o d
3
W
O P r5 ffi
c^ C ^ o -^ o
o S < H 2 <
^ < ^ d
Q W < K
> >H ^ O
K^ G Q fc
o a u w
o
O
o
•-^ *^J l-H
< O Pi
n p^ Q Z i< <1
5 00 S 3 ?< U •
QSQH
00 Q r" -^
w w b q
Q -1 ^ W
"S i^
5 o
?- o
»>
'5
VM O
O yi
e o
^ a
II o
g-
O
a
o
cd
g
< d)
w::
D cd
a. o
o o
-^ Cd
1 #
cd 'S
.a <
73 "5
cd ^
<5
.3 D
O </3
OH
O
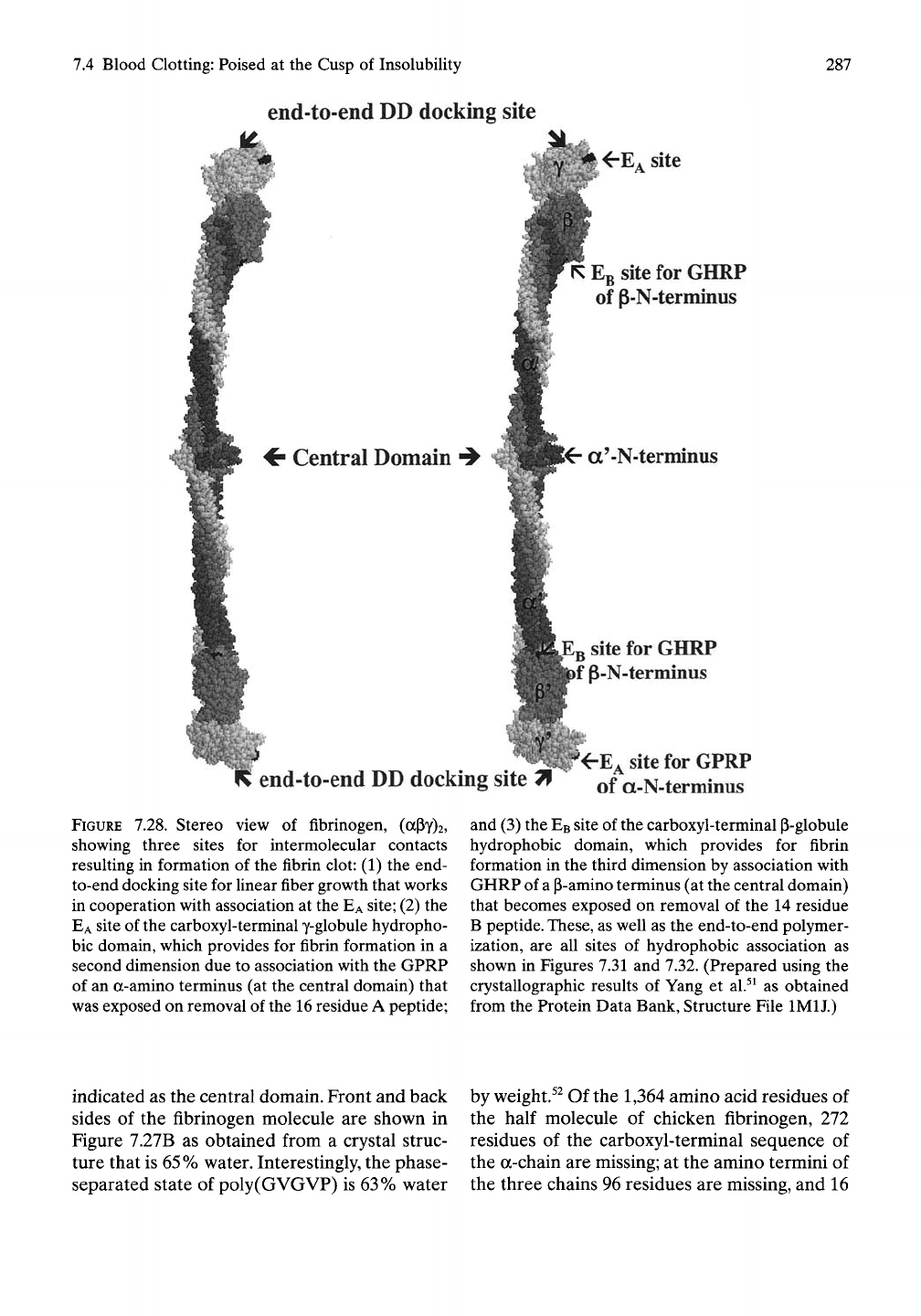
7.4 Blood Clotting: Poised at the Cusp of Insolubility
end-to-end DD docking site
287
^^E^site
Pv
Eg site for CHRP
of p-N-terminus
Central Domain
tir a'-N-terminus
site for CHRP
rf P-N-terminus
end-to-end DD docking site
^<-E^
site for GPRP
of a-N-terminus
FIGURE 7.28. Stereo view of fibrinogen, (aPY)2,
showing three sites for intermolecular contacts
resulting in formation of the fibrin clot: (1) the end-
to-end docking site for linear fiber growth that works
in cooperation with association at the
EA
site; (2) the
EA
site of the carboxyl-terminal y-globule hydropho-
bic domain, which provides for fibrin formation in a
second dimension due to association with the GPRP
of an a-amino terminus (at the central domain) that
was exposed on removal of the
16
residue A peptide;
and (3) the
EB
site of the carboxyl-terminal P-globule
hydrophobic domain, which provides for fibrin
formation in the third dimension by association with
GHRP of
a
p-amino terminus (at the central domain)
that becomes exposed on removal of the 14 residue
B
peptide.
These,
as well as the end-to-end polymer-
ization, are all sites of hydrophobic association as
shown in Figures 7.31 and 7.32. (Prepared using the
crystallographic results of Yang et al.^^ as obtained
from the Protein Data Bank, Structure File IMIJ.)
indicated as the central domain. Front and back
sides of the fibrinogen molecule are shown in
Figure
7.27B
as obtained from a crystal struc-
ture that is 65% water. Interestingly, the phase-
separated state of poly(GVGVP) is 63% water
by weight.^^ Of the 1,364 amino acid residues of
the half molecule of chicken fibrinogen, 272
residues of the carboxyl-terminal sequence of
the a-chain are missing; at the amino termini of
the three chains 96 residues are missing, and 16

288
7.
Biology Thrives Near a Movable Cusp of Insolubility
residues are not seen at the carboxyl terminus
of the y-chain. These missing sequences are
so disordered as to provide no diffraction
pattern.
The absence of the residues noted does not
appear to block an understanding of the
key elements of fibrin formation as much as
might have been initially expected. Binding of
the key residue sequences that become exposed
on removal of the A and B peptides identifies
the
EA
and
EB
sites for intermolecular associa-
tion.
The sequence GPRP, w^hich binds at the
EA
site,
and the sequence GHRP, which binds at
the EB site, are found in the expected sites as
labeled in Figure 7.27B, w^here they look much
like black dots in this whole-molecule view.
The primary message of this consideration of
blood clotting in relation to the movable cusp
of insolubiUty becomes apparent in this whole-
molecule view of fibrinogen in Figure 7.27C.
Even at this level of inspection with the most
hydrophobic amino acid residues given in black
in Figure 7.27C, the greater hydrophobic char-
acter of the carboxyl-terminal globular units of
the y-chain and p-chains is apparent. This aspect
is pursued in more detail below when consid-
ering the double fragment D from human fibrin
itself,
which is the end-to-end association of the
y-chain globular units.
7.4.2.2
Tf-based Hydrophobicity Plots of
Human Fibrinogen Chains
The single residue Tt-based hydrophobicity
plot and the mean residue hydrophobicity plot
reported for the chains of fibrinogen provide
interesting insights. A gUmpse of the single
residue plot provides immediate sequence
insights that are easier to glean from such a plot
than from the sequences as listed in Table 7.1.
The mean residue hydrophobicity plot pro-
vides an opportunity to visualize hydrophobic
weightings of sequences and to recognize
pattern similarities between chains that may be
associated with function, as seen with the
myoglobin and hemoglobin chains in Figures
7.2 and 7.9. Recall that the mean residue
hydrophobicity plot averages the value for an
11 residue sliding window with the numbered
residue as the central residue and with 37° C
being the value used entering and leaving the
sequence. For example, the mean value for
residue 1 uses TfValues of
37°
C for positions
1 to 5 and the averaged value of residues 1
through 6; and the mean Tt-value for residue 6
is the average value for the first 11 residues.
7.4.2.2.1 y-Fibrinogen—A Striking
Hydrophobic Sequence Near the
Carboxyl End
As shown in Figure 7.29, from the perspec-
tive of hydrophobic sequences, residues 337
through 379 of the y-fibrinogen chain consti-
tutes the most hydrophobic sequence, and the
mean residue hydrophobicity plot bears an
interesting pattern of hydrophobicity peaks
that bears a striking resemblance in shape and
intensity to the 401 to 448 sequence of the
BP-fibrinogen chain (Figure 7.30B). There are
additional mean residue hydrophobicity peaks
of lesser prominence centered near residues
240,
278, and 310. This provides an interesting
opportunity to look for correlations with points
of intermolecular association leading to fibrin
clot formation as represented by the crystal
structure data.
7.4.2.2.2 Aa-Fibrinogen—A Striking
Hydrophobic Sequence (-80 Residues)
at Midsequence
The most striking feature of the Aa-fibrinogen
mean residue hydrophobicity plot (plot not
included), a sequence of 610 residues, is the
central 81 residue sequence (residues 284 to
365) that is devoid of all but a single charged
residue, which is the moderate lysine (Lys, K)
residue and which contains four tryptophan
(Trp,
W) residues, the most hydrophobic
residue. The mean residue plot for Aa-
fibrinogen (plot not shown), however, is devoid
of significant hydrophobic peaks in contrast to
those observed in Figure 7.9 for the a- and P-
chains of hemoglobin A and for those of the y-
fibrinogen chain in Figure
7.29.
The presence of
the repetitive central sequence in humans, but
not in the chicken sequence, would provide a
striking opportunity for hydrophobic associa-
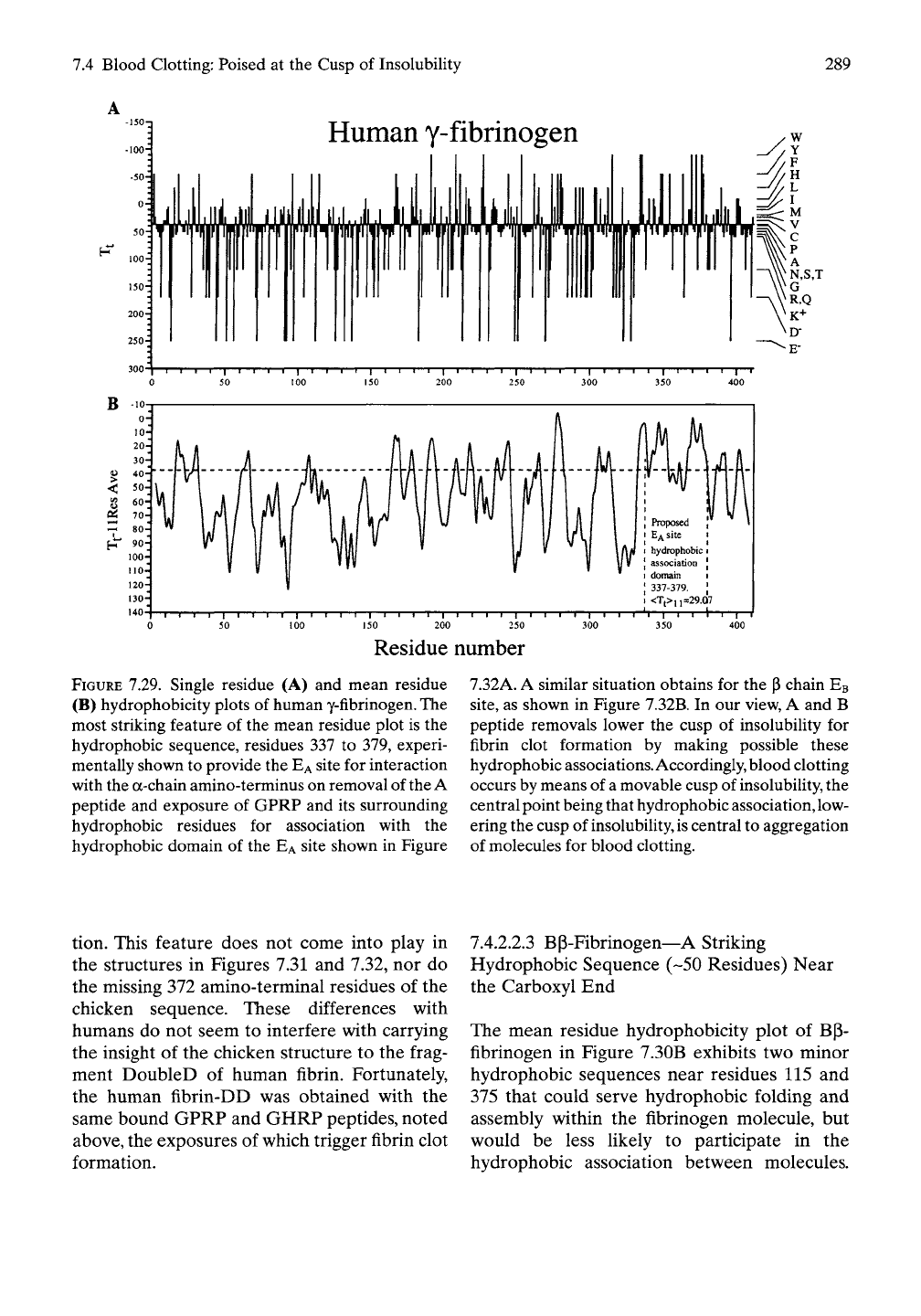
7.4 Blood Clotting: Poised at the Cusp of Insolubility
289
Human y-fibrinogen
Residue number
FIGURE 7.29. Single residue (A) and mean residue
(B) hydrophobicity plots of human y-fibrinogen. The
most striking feature of the mean residue plot is the
hydrophobic sequence, residues 337 to 379, experi-
mentally shown to provide the
EA
site for interaction
with the a-chain amino-terminus on removal of
the
A
peptide and exposure of GPRP and its surrounding
hydrophobic residues for association with the
hydrophobic domain of the
EA
site shown in Figure
7.32A. A similar situation obtains for the
(3
chain
EB
site,
as shown in Figure 7.32B. In our view, A and B
peptide removals lower the cusp of insolubility for
fibrin clot formation by making possible these
hydrophobic associations. Accordingly, blood clotting
occurs by means of
a
movable cusp of
insolubility,
the
central point being that hydrophobic association, low-
ering the cusp of insolubility,
is
central to aggregation
of molecules for blood clotting.
tion. This feature does not come into play in
the structures in Figures 7.31 and 7.32, nor do
the missing 372 amino-terminal residues of the
chicken sequence. TTiese differences w^ith
humans do not seem to interfere with carrying
the insight of the chicken structure to the frag-
ment DoubleD of human fibrin. Fortunately,
the human fibrin-DD was obtained with the
same bound GPRP and GHRP peptides, noted
above, the exposures of which trigger fibrin clot
formation.
7.4.2.2.3
BP-Fibrinogen—A Striking
Hydrophobic Sequence (-50 Residues) Near
the Carboxyl End
The mean residue hydrophobicity plot of BP-
fibrinogen in Figure
7.30B
exhibits two minor
hydrophobic sequences near residues 115 and
375 that could serve hydrophobic folding and
assembly within the fibrinogen molecule, but
would be less likely to participate in the
hydrophobic association between molecules.
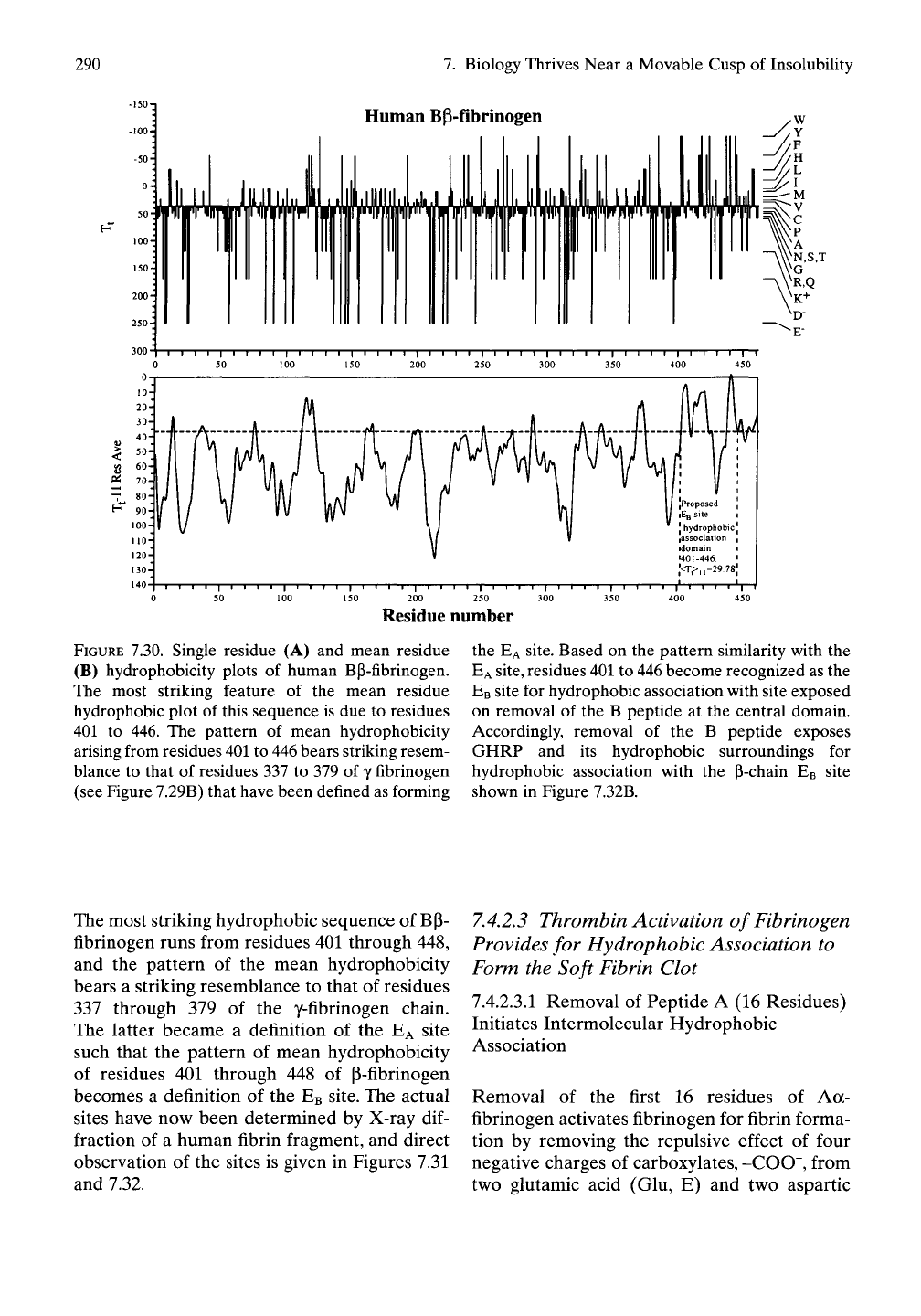
290
7.
Biology Thrives Near a Movable Cusp of Insolubility
200 250 300
Residue number
FIGURE 7.30. Single residue (A) and mean residue
(B) hydrophobicity plots of human Bp-fibrinogen.
The most striking feature of the mean residue
hydrophobic plot of this sequence is due to residues
401 to 446. The pattern of mean hydrophobicity
arising from residues
401
to 446 bears striking resem-
blance to that of residues 337 to 379 of y fibrinogen
(see Figure 7.29B) that have been defined as forming
the
EA
site. Based on the pattern similarity with the
EA
site,
residues
401
to 446 become recognized as the
EB
site for hydrophobic association with site exposed
on removal of the B peptide at the central domain.
Accordingly, removal of the B peptide exposes
GHRP and its hydrophobic surroundings for
hydrophobic association with the P-chain EB site
shown in Figure 7.32B.
The most striking hydrophobic sequence of BP-
fibrinogen runs from residues 401 through 448,
and the pattern of the mean hydrophobicity
bears a striking resemblance to that of residues
337 through 379 of the y-fibrinogen chain.
The latter became a definition of the EA site
such that the pattern of mean hydrophobicity
of residues 401 through 448 of P-fibrinogen
becomes a definition of the
EB
site. The actual
sites have now been determined by X-ray
dif-
fraction of a human fibrin fragment, and direct
observation of the sites is given in Figures 7.31
and 7.32.
7,4,2.3
Thrombin Activation of Fibrinogen
Provides for Hydrophobic Association to
Form the Soft Fibrin Clot
7.4.2.3.1 Removal of Peptide A (16 Residues)
Initiates Intermolecular Hydrophobic
Association
Removal of the first 16 residues of Aa-
fibrinogen activates fibrinogen for fibrin forma-
tion by removing the repulsive effect of four
negative charges of carboxylates, -COO", from
t^o glutamic acid (Glu, E) and two aspartic
7.4 Blood Clotting: Poised at the Cusp of Insolubility
291
acid (Asp, D) residues. This also decreases the
apolar-polar repulsive free energy of interac-
tion expressed in Equation (5.13) of Chapter 5,
section 5.7.9, and allows hydrophobic associa-
tion to proceed. The result is the exposure of
a GPRP sequence from each a-chain of the
central domain for association with
EA
sites of
y-fibrinogen.
The EA site provides for association with
another fibrinogen molecule, that is, with the
(Aa)2(B (3)2(7)2 molecule. The experimentally
defined sequence that effects polymerization of
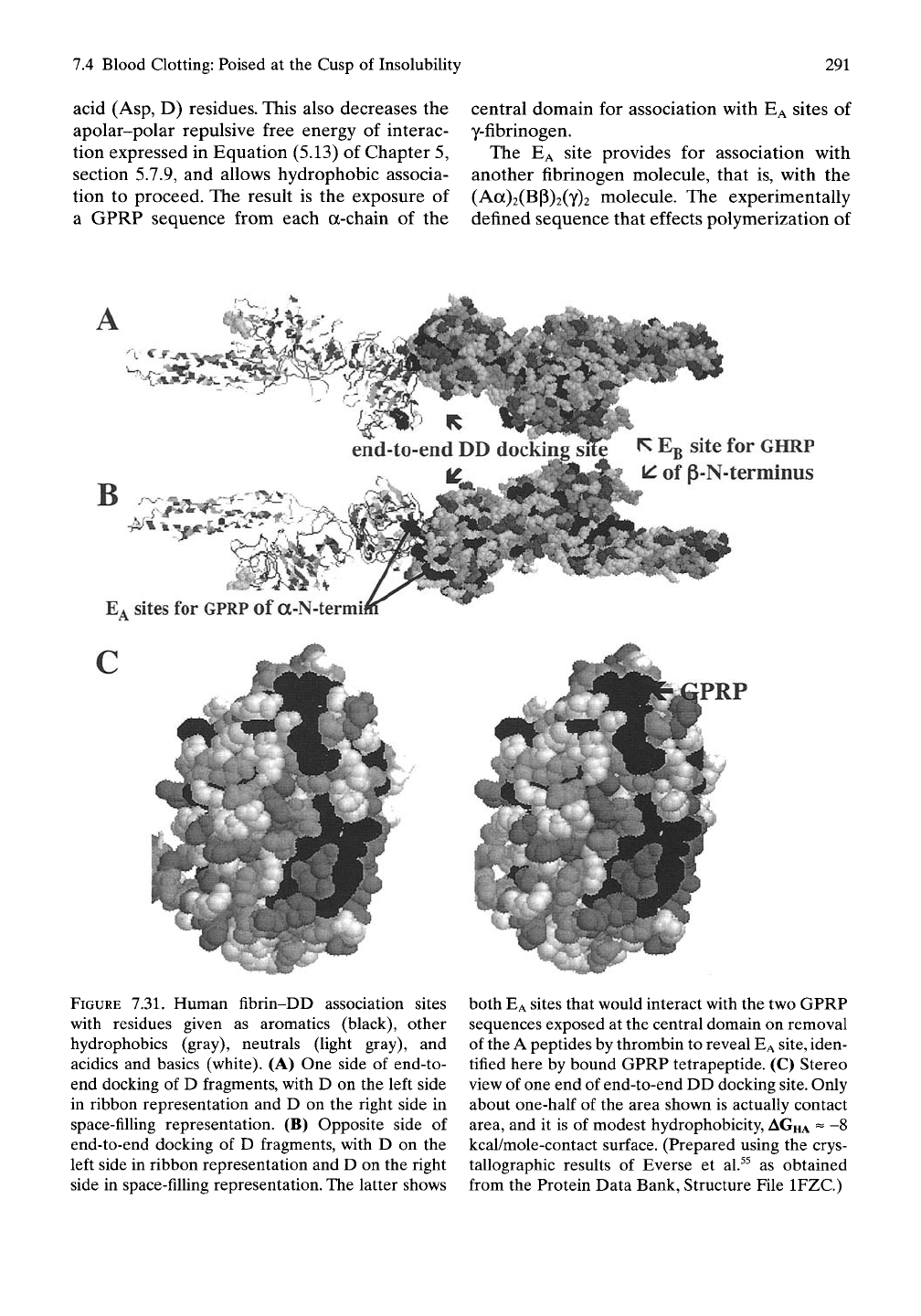
end-to-end DD docl
R Eg site for CHRP
^ of P-N-terminus
E*
sites for
GPRP
of a-N-termi
PRP
'^^i:^*
[# ^.
FIGURE 7.31. Human fibrin-DD association sites
with residues given as aromatics (black), other
hydrophobics (gray), neutrals (light gray), and
acidics and basics (white). (A) One side of end-to-
end docking of D fragments, with D on the left side
in ribbon representation and D on the right side in
space-filling representation. (B) Opposite side of
end-to-end docking of D fragments, with D on the
left side in ribbon representation and D on the right
side in space-filling representation. The latter shows
both
EA
sites that would interact with the two GPRP
sequences exposed at the central domain on removal
of the A peptides by thrombin to reveal
EA
site,
iden-
tified here by bound GPRP tetrapeptide. (C) Stereo
view of one end of end-to-end DD docking
site.
Only
about one-half of the area shown is actually contact
area, and it is of modest hydrophobicity,
AGHA
~ -8
kcal/mole-contact surface. (Prepared using the crys-
tallographic results of Everse et al.^^ as obtained
from the Protein Data Bank, Structure File IFZC.)
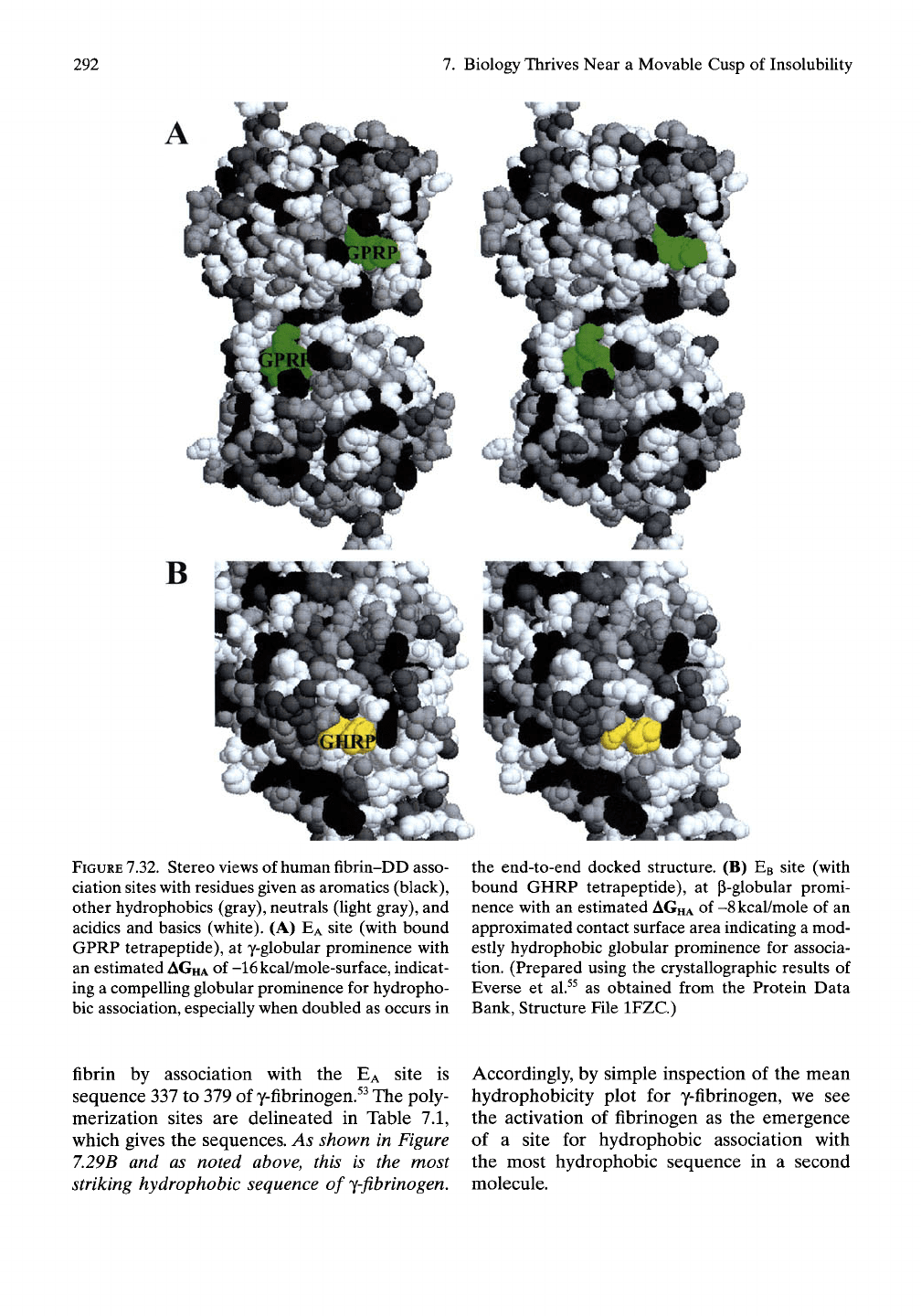
292
7.
Biology Thrives Near a Movable Cusp of Insolubility
FIGURE
7.32.
Stereo views of human fibrin-DD asso-
ciation sites with residues given as aromatics (black),
other hydrophobics (gray), neutrals (light gray), and
acidics and basics (white). (A)
EA
site (with bound
GPRP tetrapeptide), at y-globular prominence with
an estimated
AGHA
of -16kcal/mole-surface, indicat-
ing a compelling globular prominence for hydropho-
bic association, especially when doubled as occurs in
the end-to-end docked structure. (B)
EB
site (with
bound GHRP tetrapeptide), at p-globular promi-
nence with an estimated AGHA of -8kcal/mole of an
approximated contact surface area indicating a mod-
estly hydrophobic globular prominence for associa-
tion. (Prepared using the crystallographic results of
Everse et al.^^ as obtained from the Protein Data
Bank, Structure File IFZC.)
fibrin by association with the EA site is
sequence 337 to 379 of y-fibrinogen.^^ The poly-
merization sites are delineated in Table 7.1,
which gives the sequences. As shown in Figure
7.29B
and as noted above, this is the most
striking hydrophobic sequence of y-fibrinogen.
Accordingly, by simple inspection of the mean
hydrophobicity plot for y-fibrinogen, we see
the activation of fibrinogen as the emergence
of a site for hydrophobic association with
the most hydrophobic sequence in a second
molecule.

7.4 Blood Clotting: Poised at the Cusp of Insolubility
293
Furthermore, as seen in Figure 7.28, the EA
site is approximately at right angles to and
shares a common edge with the end-to-end
DoubleD association site such that these sites
could cooperate in initiation of the hydropho-
bic associations for soft fibrin clot formation.
7.4.2.3.2 Removal of Peptide B (14 Residues)
Allows for Further Intermolecular
Hydrophobic Association
Removal of the first 14 residues of Bp-
fibrinogen activates fibrinogen for fibrin forma-
tion by removing the repulsive effect of three
negative charges of carboxylates, -COO", from
two glutamic acid (Glu, E) residues and one
aspartic acid (Asp, D) residue. Removal of the
B peptide creates or exposes a GHRP site near
the central domain defined in Figure 7.27 that
associates with the carboxyl-terminal end of
the p-fibrinogen, the EB site, chain of another
fibrinogen molecule.^^ This sequence has not
been specifically delineated as above for y-
fibrinogen. Nonetheless, based on the structural
homology between y-fibrinogen and p-
fibrinogen, as demonstrated by the P-chain and
y-chain topologies given in Figure 3a of Sprag-
gon et
al.,^"*
and particularly based on the mean
residue plot of the y-chain (Figure 7.29B) with
its striking similarity to the mean residue plot
of the p-chain (Figure 7.30B), the correspond-
ing sequence for association with the EB site
would involve residues within the sequence
from residues 401 through 448. The mean
residue hydrophobicity plot for residues 401
through 448 in B P-fibrinogen demonstrates
hydrophobic homology to sequences 337 to 379
of y-fibrinogen (Figure 7.29B). Accordingly, by
simple inspection of a mean hydrophobicity
plot, this time for P-fibrinogen, we again see a
dominantly hydrophobic sequence and recog-
nize hydrophobic association as the basis for
soft fibrin clot formation.
7.4.2,4
Hydrophobic Associations of
Fragment DoubleD from Human Fibrin
When the fibrin clot is cleaved by the protease
plasmin, it fragments into two parts, a D frag-
ment and a smaller E fragment. Although no
crystal structures have been obtained for the E
fragment, DooUttle and coworkers have
obtained crystal structures for a factor XIII
(thrombin-activated transglutaminase) cross-
linked DoubleD fragment in which D frag-
ments are attached end to end.^"^ Fortunately,
these crystal have been obtained and the struc-
tures solved with bound GPRP and GHRP
peptides such that the EA and EB sites have
been identified.^^ With these accomplishments
of the Doolittle group, it becomes possible to
characterize the associations of chains that
result in blood clotting. The objective is to
assess the hydrophobicity of the association
sites to challenge further our perspective that
"biology thrives at the cusp of insolubiUty,"
which cusp of insolubility represents the
process of hydrophobic association.
7.4.2.4.1 Hydrophobicity of the Points
of Association
Three key points of association become clear.
They are the EA and
EB
sites identified on the
fibrinogen molecule of Figures 7.27B and 7.28
and demonstrated in the human fragment
DoubleD of Figure 7.31 A,B and the end-to-end
docking site found in the human fragment
DoubleD. This provides the opportunity to
examine directly the hydrophobicity of these
three sites of aggregation for soft fibrin clot for-
mation and to estimate hydrophobicity in terms
of estimates of relative values for the Gibbs
free energy of hydrophobic association, AGHA,
of the contact faces or prominences.
7.4.2.4.2 End-to-End Docking Uses the
Carboxyl-terminal Globular Component
of the y-chain
With the crystal structure, the end-to-end
docking prominence becomes defined. The
docking site is shown in side views in Figure
7.31A,B and end on in Figure 7.31C. This is a
site of modest hydrophobicity that involves
using only about half of the end observed in
Figure 7.31C. One estimates the Gibbs free
energy of hydrophobic association,
AGHA,
to
be -8kcal/mole of contact surface. It should
be appreciated that this is a gross estimate
that should be improved once appropriate
programs have been prepared. The calculation

294
7.
Biology Thrives Near a Movable Cusp of Insolubility
utilizes the values,
AGHA,
in Table 5.3 for the
amino acid residues and subjectively reduces
those values with crude estimates of the
amount of exposure of the side chain to the
surface concerned and a further reduction of
the contributions of charged residues by half
when ion paired.
7.4.2.4.3 Hydrophobicity of the
EA
Site and
a Presumed Cooperative Binding with
End-to-End Docking
As is apparent in Figure 7.31B, the EA site
occurs as a double site at the junction of two
molecules. Coarse calculations of the free
energy for hydrophobic association at each of
the EA sites in Figure 7.32A indicate an espe-
cially hydrophobic surface with an estimate for
AGHA
of -16kcal/mole-EA site, which would be
twice that number for the double site. It seems
reasonable to expect that two sites of the
fibrinogen molecule exposed at the central
domain on removal of the A peptides from the
amino terminus of each a-chain would them-
selves cooperatively bind with both EA sites.
The process of assembly of the two
EA
sites on
different fibrinogen molecules with the two
thrombin exposed GPRP-containing sites in
proper relationship at the central domain could
be expected to cooperatively facilitate the end-
to-end docking. In this way thrombin activation
of fibrinogen would initiate fibrin clot forma-
tion. Initiation of fibrin clot formation becomes
the stitching together of hydrophobic domains.
"Biology thrives at the movable cusp of
insolubiUty...."
7.4.2.4.4 Hydrophobicity of the Eg Site
The
EB
site gains its potential for hydrophobic
association primarily by the unusual clustering
of the most hydrophobic residue, tryptophan,
specifically residues W424, W440, W444, and
W385,
as well as the additional very hydropho-
bic aromatic residues F374, F457, Y345, and
Y445.
Five of those residues contribute to the
mean residue hydrophobicity pattern in Figure
7.30B, and small adjustments in surface expo-
sure on association could create the most
hydrophobic single site for fibrin assembly.
Perhaps the 272 missing carboxyl-terminal
residues of the a-chains serve to cover the
hydrophobic
EB
and
EA
sites until pushed aside
by the much greater hydrophobic association
potential of the sites exposed on removal of the
A and B peptides.
7.4.2.4.5 The Limited Information on
the GPRP and CHRP Sites at the
Central Domain
The missing 96 residues at the amino termini of
the three chains, in the vicinity of the central
domain, preclude definition of the hydrophobic
domains that pair with the
EA
and
EB
sites, but
one predicts the capacity of these missing
residues to arrange in formation of hydropho-
bic domains with which to pair with the total of
four
EA
and
EB
sites.
A description of the general conclusion of
blood clotting as driven by hydrophobic associ-
ation follows.
7.4,2,5
Cross-linking to Form the Hard
Fibrin Clot
The hydrophobic associations with the EA site
of the hydrophobic 337 to 379 sequence of the
y-chain and with the
EB
site of the hydrophobic
409 to 451 sequence of the p-chain result in
antiparallel alignment of the y-chains from
dif-
ferent fibrin molecules near the end-to-end
y-chain-y-chain docking site. In particular,
by means of a transamidase, glutamine-399 of
one y-chain cross-links to the lysine-406 of the
abutting second y-chain, and glutamine-399 of
the second y-chain cross-Unks with the
lysine-406 of the first y-chain to form two £-(y-
glutamyl)lysine cross-links. These chemical
cross-links transform the soft fibrin clot irre-
versibly into the hard fibrin clot, which can only
be disassembled by subsequent proteolytic
enzymes.
ThuSy
the key process of fibrin clot formation
is one of cleaving peptide sequences, the result of
which is to lower the cusp of insolubility^ repre-
senting the hydrophobic association between
molecules, from above to below physiological
temperature.
