Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

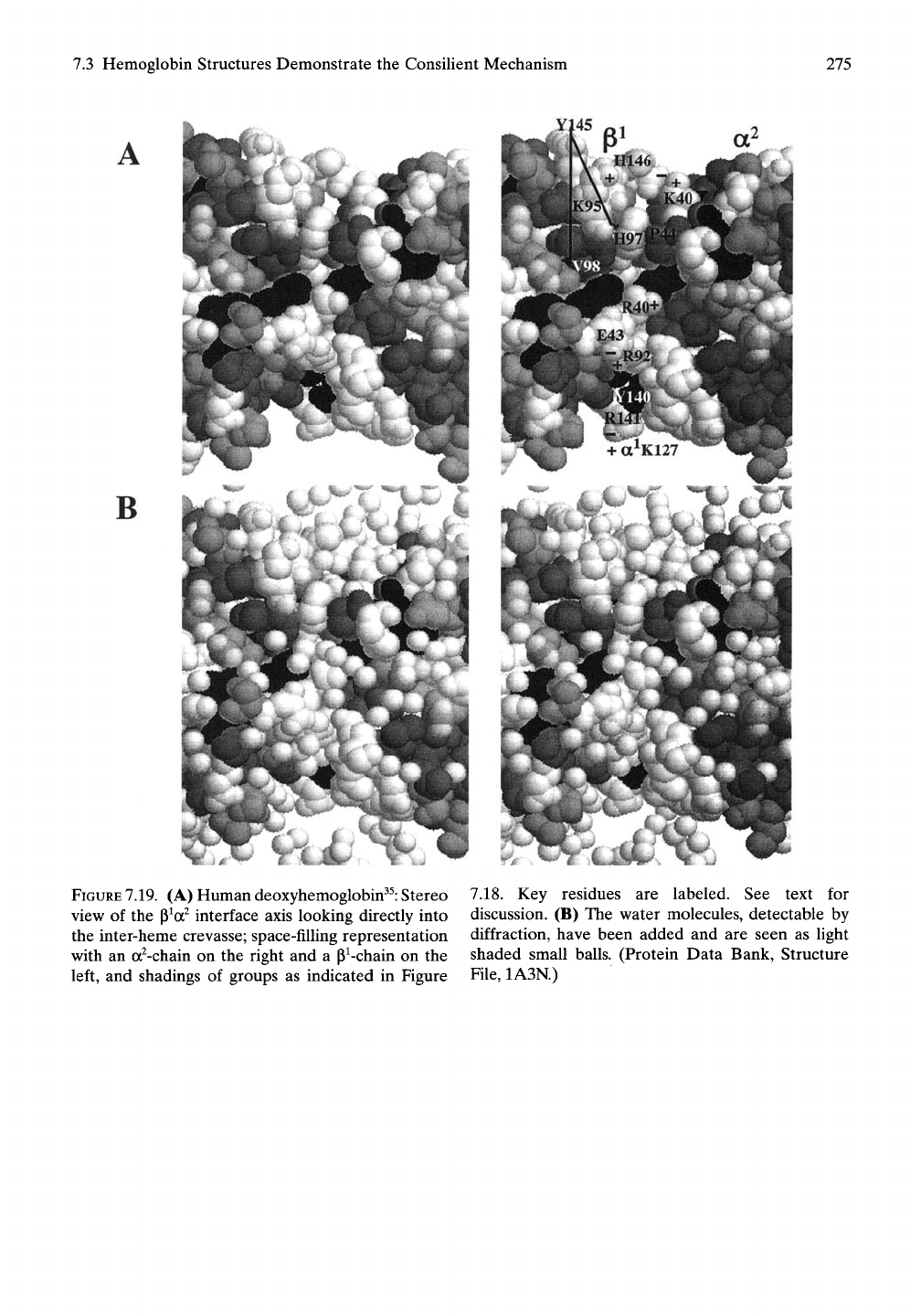
7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
275
B
FIGURE
7.19.
(A) Human deoxyhemoglobin^^: Stereo
view of the p^a^ interface axis looking directly into
the inter-heme crevasse; space-filling representation
with an a^-chain on the right and a P^-chain on the
left, and shadings of groups as indicated in Figure
7.18. Key residues are labeled. See text for
discussion. (B) The water molecules, detectable by
diffraction, have been added and are seen as light
shaded small balls. (Protein Data Bank, Structure
File,
1A3N.)
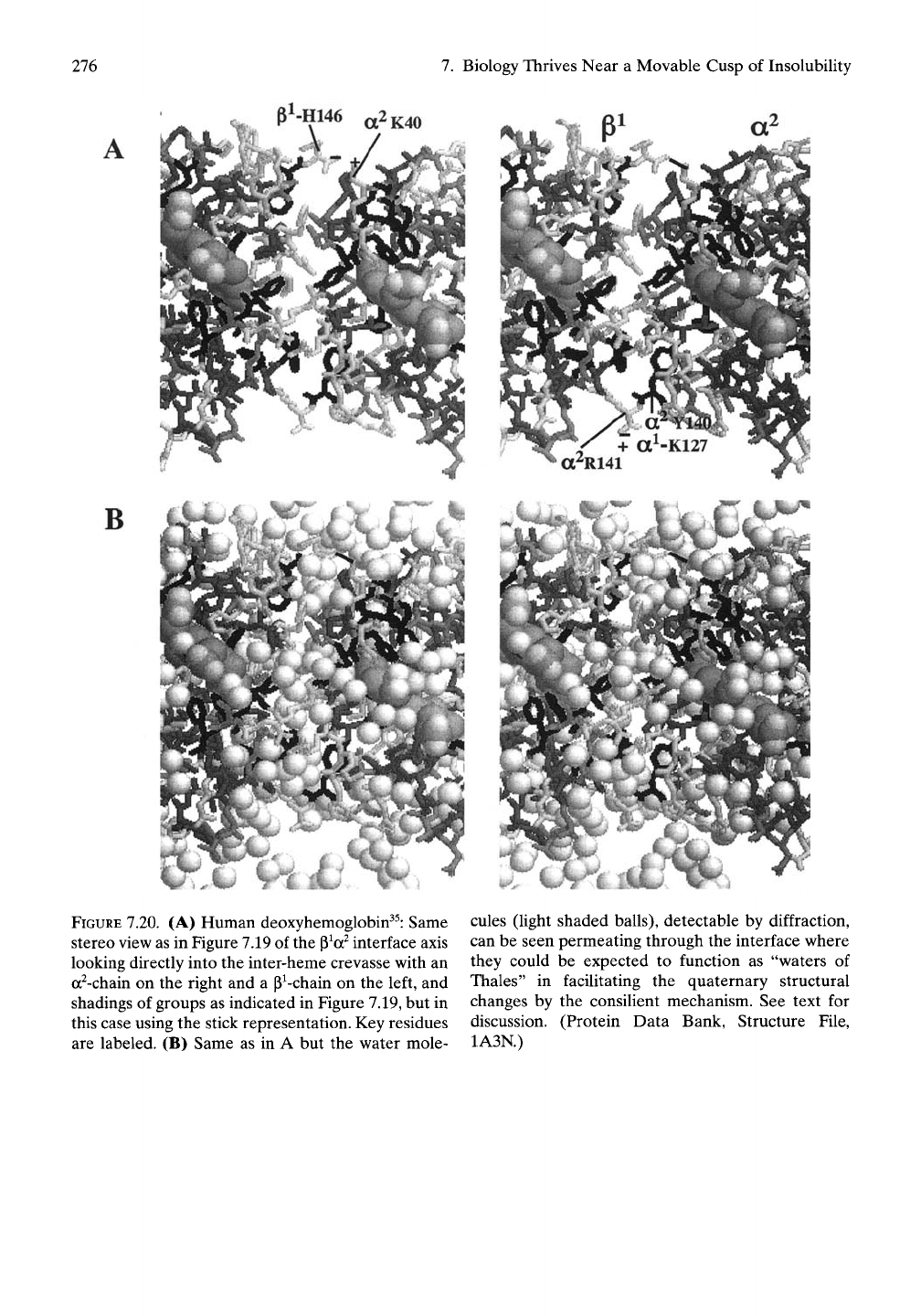
276
7.
Biology Thrives Near a Movable Cusp of Insolubility
pl.H146
„2K40
B
FIGURE 7.20. (A) Human deoxyhemoglobin^^: Same
stereo view as in Figure 7.19 of the
p^a^
interface axis
looking directly into the inter-heme crevasse with an
a^-chain on the right and a P^-chain on the left, and
shadings of groups as indicated in Figure 7.19, but in
this case using the stick representation. Key residues
are labeled. (B) Same as in A but the water mole-
cules (light shaded balls), detectable by diffraction,
can be seen permeating through the interface where
they could be expected to function as "waters of
Thales" in facilitating the quaternary structural
changes by the consilient mechanism. See text for
discussion. (Protein Data Bank, Structure File,
1A3N.)
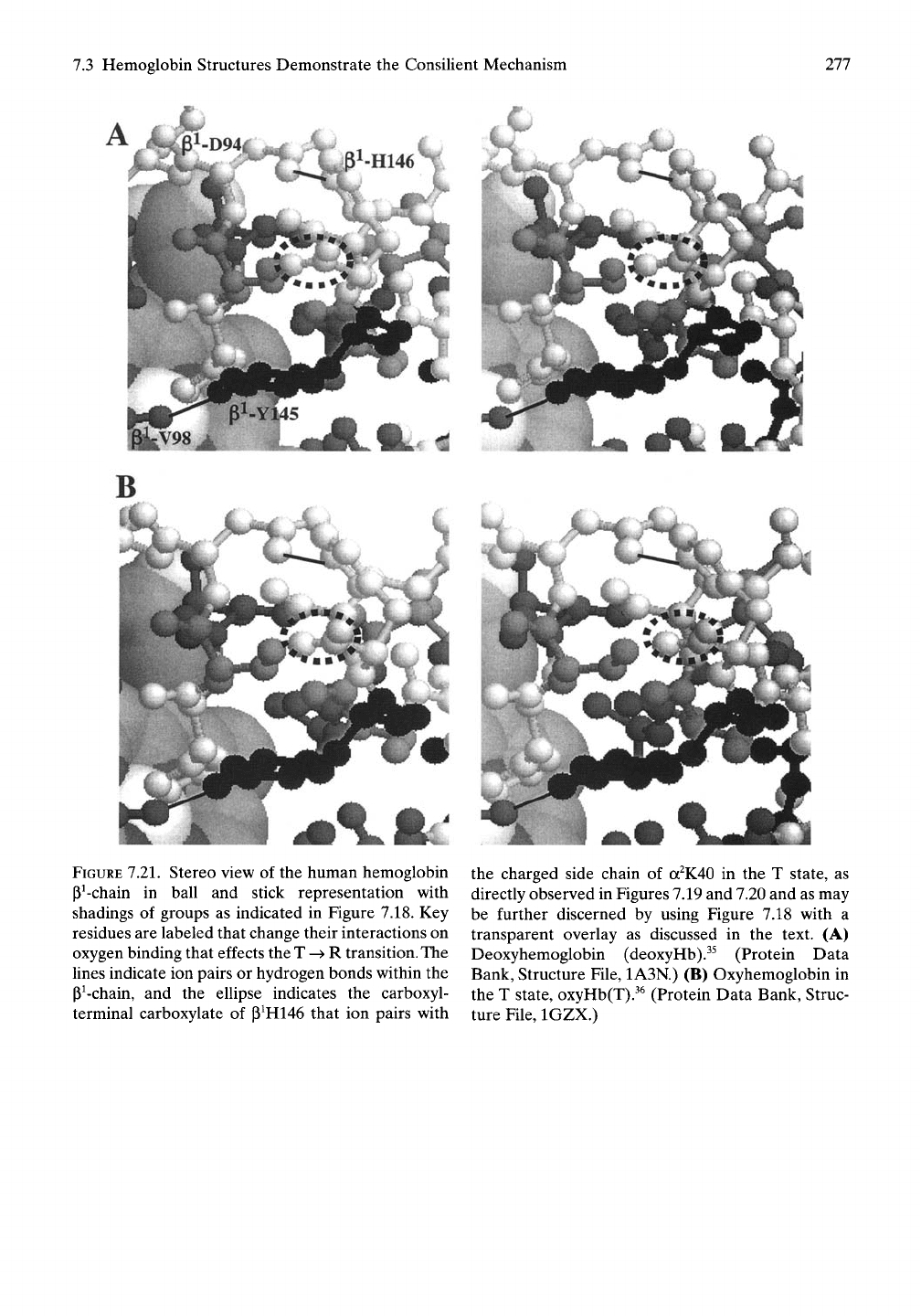
7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
277
FIGURE
7.21.
Stereo view of the human hemoglobin
P^-chain in ball and stick representation with
shadings of groups as indicated in Figure 7.18. Key
residues are labeled that change their interactions on
oxygen binding that effects the T -^ R
transition.
The
lines indicate ion pairs or hydrogen bonds within the
P^-chain, and the ellipse indicates the carboxyl-
terminal carboxylate of P^H146 that ion pairs with
the charged side chain of
a^K40
in the T state, as
directly observed in Figures 7.19 and 7.20 and as may
be further discerned by using Figure 7.18 with a
transparent overlay as discussed in the text. (A)
Deoxyhemoglobin (deoxyHb).^^ (Protein Data
Bank, Structure File, 1A3N.) (B) Oxyhemoglobin in
the T state, oxyHb(T).^^ (Protein Data Bank, Struc-
ture File, IGZX.)

278
7.
Biology Thrives Near
a
Movable Cusp
of
Insolubility
7.3.5
The
Consilient Mechanism
Summarized
as the
Source
of
the Sigmoid Oxygen Binding
of Hemoglobin
7.3.5.1
Positive Cooperativity
in
Hemoglobin
by the
Consilient Mechanism
7.3.5.1.1
The
Interheme Hydrophobic
Crevasse, Filled with Hydrophobic Hydration,
Repulses Charge
and the
Approach
of
Polar Oxygen
From
the
perspective
of the
consilient mecha-
nism,
the
approach
of an
oxygen molecule
to a
heme
of
deoxyhemoglobin faces loss
of
hydra-
tion
as it
enters
the
sphere
of
hydrophobic
hydration emanating from
the
hydrophobic
heme group
and its
hydrophobic surroundings,
including that
of the
interheme crevasse. This
constitutes AGap
and
kinetic barriers.
7.3.5.1.2 Binding
of
Initial Oxygen
Is
Poor
Due
to
Repulsive AGap
The work performed
by the
now-bound oxygen
molecule
in
disrupting hydrophobic hydration
also results
in a
lowered binding affinity
because
of the
expended AGap.
An
important
part
of
that work involves
the
disruption
of ion
pairs
and
reorientation
of the
charged side
chains
of the
crevasse
on
oxygen binding.
7.3.5.1.3 Oxygen Binding Increases Heme
Polarity
and
Results
in
Hydration
of
Dissociating Polar Groups
and of
Dissociating
Hydrophobic Groups with
the
Consequence
of
a
Generalized Water Uptake
The increased polarity
due to
oxygen binding
relaxes
ion
pairs
and
effects dissociation
of
hydrophobic groups.
It
does
so,
because
it
allows polar species
to
gain
in the
competition
for hydration with hydrophobic groups. Some
of
the
hydrophobic hydration that previously
formed
on
hydrophobic dissociation
can now
be recruited
by the
emerging polar groups,
and
the dissociations
of
both stand.
The
result
is
swelling,
as
reflected
in the
experimentally
measured uptake
of
water
and in the
shatter-
ing
of
crystalline deoxyHb
on
efforts
to
bind
oxygen. (These
are
generalized
and
fundamen-
tal properties
of
increasing
the
polarity
of
heme
groups that
can be
expected
to
extend
to
oxi-
dation
of
cytochromes
of the
electron transport
chain.)
7.3.5.1.4 Each Oxygen That Binds Facilitates
Binding
of the
Subsequent Oxygen
Communication between hemes readily occurs
through
the
interheme crevasse.
The
decrease
in hydrophobicity
of the
oxygen-bound heme
allows separation
of ion
pairs
and
reorientation
of charges associated with
the
interheme
crevasse. This increase
in
polarity propagates
to
the second heme.
Now the
work
to be
per-
formed
by
oxygen binding
to the
second heme
decreases
and
affinity increases.
With
the
proximity
of
the
p^
H146-a^ K40
ion
pair
to the P^a^
interheme crevasse, shown
in
Figure 7.19A,
it is not
unreasonable
to
suppose
that oxygen binding
to one or
both hemes
of
the
P^a^
heme pair would facilitate this
ion
pair separation that
is one of the two
keys
to
the
quarternary structure change
on
oxygen
binding. Furthermore,
it is not
unreasonable
to
suggest that
the a}
R141-a^
K127 ion
pair, also
shown
in
Figure 7.19A, might
be
influenced
by
oxygen binding
at the
P^a^-heme pair. Similar
relationships occur
at the
a^p^-heme pair where
the
key ion
pairs would
be p^
H146(COO")-a^
K40
and the a^
R141(COO-)-a^
K127
with
similar proximities
to
those
of the
p^a^-heme
pair
in
Figure 7.19A. These relationships
can be
visualized
by a
combination of Figure
7.19A and
the transparency overlay
in
Figure
7.18.
7.3.5.2
Melding
of the
Perutz
and
Consilient Mechanisms
Should oxygen binding alter
the
tertiary struc-
ture
of an
individual globin chain such that
the
above-noted
set of
four
ion
pairs
at the
inter-
face between
the ap
dimers
(a^P^ and a^p^)
could
no
longer sterically form, then
the
separated ions could
be
expected
to
disrupt
hydrophobic hydration,
and the
free energy
of
hydrophobic association would become less
favorable.
The
small change
in
His-Fe distance
and
in
angles
at the
heme, transmitted
by the
"gears
and
levers"
as
proposed
in the
Perutz
mechanism, could readily effect separation
of

7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
279
the ion pairs and hydrogen bonds in the switch
and joint regions. Given the relatively rigid
structures of the a- and the (i-chains, the effect
of the small conformational change would be to
reduce the complementarity between the a^P^
and a^P^ interfaces.
This would constitute merging of the con-
silient mechanism with the Perutz mechanism.^^
The competition for hydration between polar
(e.g.,
charged) and hydrophobic groups respon-
sible for positive cooperativity would remain
at these sites, but in the Perutz mechanism it
would have been the result of the tertiary struc-
tural change rather than arising directly from
changes in the balance of the competition for
hydration at the p^a^- and a^P^-heme pairs that
result from oxygen binding.
charged carboxylate-containing E6 residue, by
a modestly hydrophobic V6 residue (see the
Genetic Code in Table 6.2 for the single base
change required for the replacement of Glu by
Val).
This substitution shifts the hemoglobin
tetramer toward hydrophobic association, that
is,
lowers the cusp of insolubility from above to
below physiological temperature, when in the
deoxygenated state. Such a simple substitution
lowers the Tt-(solubility/insolubiUty)divide for
the aggregation of the more hydrophobic
tetrameric deoxyhemoglobin S molecules into
fibers shown in Figure 7.23. The Tt-divide,
however, remains above physiological temper-
atures as long as there is a high enough level of
oxygen to maintain the more polar oxyhemo-
globin state.
7.3.6 Sickle Cell Anemia: Lowers the
Temperature Interval of the "Cusp of
InsolubiUty" with the Consequences
of Disease and Death
The movable "cusp of insolubiUty" depicted in
Figure 7.1 provides meaningful visualization of
the disease, sickle cell anemia. A single muta-
tion in the (i-chain of the normal adult human
hemoglobin, hemoglobin A, to produce hemo-
globin S lowers the "cusp of insolubiUty" for
deoxyHbS from above to below physiological
temperatures. Under conditions of low oxy-
genation, the hemoglobin S aggregates and
causes the red blood cell to sickle, as shown in
Figure 7.22. Without treatment, homozygous
individuals, in which all P-chains contain the
mutation, suffer from acute onset of abdominal
pain and ulcerations of the lower extremities
and have a life expectancy of about 45 years."^
Heterozygous individuals, in which half of their
p-chains contain the mutation and the other
half are normal, escape such dire symptoms.
73.6,1 Hemoglobin S
7.3.6.1.1 Hemoglobin S Results from the
Mutation of Glu^ (E6) to Val^ (V6) in the
P-chain
Hemoglobin S is the result of a single base
mutation in the DNA sequence for the P-
chain that replaces the most polar residue, the
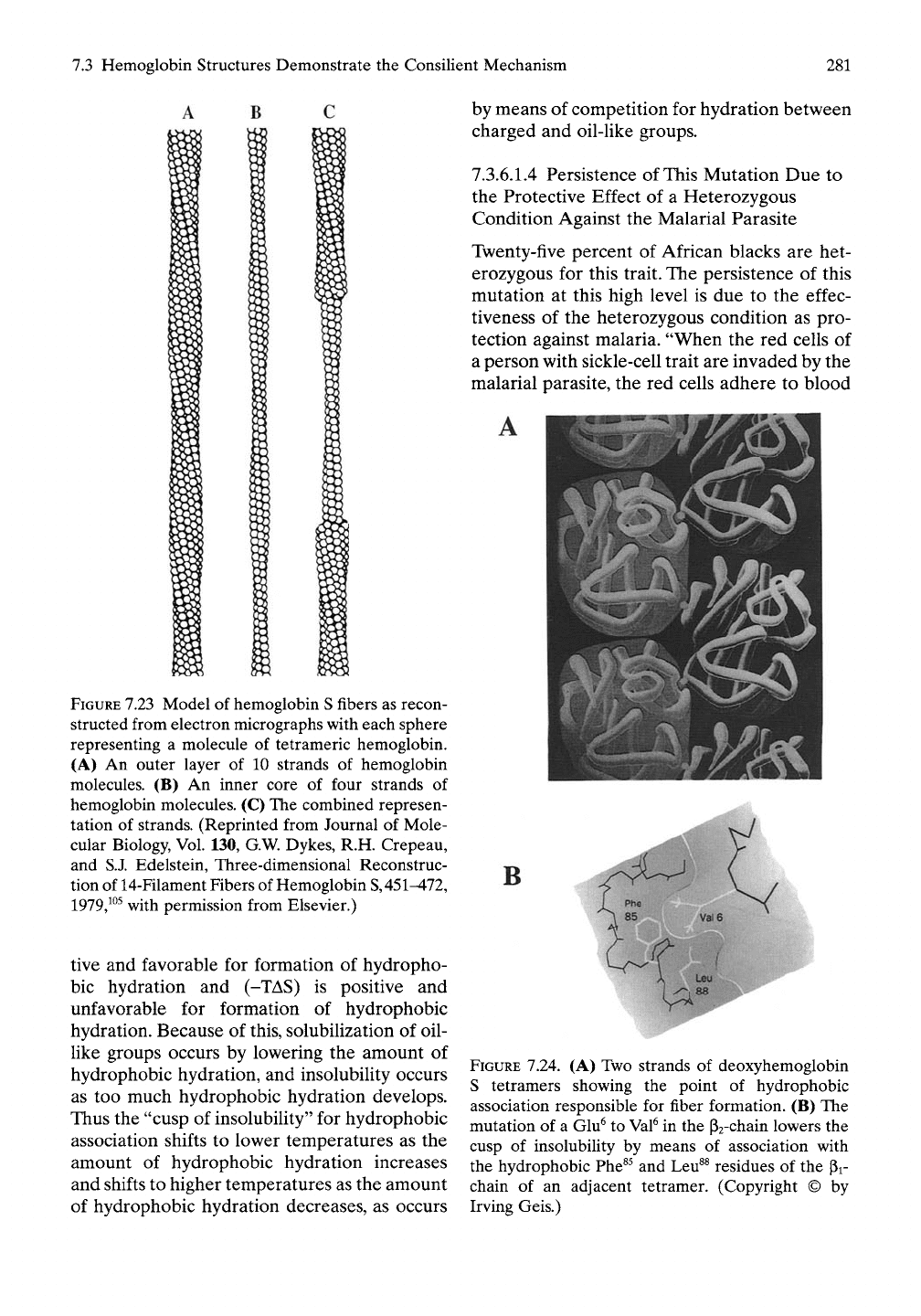
7.3.6.1.2 Hydrophobic Association of
Hemoglobin S Tetramers (Insolubilization)
The critical intertetramer contact that lowers
the Tt-divide for aggregation directly involves
the mutated hydrophobic residue, V6, of a P2-
chain. As shown in Figure 7.24, the oil-like
Val^ side chain, -CH-(CH3)2 of a P2-chain
associates with the very oil-like F8 side
chain, -CH2-C6H5 and L89 side chain,
-CH2-CH-(CH3)2, of the pi-chain in an adja-
cent tetramer. This hydrophobic interaction
lowers the cusp of insolubility for association
of deoxyhemoglobin S to below physiological
temperatures. The hydrophobic association
between tetramers propagates fiber formation,
illustrated in Figure
7.23,
and distorts the hemo-
globin transporting cell, the red blood cell, as
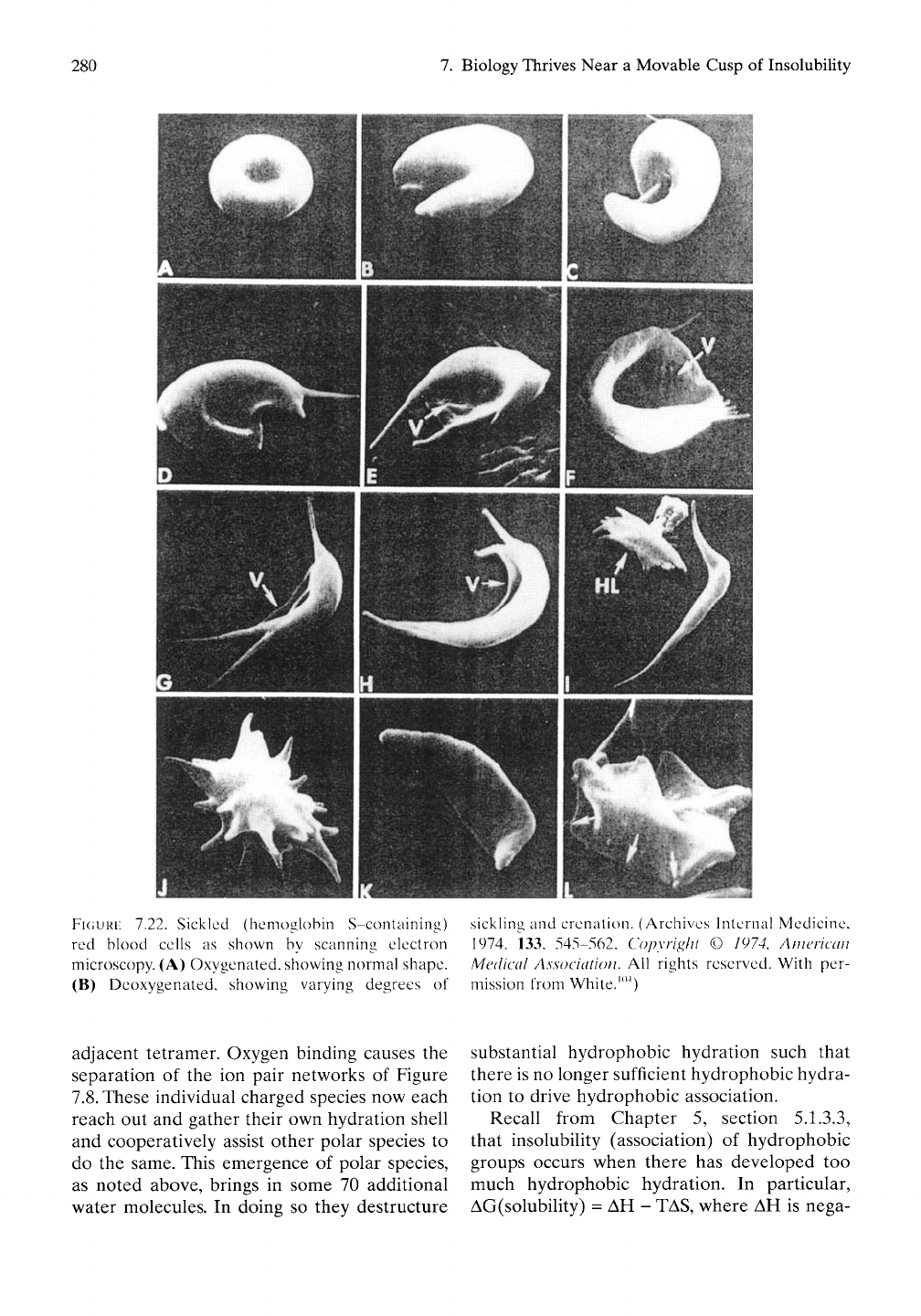
shown in Figure 7.22.
7.3.6.1.3 Manifestation of the Oxygenated
State that Prevents Hydrophobic Association
of Hemoglobin
S
Tetramers
Qualitatively, oxygen binding to hemoglobin
forms a more polar
state.
The more polar state
in its thirst for hydration destructures the
hydrophobic hydration that would otherwise
form around the oil-like V6 side chain,
-CH-(CH3)2 of the P2-chain and the very oil-
like Phe^^ side chain, -CH2-C6H5 and L89 side
chain, -CH2-CH-(CH3)2, of the pi-chain of an
280
7. Biology Thrives Near a Movable Cusp of Insolubility
FIGURE
7.22. Sickled (hemoglobin S-conlaining) sickHng and crenalion. (Archives Internal Medicine,
red blood cells as shown by scanning electron 1974, 133, 545-562, Copyright © 1974, American
microscopy. (A) Oxygenated, showing normal shape. Medical Association. AW rights reserved. With per-
(B) Deoxygenated, showing varying degrees of mission from White.'"^)
adjacent tetramer. Oxygen binding causes the
separation of the ion pair networks of Figure
7.8.
These individual charged species now each
reach out and gather their own hydration shell
and cooperatively assist other polar species to
do the same. This emergence of polar species,
as noted above, brings in some 70 additional
water molecules. In doing so they destructure
substantial hydrophobic hydration such that
there is no longer sufficient hydrophobic hydra-
tion to drive hydrophobic association.
Recall from Chapter 5, section 5.1.3.3,
that insolubility (association) of hydrophobic
groups occurs when there has developed too
much hydrophobic hydration. In particular,
AG(solubility) = AH - TAS, where AH is nega-
7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
281
FIGURE 7.23 Model of hemoglobin
S
fibers
as recon-
structed from electron micrographs with each sphere
representing a molecule of tetrameric hemoglobin.
(A) An outer layer of 10 strands of hemoglobin
molecules. (B) An inner core of four strands of
hemoglobin molecules. (C) The combined represen-
tation of strands. (Reprinted from Journal of Mole-
cular Biology, Vol. 130, G.W. Dykes, R.H. Crepeau,
and S.J. Edelstein, Three-dimensional Reconstruc-
tion of 14-Filament Fibers of Hemoglobin
S,
451^72,
1979,^°^
with permission from Elsevier.)
tive and favorable for formation of hydropho-
bic hydration and (-TAS) is positive and
unfavorable for formation of hydrophobic
hydration. Because of this, solubilization of oil-
like groups occurs by lowering the amount of
hydrophobic hydration, and insolubility occurs
as too much hydrophobic hydration develops.
Thus the "cusp of insolubility" for hydrophobic
association shifts to lower temperatures as the
amount of hydrophobic hydration increases
and shifts to higher temperatures as the amount
of hydrophobic hydration decreases, as occurs
by means of competition for hydration between
charged and oil-like groups.
7.3.6.1.4 Persistence of This Mutation Due to
the Protective Effect of a Heterozygous
Condition Against the Malarial Parasite
Twenty-five percent of African blacks are het-
erozygous for this trait. The persistence of this
mutation at this high level is due to the effec-
tiveness of the heterozygous condition as pro-
tection against malaria. "When the red cells of
a person with sickle-cell trait are invaded by the
malarial parasite, the red cells adhere to blood
B
FIGURE
7.24. (A) Two strands of deoxyhemoglobin
S tetramers showing the point of hydrophobic
association responsible for fiber formation. (B) The
mutation of a Glu^ to
Val^
in the Pa-chain lowers the
cusp of insolubility by means of association with
the hydrophobic Phe^^ and Leu^^ residues of the Pi-
chain of an adjacent tetramer. (Copyright © by
Irving Geis.)

282
7.
Biology Thrives Near a Movable Cusp of Insolubility
vessel walls, become deoxygenated, assume the
sickled shape, and then are destroyed, the
parasite being destroyed with them.'"^'*
73,62 Phase Diagrams of Hemoglobins
S and A
7.3.6.2.1 Temperature Effect Diagnostic of
Consilient Mechanism
The most fundamental characteristic of the
consilient mechanism, based as it is on proteins
that exhibit inverse temperature transitions, is
the property of aggregation on raising the tem-
perature and dissolution on lowering the tem-
perature. That the consilient mechanism applies
to hemoglobin S can be appreciated in the
statement of Eaton and Hofrichter^^ that "the
most important characteristic of hemoglobin S
polymerization is that a gel can be prepared by
heating a Uquid solution at the appropriate con-
centration and 'melted' by cooling." Of course,
the gel is the state of hydrophobically associ-
ated fibers, and melting is the process of fiber
dissolution.
7.3.6.2.2 The Phase Diagram of the Elastic
Model Protein (GVGVP)25i
The phase diagrams of the several model pro-
teins given in Figure 5.3 show the binodal
or coexistence lines for each that we here
call the Tfdivide. As shown specifically for
(GVGVP)25i in Figure 7.25, there is a second
line,"^^'"^^
the spinodal line (Tsp), and it becomes
coincident with the Tt-divide for this model
protein at modest concentrations. The spinodal
line is obtained by extrapolation of data from
lower temperatures as indicated in the insert.
The spinodal line exhibits the particular advan-
tage that it can be determined for aggregations
that would occur at higher temperatures were
it not for other effects such as thermal denatu-
ration. As demonstrated by the insightful work
of San Biagio and Palma,'*^'^^ this provides
substantial advantage in the comparison of
hemoglobins S and A.
7.3.6.2.3 Inverted Phase Diagrams of
Hemoglobins A and S: A Further Diagnostic
of Inverse Temperature Transition Using the
Spinodal Line
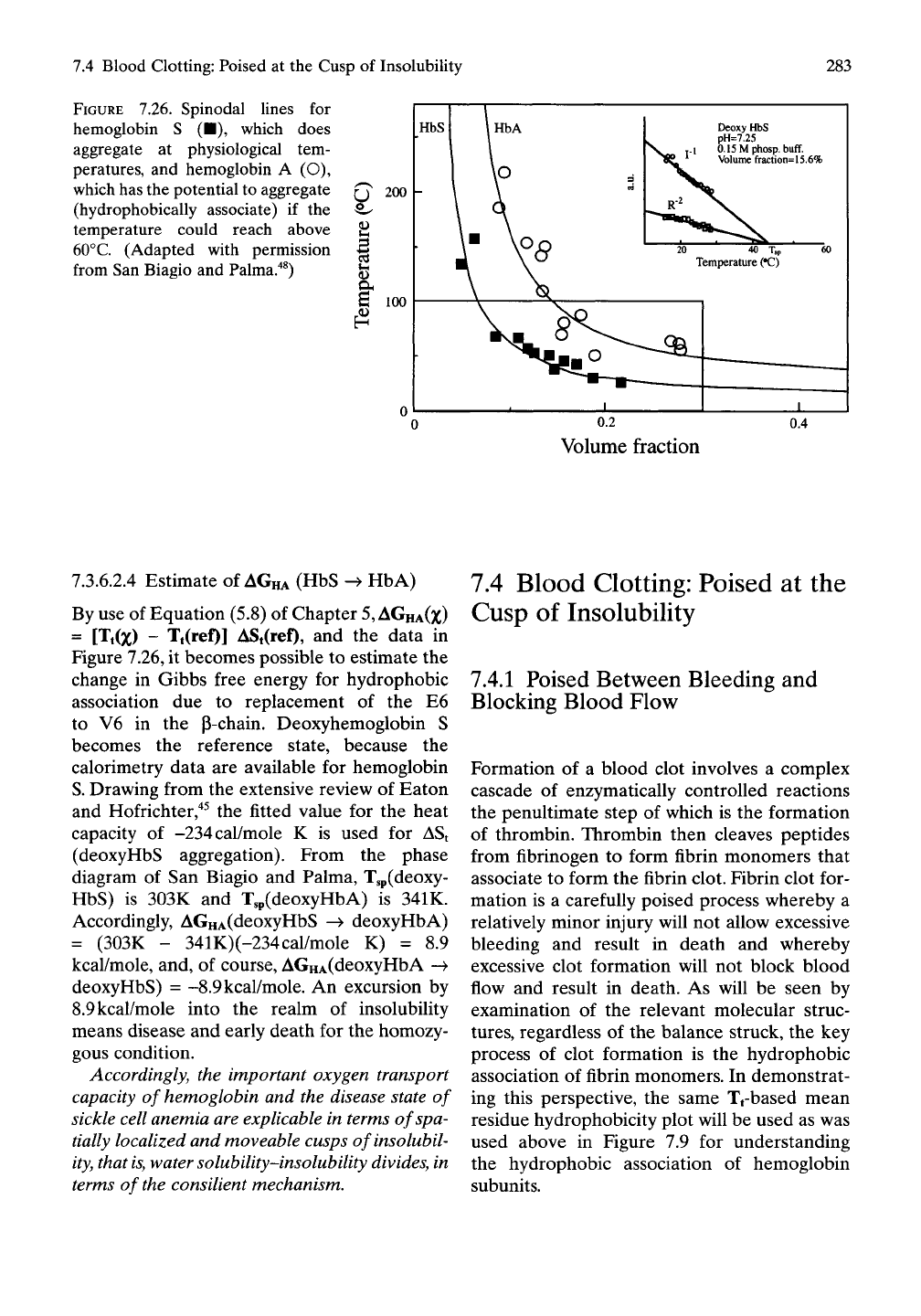
As shown in Figure 7.26, San Biagio and
Palma"^^'"*^ determined the spinodal lines for
the aggregation of hemoglobins S and A and
demonstrated the occurrence of inverse tem-
perature transitions. Rather than being domed
(concave with respect to the horizontal axis), as
occurs for the most common petroleum-based
polymers, the transition line is shaped valley-
like (convex with regard to the horizontal axis).
This indicates that intermolecular hydrophobic
association dominates the aggregation, as
shown in Figure 7.24 and as occurs for
(GVGVP)n and the other polymers in Figure
5.3.
TCC)
100
1
80
60
40 I
20
0.00
r ^
r -e 2
[ w
F
°
Tcloud = 27.3 'C
"%- Ts=40.5'C"
^^1^ <D =
0.02
20 j^.^^ 40
1 a 8 D B
1
>
111111111111
0.04
E
c
0.02 ^
0.00
' • ' •
0.05
0.10
0.15 0.20
0)
0.25
FIGURE
7.25.
Phase diagram of (GVGVP)25i showing
the spinodal line in addition to the usual binodal
(coexistence) line that we call the Tfdivide. Inset
shows experimental determination of the spinodal
line by extrapolation to the x-axis intercept of data
on the temperature dependence of concentration
fluctuations obtained at lower temperature. This
means that critical data can be obtained for phase
separations that would occur at elevated tempera-
tures if denaturation did not occur. Note that spin-
odal and binodal lines overlap for part of the volume
fraction axis. (Reproduced with permission from
Manno et al.^^)
7.4 Blood Clotting: Poised at the Cusp of Insolubility
283
FIGURE 7.26. Spinodal lines for
hemoglobin S (•), which does
aggregate at physiological tem-
peratures, and hemoglobin A (O),
which has the potential to aggregate
(hydrophobically associate) if the
temperature could reach above
60°C.
(Adapted with permission
from San Biagio and Palma."*^)
(J 200
I
100
HbS
Deoxy HbS
pH=7.25
y.l O.lSMphosp.
buff.
^ Volume fraction=15.6%
0.2
Volume fraction
0.4
7.3.6.2.4 Estimate of
AGHA
(HbS -^ HbA)
By use of Equation (5.8) of Chapter
5, AGHA(Z)
= [Tt(x) - Tt(ref)] ASt(ref), and the data in
Figure 7.26, it becomes possible to estimate the
change in Gibbs free energy for hydrophobic
association due to replacement of the E6
to V6 in the p-chain. Deoxyhemoglobin S
becomes the reference state, because the
calorimetry data are available for hemoglobin
S. Drawing from the extensive review of Eaton
and Hofrichter,"*^ the fitted value for the heat
capacity of -234cal/mole K is used for ASt
(deoxyHbS aggregation). From the phase
diagram of San Biagio and Palma, Tsp(deoxy-
HbS) is 303K and T,p(deoxyHbA) is 341K.
Accordingly, AGHA(deoxyHbS -^ deoxyHbA)
= (303K - 341K)(-234cal/mole K) = 8.9
kcal/mole, and, of course, AGHA(deoxyHbA ->
deoxy HbS) = -8.9
kcal/mole.
An excursion by
8.9 kcal/mole into the realm of insolubility
means disease and early death for the homozy-
gous condition.
Accordingly, the important oxygen transport
capacity of hemoglobin and the disease state of
sickle cell anemia are explicable in terms of spa-
tially localized and moveable cusps of insolubil-
ity , that
is,
water solubility-insolubility divides, in
terms of the consilient mechanism.
7.4 Blood Clotting: Poised at the
Cusp of Insolubility
7.4.1 Poised Between Bleeding and
Blocking Blood Flow
Formation of a blood clot involves a complex
cascade of enzymatically controlled reactions
the penultimate step of which is the formation
of thrombin. Thrombin then cleaves peptides
from fibrinogen to form fibrin monomers that
associate to form the fibrin clot. Fibrin clot for-
mation is a carefully poised process whereby a
relatively minor injury will not allow excessive
bleeding and result in death and whereby
excessive clot formation will not block blood
flow and result in death. As will be seen by
examination of the relevant molecular struc-
tures,
regardless of the balance struck, the key
process of clot formation is the hydrophobic
association of fibrin monomers. In demonstrat-
ing this perspective, the same Tfbased mean
residue hydrophobicity plot will be used as was
used above in Figure 7.9 for understanding
the hydrophobic association of hemoglobin
subunits.

284
7.
Biology Thrives Near a Movable Cusp of Insolubility
7.4,1,1
Thrombus Formation
Leading to Heart Attack, Stroke,
and Phlebitis
Excess clot (thrombus) formation in the blood
vessels results in disease and death. When
thrombus forms in the coronary arteries that
service the heart muscle, occlusion of a coro-
nary artery can result in a heart attack with
death of a region of heart muscle, a myocardial
infarction. When the blockage destroys a large
enough or critical portion of the heart, pumping
function is lost and death results.
When thrombus forms in an artery of
the brain, referred to as apoplexy or a cere-
brovascular accident, a portion of brain
tissue dies. Again, the size and location of the
artery blocked determines the amount of lost
brain tissue and whether the lost function
results in partial paralysis, loss of speech, or
death.
When blood clot forms in a vein, without
inflammation and called phlebothrombosis or
in response to inflammation and termed throm-
bophlebitis, localized pain, swelling, redness,
and heat develop with resulting loss of function.
From the perspective of an inverse temperature
transition as the basis for clot formation, the
natural increase in temperature would aggra-
vate the problem.
7,4,1,2
Hemophilia
(Bleeding Diseases)
Hemophilia arises from any of a number of
factors (commonly due to sex-linked inheri-
tance) required for thrombin formation such
that proper fibrin clot does not form. In infancy
anemia and death can occur. Bruises occur
when the source of the bruise was so innocuous
as not to have been noticed. Internal bleeding
occurs in the mouth, nose, gastrointestinal tract,
and in the joints with swelling and impairment
of function. As we will discuss, all of this occurs
because the hydrophobic association required
for fibrin clot formation cannot happen. All
of this is the result of loss of control of the
movable "cusp of insolubility."
7.4.2 Control of Fibrin Clot
(Thrombus) Formation
7,4.2,1
Structure of Fibrinogen,
the Precursor Protein
7.4.2.1.1 The Subunit Structure of Fibrinogen
The fibrinogen molecule is comprised of three
chains—Aa, B|3, and y—that are cross-linked
by disulfide bridges among themselves and to a
second set of three chains to give a multiply
disulfide-bridged structure, (Aa)2(B(3)2(7)2, as
schematically represented in Figure 1,21
A,
The
A stands for 16 residues at the amino end of
the a-chain, and B represents 14 residues at the
amino end of the p-chain. Note that all six
chains are associated amino end to amino end
and held in that position by numerous disulfide
cross-hnks, indicated by the interconnecting
fines. The A and B sequences are so defineated
because their removal initiates fibrin clot
formation. The removal of the A peptide for
activation represents the removal of two A
peptides at the amino-end-amino-end junction
that then hydrophobically associate with a pair
of end-to-end associated globular y domains.
7.4.2.1.2 The Primary Structure of the a, P,
and y Chains of Human Fibrinogen
The amino acid sequences of the Aa (610
residues), BP (461 residues), and y (411
residues) chains of human fibrinogen are given
in Table
7.1.^^
Having the amino acid sequences
directly available will be helpful in discussing
the peptide cleavages and the interchain inter-
actions relevant to fibrin formation.
7.4.2.1.3 The Crystal Structure of
Chicken Fibrinogen
The crystal structure of approximately 70% of
the residues of chicken fibrinogen has become
available from the laboratory of Doolittle and
coworkers^^ as shown in Figure 7.27B. The
(Aa)2(Bp)2(y)2 subunit structure combines to
form a flattened sigmoidal or
S-like
shape,
which with the missing sequences will be indi-
cated as (apya'pY)-The amino termini of all six
chains, the two chains—aa^ PP', and y/—all
meet in the central part of the structure.
