Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


8.1 Thesis 345
in extension, is completely recovered on release
of the deforming force, that is, on relaxation.
On the other hand, for a nonideal elastomer, all
of the energy expended in deformation is not
recovered on relaxation. In this case, the
stress-strain curve is said to exhibit hysteresis.
8.1.9.3.3
Performance of Mechanical Work
with an Isometric Contraction Followed by
an Isotonic Contraction with Relaxation to
Initial Force
During an isometric contraction, for example,
due to hydrophobic association, force develops
by the damping of internal chain dynamics
of an interconnecting elastic chain segment
(see Figure 5.8). To the extent that the chain
segment exhibits hysteresis, on allowing the
decrease in chain length to ensue (as in an
isotonic contraction), only a part of the energy
of deformation is recovered. Such a
mechanochemical engine could be no more
efficient than allowed by the nonideal (hys-
teretic) elastic chain segment.
In our analysis of hysteresis, appreciation was
clear for an elastic segment that stores all of the
deforming energy in the chain backbone. Thus,
a mechanochemical motor, in which loss of
deformation energy into associated chains
occurred, would decrease efficiency for the con-
version of chemical energy into mechanical
work and vice versa. This begins to place con-
straints on the mechanism of efficient biologi-
cal motors, and it begins to limit the utilization
of levers, ratchets, and physical pushing derived
from mechanical interactions between chains
when searching for a more efficient mechanism
for the performance of mechanical, chemical, or
even electrical work.
8.1.10
The Electrostatic Argument
of a Low Dielectric Constant Versus
AGap to Explain pKa Shifts and
Ion-pair Formation
8J. 10.1 Why Ion Pairs Form in
Protein-based Polymers
Salts dissolve in water because the free energy
of the separated hydrated ion is lower than
when associated with other ions within the salt
crystal. Ionized side chains of proteins remain
in water as long as they retain adequate hydra-
tion. Therefore, any process that decreases
effective hydration of ionic side chains of pro-
teins becomes a driving force for ion pairing.
Commonly, hydration has been treated by
assuming a uniform medium of a given dielec-
tric constant, e, where the force, f, between two
charges, q and q', is given by the product, qq',
divided by the distance, r, between charges
squared times the dielectric constant, that is, f =
qqVer^. When the charges are on a pair of oppo-
sitely charged plates, with water between them,
the value of
8
for water is experimentally deter-
mined to be about 80 at room temperature.
Thus,
by this argument, and consistent with
limited water, ions pair only when 8 becomes
sufficiently small. In calculations of protein, an
8 as low as 5 or less is used, and in calculations
of small peptides where there are partial
charges on atoms instead of a charge of one or
more, 8 has been commonly taken as unity.^^'^^
Qualitatively, the argument for using a low
dielectric constant seems reasonable. The
low dielectric constant comes into question,
however, on closer scrutiny of experimental
results on model proteins for which control of
composition is possible, and it makes difficult
what must be subjective judgments of a correct
dielectric constant for water-filled clefts and
crevices of various dimensions found in many
protein-based machines.
8.1.10.2 The Usual Electrostatic Argument
of a Low Dielectric Constant as
Responsible for pKa Shifts
The usual electrostatic consideration of pKa
shifts has been to assume that the ionizable
function resides in a location within the protein
that is of low dielectric constant. As the rea-
soning goes, because of the low dielectric locale,
the carboxyl function does not ionize to form
carboxylate until the pH is much higher, that is,
the carboxyl exhibits a pKa shift. A simple
means of relating pKa shift to dielectric con-
stant uses the expression for solvation energy,
SE,
due to Born, SE = [(ze)^/2r](l - 1/8), where
z is the charge on the ion, e is the unit electron
charge, 8 is the dielectric constant of the sur-
rounding medium, and r now stands for the
radius of the ion. As treated in Chapter 5,

346
8. Consilient Mechanisms for Protein-based Machines of Biology
section 5.7.8.3, the interest is in fitting the
series of experimental data points due to pKa
shift as a function of mole fraction,
/K,
of the
lysine functional group in the polymer,
poly[/v(GVGIP),/x(GXGIP)] with X = Lys(K)
and /K varied from 1.0 to 0.06.^^ The data are
included in Figure 5.30 along with the data for
poly[/v(GVGIP),/x(GXGIP)] with X = Glu(E),
and /x again varied from 1.0 to 0.06.^^
Interest centers on the change in solvation
energy, ASE = Q[(£i-i - 8i)/8i_i8i], where Q is
substituted for the coefficient [(ze)V2r]. To
fit the data in Figure 5.30A,B, a dielectric
constant of 5 or less had to be used. Yet from
experimental determination of the polymer,
poly(GVGIP), at the phase-separated concen-
tration of 61% polymer and 39% water by
weight, the minimal value for the dielectric
constant was 65.^^ Because the polymer is a
repeating pentapeptide sequence, because the
barrier to backbone mobility is between 1 and
1.5kcal/mole, and because any introduction of
a charged functional group must raise the value
of the dielectric constant, there is no way that
the functional group can be held in a locale of
low dielectric constant. There must be another
physical basis for the hydrophobic-induced pKa
shifts in Figure 5.30. As developed in Chapter 5
and reviewed briefly above, the physical basis
is the competition for hydration between
hydrophobic and charged groups, called AGap,
the apolar-polar repulsive free energy of
hydration.
Accordingly, one should maintain a healthy
skepticism of the results of calculations of
protein structure and function where low
dielectric constants become the basis to drive
ion-pair formation. This is especially the case
when there are water-filled crevices and clefts
on the surface of proteins and indeed coursing
through the protein structure. The "waters of
Thales" are there as an integral part of protein
structure and function. To assume otherwise
has been a useful approximation in the past, but
it would seem no longer to be the most pro-
ductive approach.
Another limitation of the assumption of low
dielectric constants to explain pKa shifts is the
absence of an explanation for the obligatory
coupling of pKa shifts to positive cooperativity
shown in Figures 5.20,5.23,5.31, and 5.34. Also,
as argued above, positive cooperativity is a
central aspect of efficient energy conversion by
protein-based machines and naturally arises
out of a competition for hydration between
hydrophobic and charged groups.
Positive cooperativity arises from a competi-
tion for hydration between hydrophobic and
charged residues just as do pKa shifts. On the
other
hand,
the classic electrostatic argument
would appear to have no obvious way to become
integral with positive cooperativity: Further-
more, in Figure 1.4 a residual pKa shift of 1.7
pH units is found under circumstances where the
model protein is completely unfolded. Under
such circumstances one is compelled by the elec-
trostatic analysis to choose a dielectric constant
approaching 80 where there would be no sig-
nificant energy of interaction. In our view,
the classic electrostatic argument is simply left
wanting, unable to describe the extensive data on
elastic-contractile model proteins.
8.1.10.3 Electrostatics Become Significant
in Protein Structure and Function When
Charges Are Under the Influence of
Hydrophobic Domains
Electrostatic interactions, considered here
simply as the interactions of charged species,
become significant in proteins in aqueous media
when under the influence of hydrophobic
domains (see Figures 5.30A,B), that is, when
competing for adequate hydration with hydro-
phobic groups. In particular, pKa shifts and
changes in reduction potential do not necessar-
ily result from being buried in a localized region
(medium) of low dielectric constant within the
protein or from direct electrostatic interactions,
but rather result from competition for hydration
between hydrophobic and polar groups.
It is our behef that the presence of hydrated
hydrophobic side chains limits the available
hydration for ionic side chains and increases
the probability of ion pairing. Thus, while the
formation of ion pairs may be considered an
electrostatic event, it occurs in the aqueous
milieu of protein folding, assembly, and function
due to the dominance of the presence of, or
potential for, hydrophobic hydration.

8.1 Thesis
347
Above, in section 8.1.9.1, we considered a
purely electrostatic mechanism for chemo-
mechanical transduction. A high density of
charged groups dominated a relatively minimal
presence of hydrophobic groups in the cross-
linked polymer PMA. Its efficiency for chemo-
mechanical transduction was compared with
the inverse circumstance of limited charge
under the influence of a dominantly hydro-
phobic model protein. The latter proved to be
much more efficient even though the functional
group was the same, the -COOH/-COO"
chemical couple.
8.1.11
ATP: Biology's
Energy Currency
The apolar-polar repulsive free energy of
hydration, AGap, as reviewed above, results
from a competition for hydration between
charged species and hydrophobic groups. In
the most extreme case reported thus far, this
competition raised the pKa of a carboxyl from
about 4 to about 11.^^ This constitutes an
amount sufficient to raise the free energy of
carboxylates by 8 to lOkcal/mole. The basic
issue to be addressed here is whether there is
reason to believe that the free energy of the
phosphate group would also be subject to
competition for hydration with hydrophobic
groups.
Simply stated, is the free energy of hydroly-
sis of ATP and other related phosphoanhy-
drides sensitive to limitations in hydration? If
the answer is no, then the apolar-polar repul-
sive free energy of hydration, AGap, would not
be relevant to ATP's role in energy conversion.
If the answer is yes, then phosphates would
indeed be sensitive to the competition for
hydration with hydrophobic groups, as exten-
sively documented experimentally with the car-
boxylate group. Again, if the answer is yes, then
the development, within Chapter
5,
most
specif-
ically section 5.7 and the associated data in
Figures 5.23 through 5.32, of the concept of an
apolar-polar repulsive free energy of hydration
would be fundamentally congruent with the
energizing and functioning of high-energy
phosphates in biology.
In the process of building the case for this
equivalence of energizing carboxylates and
phosphates, a number of quotations will be
drawn from the literature rather than relying in
all cases on our interpretation of the literature.
In doing so, the case for high-energy phos-
phates resulting from limited hydration will
become very evident, and the energizing of
phosphates, as with the energizing of carboxy-
lates as evidenced by hydrophobic induced pKa
shifts,
becomes another illustration of con-
silience, that is, of a "common groundwork of
explanation."
In terms of the molecular process whereby
ATP forms and functions as biology's energy
coin, there are two aspects—the protonation/
deprotonation of carboxylates due to a proton
concentration gradient that drives a rotor to
form ATP from ADP and Pi, as in the ATP syn-
thase, and the molecular process whereby the
breakdown of ATP to ADP with release of Pi
drives the protein-based machines of biology, as
in the myosin II motor of muscle contraction.
8,1.11,1 Standard Free Energies of
Hydrolysis of Phosphate Bonds
In this section the standard free energy
of hydrolysis of ATP (and its equivalent
nucleotide triphosphates, NTPs) to ADP and
AMP and related biologically phosphorylated
molecules are noted.^'^The standard free energy
of hydrolysis of ATP to form ADP and Pi can
be obtained from the equilibrium constant for
the reaction
ATP ^ ADP-h Pi
to give the equilibrium constant, Keq,
Ke,-([ADP][PJ)/[ATP]
(8.1)
(8.2)
The reference conditions for the reaction must
be carefully defined and maintained, especially
the availability of water and the presence of
salts,
when making comparisons of different
high-energy compounds. From equilibrium
theory, Keq can be written in terms of the
change in Gibbs free energy AG for the
reaction
K
_^-AG/RT
eq ~ ^
(8.3)
348
8. Consilient Mechanisms for Protein-based Machines of Biology
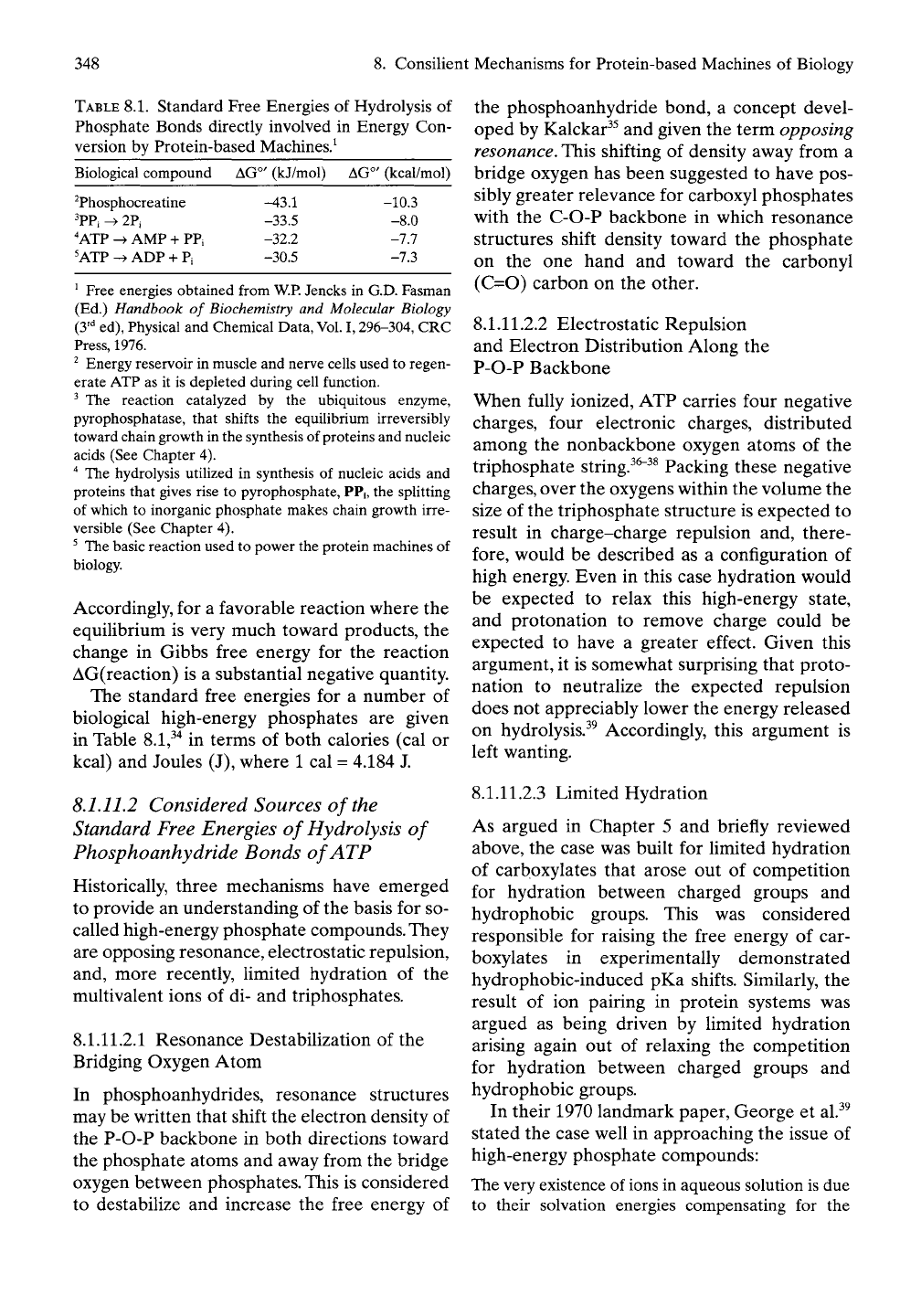
TABLE
8.1. Standard Free Energies of Hydrolysis of
Phosphate Bonds directly involved in Energy Con-
version by Protein-based Machines.^
Biological compound AG°' (kJ/mol) AG°' (kcal/mol)
^Phosphocreatine
^PPi ^ 2Pi
'ATP -^ AMP + PPi
'ATP -^ ADP + Pi
-43.1
-33.5
-32.2
-30.5
-10.3
-8.0
-7.7
-7.3
^ Free energies obtained from
W.P.
Jencks in G.D. Fasman
(Ed.) Handbook of
Biochemistry
and Molecular Biology
(y^ ed), Physical and Chemical Data,
Vol.
1,296-304, CRC
Press,
1976.
^ Energy reservoir in muscle and nerve cells used to regen-
erate ATP as it is depleted during cell function.
^ The reaction catalyzed by the ubiquitous enzyme,
pyrophosphatase, that shifts the equilibrium irreversibly
toward chain growth in the synthesis of proteins and nucleic
acids (See Chapter 4).
"^
The hydrolysis utilized in synthesis of nucleic acids and
proteins that gives rise to pyrophosphate, PPi, the splitting
of which to inorganic phosphate makes chain growth irre-
versible (See Chapter 4).
' The basic reaction used to power the protein machines of
biology.
Accordingly, for a favorable reaction where the
equilibrium is very much toward products, the
change in Gibbs free energy for the reaction
AG(reaction) is a substantial negative quantity.
The standard free energies for a number of
biological high-energy phosphates are given
in Table
8.1,^"^
in terms of both calories (cal or
kcal) and Joules (J), where 1 cal = 4.184 J.
8J.11.2 Considered Sources of the
Standard Free Energies of Hydrolysis of
Phosphoanhydride Bonds of ATP
Historically, three mechanisms have emerged
to provide an understanding of the basis for so-
called high-energy phosphate
compounds.
They
are opposing resonance, electrostatic repulsion,
and, more recently, limited hydration of the
multivalent ions of di- and triphosphates.
8.1.11.2.1 Resonance Destabilization of the
Bridging Oxygen Atom
In phosphoanhydrides, resonance structures
may be written that shift the electron density of
the P-O-P backbone in both directions toward
the phosphate atoms and away from the bridge
oxygen between phosphates. This is considered
to destabilize and increase the free energy of
the phosphoanhydride bond, a concept devel-
oped by Kalckar^^ and given the term opposing
resonance.
This shifting of density away from a
bridge oxygen has been suggested to have pos-
sibly greater relevance for carboxyl phosphates
with the C-O-P backbone in which resonance
structures shift density toward the phosphate
on the one hand and toward the carbonyl
(C=0) carbon on the other.
8.1.11.2.2 Electrostatic Repulsion
and Electron Distribution Along the
P-O-P Backbone
When fully ionized, ATP carries four negative
charges, four electronic charges, distributed
among the nonbackbone oxygen atoms of the
triphosphate string.^^^^ Packing these negative
charges,
over the oxygens within the volume the
size of the triphosphate structure is expected to
result in charge-charge repulsion and, there-
fore,
would be described as a configuration of
high energy. Even in this case hydration would
be expected to relax this high-energy state,
and protonation to remove charge could be
expected to have a greater effect. Given this
argument, it is somewhat surprising that proto-
nation to neutralize the expected repulsion
does not appreciably lower the energy released
on hydrolysis.^^ Accordingly, this argument is
left wanting.
8.1.11.2.3 Limited Hydration
As argued in Chapter 5 and briefly reviewed
above, the case was built for limited hydration
of carboxylates that arose out of competition
for hydration between charged groups and
hydrophobic groups. This was considered
responsible for raising the free energy of car-
boxylates in experimentally demonstrated
hydrophobic-induced pKa shifts. Similarly, the
result of ion pairing in protein systems was
argued as being driven by Umited hydration
arising again out of relaxing the competition
for hydration between charged groups and
hydrophobic groups.
In their 1970 landmark paper, George et al.^^
stated the case well in approaching the issue of
high-energy phosphate compounds:
The very existence of ions in aqueous solution is due
to their solvation energies compensating for the

8.1 Thesis 349
large amount of energy required to disrupt the
crystal lattice in the dissolution of an ionic crystal, or
to break the covalent bond to hydrogen and bring
about electron transfer in the ionization of an acid.
... Hence even in the absence of values for the high
energy phosphate compounds the conclusion is
inescapable that at biological pH an important term
contributing to their thermodynamic reactivity will
be the difference between the solvation energies for
the multi-charged reactant and product ions.
Drawling from their thermodynamic data and
calculations on ATP, ADP and AMP hydroly-
ses,
George et al.^^ concluded that the source of
release of high energy on hydrolysis of ATP and
ADP is a Umitation of hydration in the reac-
tants,
for example, in ATP.
Almost two decades later Hayes et al."^^
concluded.
Although intramolecular (opposing resonance and
electrostatic) effects play an important role in deter-
mining the energy of hydrolysis in some of these
reactions, it is concluded that in those hydrolyses of
most importance in energy storage and transduction
(ATP -> ADP + orthophosphate, and phosphocrea-
tine + ADP -^ creatine + ATP), relative solvation
energies of reactant and products are by far the most
important factors in determining these energies.
From de Mies,^^
However, from the values of Table IV, it can be
inferred that a small change in the organization of
solvent around the molecules of reactants and prod-
ucts might easily lead to a significant change in the
thermodynamic parameters of a reaction.
This brings us directly to our argument that,
from these data Hayes et
al."^^
concluded that sol-
vation energies of reactants and products are by
far the most important factors in determining
the energies of hydrolysis of phosphate com-
pounds that are used for energy storage and
transfer in the living cell. The same conclusion
has been reached recently by Ewig and Van
Wazer"^^
w^ho
calculated the energy of hydrolysis
of pyrophosphate in the gas
phase.
Furthermore,
due to intramolecular competition for hydra-
tion, even greater effects of limited solvation
energy can be expected for the phosphoanhy-
drides of biology such as ATP and ADP that
also contain the adenine aromatic hydrophobic
group than for the inorganic phosphate prod-
ucts calculated by Ewig and Van Wazer."^^
8.1.11.3 Means of Energizing a Phosphate
Sufficient for Addition to ADP
8.1.11.3.1 Our View: Competition for
Hydration with Hydrophobic Moieties
Energizes Polar Species
From an analysis of the hydrophobic-induced
pKa and reduction potential shifts with the
associated destruction of hydrophobic hydra-
tion on formation of carboxylates (see Figure
5.25B), we argue that the solvation limitation
of phosphates can be made critical by the
competition for hydration between polar and
hydrophobic domains.
8.1.11.3.2 Analogy Between Formation
of High-energy Phosphate Bonds and
Hydrophobic-induced pKa Shifts:
The General Argument
The magnitude of this apolar-polar repulsive
free energy of hydration, when the polar
species is carboxylate, has been demonstrated
experimentally to approach the order of 40 kJ/
mole or 8 to lOkcal/mole. This would be suffi-
cient to raise the free energy of an inorganic
phosphate for addition to an ADP, and in
general to destructure hydrophobic hydration
and open hydrophobic folds.
8.1.11.3.3 Question: How is the Free Energy
of Phosphate Raised Sufficiently for Addition
to ADP with Formation of ATP?
The free energy of phosphate is raised by the
forced confrontation of the site containing
ADP and Pi (or R-Pi) with a hydrophobically
hydrated surface, as argued below for ATP
synthase.
8.1.11.3.4 Question: How Can the Binding of
ATP Drive a Conformational Change?
The binding of ATP to a site in sufficient prox-
imity to a spatially localized hydrophobic asso-
ciation can result in the separation of associated
hydrophobic domains. In the absence of ATP,
the site exhibits transient partial openings due
to normal thermal fluctuations. During such a
fluctuation, the build up of too much hydropho-
bic hydration causes the hydrophobic associa-

350
8. Consilient Mechanisms
for
Protein-based Machines
of
Biology
tion
to
re-form, because
the
Tt-divide
is
below
the operating temperature
for
that spatially
localized association.
If
during
the
fluctuation
ATP should become proximal,
the
incipient
hydrophobic hydration
is
destructured; there
is
no longer
a
driving force
for
closure,
and the
conformational change involving separation
of
associated hydrophobic domains occurs.
The
cross-bridge
of the
myosin
II
motor demon-
strates such features
in
its interaction with actin
and
in the
interaction
of the
head
of the
lever
arm with
the
amino-terminal domain
(see
Figures 2.17
and
8.53).
8.1.11.3.5 Question: Over What Distances
Can such Destruction
of
Hydrophobic
Hydration
Be
Effective?
The destructuring
of
hydrophobic hydration
by polar species
is a
cooperative process. This
allows
the
opening
of a
hydrophobic associa-
tion that includes
an
ion pairing
to
proceed with
the result
of
adding
the
separated positive
and
negative charges
for
each
to
function
in
their
own right
as
polar species
for
disruption
of
further removed hydrophobic associations
in a
sort
of
propagating domino
or
unzipping effect.
Thus,
the
distance over which
the
destabilizing
of hydrophobic associations
can
occur depends
on
the
structure involved,
but
could readily
become several nanometers,
as is the
case
for
the GroEL/GroES chaperonin (see Chapter 7).
The specific distance would depend
on the
details
of the
distances between
the
newly
emerged charges
on
dissociation
of the ion-
pair-containing hydrophobic domains.
If we
consider
20
Mrad y-irradiation cross-linked
Model Protein
i of
Table
5.5,
(GVGVP
GVGVP GEGVP GVGVP GVGVP
GVGVP)39(GVGVP),
in the
contracted state,
the density
of
carboxyls
is
such that
the car-
boxyls
are
separated
by
more than 2nm.
By the
time 50% ionization
has
occurred,
the
volume
has increased 10-fold, the maximal allowed with
20 Mrad y-irradiation cross-linking. This occurs
with
a
mean distance between charges
of
more
than
4nm.
Contraction
due to
hydrophobic
association begins
as
carboxylates become less
than one every
4
nm. Thus the effect
of
a charged
carboxylate
can be
said
to
reach
out a
distance
of more than
2nm in its
capacity
to
destructure
hydrophobic hydration.
The
domino effect
of
separating ion pairs can
be
expected
to
increase
the distance further.
The multivalent phosphates, already signifi-
cantly limited
in
hydration,
can be
expected
to
reach
out
substantially further than carboxy-
lates
in
their search
for
adequate hydration.
This effect becomes enhanced when phosphate
access
to
water
is
limited. When
the
phosphate
occurs
at the
base
of a
cleft,
the
direction
for
access
of
water becomes severely limited
and
the thirst
for
hydration
can be
directed
by the
cleft
to
target sites
of
hydrophobic association.
In other words,
the
cleft functions
as a
conduit
to direct
the
thirst
for
hydration.
By
means
of
the cleft,
the
capacity
for
disrupting hydropho-
bic hydration
to
target sites
can be
boosted
by
effecting separation
of
ion pairs enroute, which
boost
the
polar species capacity
to
disrupt
hydrophobic hydration. Accordingly,
the use of
structure
to
direct
the
forces
of
apolar-polar
repulsion becomes
a
useful design feature
in
certain ATP-driven protein-based machines,
such
as the
myosin
II
motor.
8.1.11.3.6
The
Distance Issue
Can
Also
Be
an Issue
of
Contact Area
In another context,
the
distance issue
can
become
a
matter
of
contact surface area
of
the hydrophobically associating hydrophobic
domains that
are
held together
by a
negative
AGHA- Even
if
the surface area
for
hydrophobic
contact were more than
10
nm^,
only sufficient
hydrophobic hydration would have
to be
destroyed during
a
transient fluctuation
to
cause
AGHA
to
become positive.
The
change from
a
negative to a positive AGHA
is
to shift from dom-
inantly associated
to
primarily dissociated.
8.1.11.3.7 Estimate
of
AGHA(P04
on Phosphorylation
of
Poly[30(GVGIP),(RGYSLG)]
=)
As calculated
and
discussed
in
section 5.3.4.4.4,
the
AGHA(P04")
due to phosphorylation of
poly[30(GVGIP)(GRGDSP)]
at the Ser (S)
residue raises
the
value
of Tt to an
extent
that
the
calculated increase
in
free energy
for hydrophobic association becomes about

8.1 Thesis 351
^Moving the
Tt-divide
by
whatever means
X'
AGHA (X) =
AHt (GVGIP) -
AHt
(GVGVP) (1)
where
%
is the interaction that changes AHt from that
of
the
reference polymer and
AGHA (%)
is the change
in Gibbs free energy for hydrophobic associatiob due
to the interaction.
K^l
for the following
phosphorylation
by
ATP
ATP +
poIy[30GVGIP),(RGYSLG)] =
ADP +
poly[30GVGIP)],(RGYS{PO4=} LG) (2)
Using the
slope,
ATVAGHA, defined by charged species
K +/OF-E-/OF of Figure 5-10 (6.8 cal/mol-pent/deg)
and ArKP04=) of 860°K allows an estimate of
AGHA
(P04=). Accordingly,
AGHA(P04=)
= 6.8x860 + 2.3 « 8
kcal/mol-pentamer
.*. Phosphorylation raised the free energy of the
insoluble (hydrophobically assocuated) state of the
model protein by
~
8 kcal/mol-P04~, i.e., polymer-
P04~
is in an energized state in the soluble, unfolded
model protein, as implied by K » 1.
FIGURE
8.3.
Independent estimates of
the
increase in
free energy on phosphorylation of poly[30(GVGIP),
(RGYSLG)].
8kcal/mole. Also, the equilibrium constant
for the enzymatic phosphorylation of
poly[30(GVGIP),(RGYSLG)] using ATP was
essentially one, which by Table 8.1 would be
essentially 7 to 8kcal/mole for hydrolysis of
the terminal phosphate of ATP under standard
conditions. This argument is laid out in
Figure 8.3. Accordingly, these experimental
results provided direct demonstration that the
serine-phosphate bond can be fully energized
by an increase in competition for hydration
with hydrophobic groups. From this, we con-
clude that the free energy of hydrolysis can be
a direct function of the presence of hydropho-
bic residues and that an increase in free energy
of phosphates can arise from a competition for
hydration.
8.1.11.4 Uses of ATP in Construction and
Maintenance of the Living Organism
The following is a partial list of the uses of ATP
or an energetically equivalent NTP: (1) to
produce the macromolecules nucleic acids, pro-
teins,
and polysaccharides; (2) to produce the
organelles and membranes (lipid biosynthesis);
(3) to pump ions across cell membranes against
concentration gradients to maintain the proper
electrochemical gradients across cell mem-
branes; (4) to transport nutrients into the cells
and remove waste; (5) to drive trafficking of
molecules, vesicles, and organelles (e.g., direc-
tional transport of Golgi, lysosomes, and
chloroplasts) within the cells; (6) to use molec-
ular chaperones to achieve proper protein
folding and proteasomes and cellusomes to
degrade spent protein; (7) to change supercoil-
ing (Type II topoisomerase) and correct errors
in nucleic acids; (8) to achieve motility as in cell
crawling, muscle contraction, the beating of
cilia and flagella, the actuation of hearing; (9)
to provide additional energy requirements that
include motor-dependent tasks in the cell such
as "mitosis and cytokinesis, the morphogenesis
of the endoplasmic reticulum, exo-, endo-, and
pinocytosis, the polarization of the egg, gastru-
lation, and even thought itself [neuronal
growth-cone motility and the axonal transport
of neurotransmitter-containing vesicles].'"^^
8.1.12
ATPases, Biology's Workhorse
Protein-based Machines, and AGap
8.1.12.1 The Disposition of ATP at Its
ATPase Binding Site
From the perspective of the consilient mecha-
nism and its functional component, AGap, the
most striking feature of ATPases derives from
the way in which ATP molecules attach to the
protein. ATP binds near an aqueous interface
of the protein. Even more telling is that the
ATP molecule orients such that the triphos-
phate tail resides at the aqueous interface. This
is where hydrolysis occurs. More revealing with
regard to mechanism, ATP often binds with its
phosphates at the edge of a water-filled cleft.
8.1.12.2 The Specific Case of the ATPase
of the Myosin II Motor
In particular, as a step in the functional cycle of
the myosin II motor, ATP binding occurs at a
water-filled cleft."^ In the scallop muscle, as
shown below, the water-filled cleft can be seen
to communicate in two directions. In one direc-
tion it communicates with the hydrophobic

352
8. Consilient Mechanisms for Protein-based Machines of Biology
association of an actin cross-bridge attachment
site.
In the other direction the water-filled cleft
communicates to the hydrophobic association
involving principally the head of the lever arm
and the amino-terminal domain of the cross-
bridge. In both cases the hydrophobic associa-
tions become disrupted on ATP binding. In the
first case binding results in detachment from
the actin filament. In the second case, by dis-
rupting the hydrophobic association between
the amino-terminal domain and the head of the
lever arm, ATP binding causes the cross-bridge
to reach toward its next actin site of attach-
ment. A credible mechanism requires that it
provide insight into these fundamental struc-
tural features of ATP binding to ATPases, in
general, and specifically to the ATPase that is
the myosin II motor.
By the hydrophobic consiUent mechanism
for the myosin II motor and specifically by
means of AGap, ATP binding effects both
hydrophobic dissociation from the actin
binding site and release of the hydrophobic
association at the head of the lever arm, allow-
ing the cross-bridge to move forward toward
the next attachment site. Of course, loss of
phosphate would reconstitute the hydrophobic
associations, that is, would effect hydrophobic
re-attachment to the actin binding site in
concert with re-association of the head of the
lever arm with the amino-terminal domain to
result in the powerstroke. Section 8.5.4 presents
crystal structure stereo views from which the
above-noted perspective derives.
8.1.12.3 Three Aspects of Force
Generation in ATPases
The concept of an apolar (hydrophobic)-polar
(e.g., charge) repulsive free energy of hydra-
tion, AGap, contributes to understanding the
mechanism whereby ATPases function in three
distinguishing respects. First and second, ATP
binding, and particularly on hydrolysis with for-
mation of ADP plus Pi, has the potential to
effect both "push" and "pull" components of
force. Third, release of
Pi
results in development
of an elastic "pull" component of force that is
most in evidence during isometric contractions.
These three elements of force development are
discussed immediately below.
8.1.12.4 Binding ATP Energizes a
Protein-based Machine by the Mechanical
Result of Hydrophobic Dissociation
Due to AGap
In reviewing their studies on the Fi motor of
ATP synthase, Oster and Wang"^"^ recently
stated, "In many motors, the force generating
step is associated with the binding of nucleotide
to the catalytic site. We propose that this is true
of all ATPases. In particular, for the Fi motor
this is the only way to reconcile all of the bio-
chemical and mechanical measurements with
its high mechanical efficiency." (The itaUcs are
the authors' own emphasis.) As presented here,
the hydrophobic consilient mechanism makes a
distinction between energizing and the use of
that energy in force generation. In our view, this
distinction is not so significant for the rotary
Fi motor of ATP synthase functioning as an
ATPase. In this case by the hydrophobic con-
siUent mechanism, ATP-driven rotation of the
rotor results from repulsion due to an increase
in AGap, the apolar-polar repulsive free energy
of hydration.
As discussed below in section 8.4.4.11, ATP
binding provides the major "push" component
of force, but we expect the peak in AGap to
occur at the moment of hydrolysis when the
charge concentration is greatest with the
momentary presence of both ADP and Pi. In
the synthesis function of the Fi motor of ATP
synthase, we expect that the maximum repul-
sion occurs between the most hydrophobic side
of the rotor and the ADP and Pi state and that
this maximal repulsion decreases on ATP for-
mation, which, in the consilient view, drives
ATP formation. Accordingly, because repulsion
is the force that drives the ATPase function of
the Fi motor and because repulsion drives rota-
tion, ATP binding would provide near-maximal
force generation, enhanced only at the moment
of hydrolysis to form ADP plus Pi.
8.1.12.5 Development of an Elastic "PuW
Component of Force Resulting from an
Apolar-Polar Repulsion on ATP Binding
On the basis of the consilient mechanism, and
AGap in particular, one can also consider the
development of elastic force on ATP binding to

8.1 Thesis 353
result from the damping of internal chain
dynamics by repulsion of otherwise more kinet-
ically free chains or loops. Insight into this
aspect of the consilient mechanism surfaces
with Model Protein v in Figures 1.4 and 5.31, as
considered in the associated discussions in
Chapters 1 and 5. Repulsion between the
negatively charged carboxylates and hydro-
phobic phenylalanine (Phe, F) residues results
in a repulsive free energy of 2.4kcal/mole-
carboxylate, as measured experimentally by the
residual pKa shift, after dissolution is complete.
In this case keeping the phenylalanine (Phe, F)
residues distant from the carboxylates of the
glutamate (Glu, E") limits torsion angles that
would otherwise be possible if the repulsion
were not present. This constitutes a damping of
internal chain dynamics. This repulsion limits
the freedom of backbone motion; it restricts
the backbone torsion angle combinations that
would bring carboxylates and phenylalanine
side chains too close. Such a restriction of acces-
sible torsion angles constitutes a decrease in
chain entropy that should be expressed as an
increase in elastic force between the beginning
and end points of the restricted chain segment.
If the 30mer chain segment were held at the
mean distance during repulsion, removal of the
repulsion, in this case protonation to form
-COOH, should relax the deforming force.
Based on the consilient interpretations of the
model protein data, the presence of a nega-
tively and multiply charged ATP separated by
water molecules from a nearby chain or loop
that contained hydrophobic residues would
repulse the hydrophobic groups, causing them
to reside at a greater distance and possibly in
the shadow of the backbone. This constitutes a
"deformation" that limits the freedom of
motion of the chain or loop.
Similarly, apolar-polar repulsion between a
highly charged group and very hydrophobic
side chains of a chain or loop of protein would
be expected to limit the freedom of rotation
about backbone bonds of sufficiently kineti-
cally free chain segment or loop. This increase
in AGap would decrease the number of states
accessible to the polymer and decrease the
entropy of the chain. The resulting develop-
ment of an elastic force in the chain segment
or loop of protein could be used to perform
mechanical work of changing protein structure.
The mechanism of elasticity would now take on
the description of force development due to "a
damping of internal dynamics by repulsion"
rather than by extension. In this specialized cir-
cumstance, ATP binding could provide a useful
force-generating step in an ATPase, also not
inconsistent with the suggestion of Oster and
Wang.''
Accordingly, reasoning from the consilient
mechanisms, release of phosphate has the
potential to relax the apolar-polar repulsion
and relax an entropic elastic deforming force.
Thus,
instead of an electrostatic repulsion, for
example, the less efficient charge-charge repul-
sion, there would be the apolar-polar repul-
sion, which is argued above in section
8.1.9.1
and shown in Figure 5.36 to be more than 10
times more efficient as a mechanism for chemo-
mechanical transduction. In our view, to be effi-
cient, a machine should avoid the frictional
losses inherent in latches, ratchets, and mechan-
ically driven camshafts. Entropic elastic chains
and through-solvent apolar-polar repulsive
forces provide for efficient interconversion of
chemical and mechanical energy. At present,
however, we have no example of this develop-
ment of elastic force in the myosin II motor.
Therefore, it stands as a potential source of
force development without example, as yet, in
the protein machines of biology.
8.1.12.6 Release of Pi Discharges on
Energized Protein-based Machine by the
Third Mechanical Result of a ''Puir
Component of Force Due to Re-
established Hydrophobic Association
When considering ATPases that function as
classic contractile linear motors, the distinction
between energizing and force generation
becomes much clearer. ATP binding energizes
by disrupting hydrophobic association, perhaps
usefully boosted at the moment of hydrolysis,
but then force generation does not occur until
release of Pi that allows hydrophobic associa-
tion of the contracted state to recur. This could
be thought of as a "pull" component of force
generation that under isometric conditions
expresses as the generation of an elastic force.
Thus,
ATP binding could be viewed as effecting

354
8. Consilient Mechanisms for Protein-based Machines of Biology
relaxation, whereas loss of ATP yields the ulti-
mate in contraction, approaching a rigor-like
state,
as noted in Chapter 7. Such would be the
case unless or until a sweUing pressure became
relevant.
Release of the y-phosphate from protein-
bound ATP, leaving bound ADP, restores much
hydrophobic association that existed in the
protein before ATP binding. Release of the y-
phosphate permits reconstitution of sufficient
hydrophobic hydration to drive hydrophobic
association. In the broad view of the consilient
mechanism, as regards ATPases, ATP binding
causes hydrophobic dissociation, and Pi and
ADP release re-establishes maximal hydro-
phobic association. In terms of the movable cusp
of insolubiUty, binding of the polar ATP mole-
cule raises the temperature of the movable cusp
of insolubility to give solubility, and the decrease
in polaritys on phosphate release lowers the
movable cusp of insolubility to re-establish the
insolubility of hydrophobic association
In the preceding discussion of ATPase types
of protein-based
machineSy
the hydrophobic and
elastic consilient mechanisms, by means of a
single operative component of the Gibbs free
energy of hydrophobic association, namely, that
of apolar-polar repulsion, have provided three
distinct contexts for force development. Thus, we
believe the consilient mechanisms will prove to
be fundamental to the function of the ATPases
of biology. As argued below, evidence emerges
for the underlying competition for hydration
between hydrophobic and polar (e.g., charged)
groups to be a basic element in the functioning
of key protein-based machines of biology.
8.2 Overview of Energy
Conversion in Biological
Systems
8.2.1 The Overall Energy
Conversions: Photosynthesis
and Respiration
The overall energy conversions of biology were
briefly considered in Chapter 2, the overview
chapter, and grossly represented in Figures 2.10
(for photosynthesis) and 2.11 (for respiration).
Here the statements of photosynthesis and res-
piration are given below for ready reference in
order to place in context the component energy
flows that sustain Life."*^"^^
Photosynthesis, in overview, uses light energy
from the sun to combine water and carbon
dioxide to produce carbohydrate and oxygen.
Respiration, in overview, is exactly the reverse
with the exception that, instead of an input of
light energy, the output is chemical energy, as
given in Figure 8.4 and considered below.
The light reactions themselves involve
energy steps almost 10 times greater than the
energy steps involved in the subsequent reac-
tions of photosynthesis and of respiration. In
general, there are many thousands of subse-
quent reactions, where the energy steps are 8 to
lOkcal/reaction or less. From this perspective,
the task of gaining a sense of what sustains Life
would seem to be overwhelming.
Fortunately, as will be discussed below for
respiration, there are only a few tens of reac-
tions in the oxidation of a glucose molecule to
produce 6(C02) and 6(H20). Most significantly,
there are but three products that constitute the
most immediate resulting chemical energy.
These are reduced nicotinamide adenine dinu-
cleotide (NADH, 10 molecules), reduced flavin
Photosynthesis:
The first step in biology's reversal of
the arrow of time
carbon dioxide + water
+
light energy
6{C02) 6(H20)
• carbohydrate + oxygen
(1) [C(H20)]6 6(02)
Respiration:
Animals and plants in the dark use the
chermical energy of respiration to create more diverse
and complex life forms.
carbohydrate + oxygen
[C(H20)]6 6(02)
* water
+
carbon dioxide + chemical energy (2)
6(H20) 6(C02)
But these remarkable chemical equations do not
explain how!
FIGURE 8.4. Photosynthesis and respiration repre-
sent very broad expressions of the energy conver-
sions that sustain Life.
