Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


8.2 Overview of Energy Conversion in Biological Systems
355
adenine dinucleotide (FADH2, 2 molecules),
and adenosine triphosphate (ATP, 4 mole-
cules).
As ATP is the basic energy denomina-
tion of biology, the complete conversion of one
glucose molecule to the chemical energy cur-
rency of biology comes down to the oxidation
of NADH and FADH2 in the production of an
additional 32 molecules of ATP. This involves
five protein-based machines that constitute
what has been called oxidative phosphorylation
and that occur in the mitochondria, the so-
called energy factories of biology. Four of the
protein-based machines (Complexes I through
IV) effect the pumping of protons, and the fifth
complex utilizes the return of the protons
across the inner mitochondrial membrane to
produce the 32 molecules of ATP.
In general, then, the energy conversions of
biology reduce to the production of ATP and
the uses of ATP, that is, the production of
ATP by the five protein-based machines of the
inner mitochondrial membrane and the thou-
sands of subsequent protein-based machines
that do the necessary work of the cell. This
constitutes yet an enormous task that will fill
hundreds of volumes in the future of protein-
based machines. The intention of this volume,
however, is to add a simplifying feature of a
"common groundwork of explanation" for each
of the hydrophobic and elastic consilient
mechanisms. For the function of protein-based
machines of biology, this perspective recovers
an attractive element of simplification.
8.2,1.1 Photosynthesis for the Production
of Carbohydrate (Glucose)
The following statement of photosynthesis is
essentially a question of a balanced chemical
equation where each atom among the reactants,
on the lefthand side of the equation, is
accounted for in the products on the right-hand
side.
For photosynthesis.
Carbon Dioxide + Water + Light energy
6(C02) 6(H20)
^ Carbohydrate + Oxygen (8.4)
[C(H20)],
6(02)
The remarkable simpUcity of this statement,
known for over a century, is stunning. It
involves only the atoms of carbon (C), oxygen
(O),
and hydrogen (H) with truly simple stoi-
chiometry. While there is great beauty in the
simplicity of the equation, it obscures intricate
detail of just what does happen in the process
and, importantly, how it happens.
8.2.1.2 Respiration: Oxidation of Glucose
with Ultimate Reduction of Oxygen to
Result in Water and Carbon Dioxide
The chemical products of photosynthesis
become the chemical reactants of respiration,
and the chemical products of respiration
become the chemical reactants of photosyn-
thesis.
Of course, absent the light energy for pho-
tosynthesis, there would be no products of
carbohydrate and oxygen, and absent the chem-
ical reactants of respiration, there would be no
chemical energy with which to sustain
Life.
Thus,
instead of oxidizing glucose by fire to give rise
to heat,
as
occurs in fireplaces during the burning
of wood (which is cellulose composed of strings
of glucose molecules), living organisms evolved
the capacity, step by step, to turn the oxidation
of glucose into production of the energy con-
tained within the ATP molecule. For respiration.
Carbohydrate + Oxygen ^ Water
[C(H20)],
6(02) 6(H20)
+ Carbon Dioxide + Chem Energy
6(C02) (8.5)
Again, the simplicity of the chemically balanced
equation, this time representing respiration,
belies what may seem an almost bewildering
underlying set of complex reactions. Before
addressing the key energy-converting steps of
respiration, however, another pair of analytical
expressions points in the direction of recogniz-
ing the separation of the hydrogen atom into its
proton, ff, and its electron, e~, which represent
elemental features laid bare without the blur of
molecular detail.
Respiration can be seen as a pair of
half-
reactions, which provides initial insight into the
underlying process carried out by the metaboUc
machinery of the cell. For oxidation of glucose,
the oxidative half reaction is
C6H12O6 + 6(H20) ^ 6(C02) + 24H^ + 24e-
(8.6)

356
8. Consilient Mechanisms for Protein-based Machines of Biology
For reduction of oxygen, the reductive half
reaction is
6(02) + 24H^ +24e- ^ 12(H20) (8.7)
Addition of the two half-reactions gives the
expression for respiration on explicitly in-
cluding the statement for the chemical energy
obtained. The analytical simplicity of the
half-
reactions lays out the underlying essential result
of the biological electron transport chain.
Indeed, the electron transport chain of the mito-
chondrion achieves the separation into protons,
¥t, and electrons, e~. (For the structure of the
mitochondrion, refer to Figure 8.5, below.)
The protons are released to one side of an
otherwise generally proton-impermeable inner
mitochondrial membrane to collect the protons
in the space between the inner and outer
membranes of the mitochondrion. The resulting
proton concentration gradient then drives for-
mation of ATP by the quintessential protein-
based machine, ATP synthase, as the protons
flow back through the inner mitochondrial
membrane by means of another special path
effecting proton permeability. Thus there are
two fundamental questions. The first is, how
does electron flow within the membrane
achieve unidirectional proton flow across the
membrane? The second is, how does the return
flow of protons result in the formation of ATP,
the energy coin of biology?
Incredibly, the marvelous work of many bio-
chemists, biophysicists, and crystallographers
has provided reaction stoichiometries, mecha-
nistic details, and structures. It is a beautiful
evolving story into which we would like to
introduce the reasoning of the consilient
mechanisms. As presented in Chapter 5, the
hydrophobic and elastic consilient mechanisms
developed from inverse temperature transi-
tions of hydrophobic association exhibited by
elastic-contractile model proteins functioning
as protein-based machines capable of inter-
converting the same energies interconverted by
living organisms. In particular, we introduce
the insight of an apolar-polar repulsion into
considerations of understanding molecular
mechanism. First to be considered, however, are
a number of intermediate molecular players
and processes in the early oxidation stages of
glucose in preparation for utilization of the
reduced molecules in the biological electron
transport chain.
8.2,1.3 Products of Glucose Oxidation by
the Reactions of Intermediary Metabolism
Despite what at initial consideration may
appear as maze-like detail, the many reactions
that accomphsh respiration, given as the oxida-
tion of glucose to CO2 and water, group as two
cycles tied together by a single reaction. The
first cycle is the glycolysis cycle that by way
of the transition reaction feeds into the citric
acid cycle, also called the Krebs cycle or the
tricarboxylic acid cycle. As briefly enumerated
below, the products of these reactions either are
or become convertible to the universal energy
currency of biology, ATP.
8.2.1.3.1
Glycolysis Cycle
By the glycolysis cycle, the oxidation of glu-
cose yields two molecules of pyruvate, two
molecules of reduced nicotinamide adenine
dinucleotide (2 NADH), and two molecules of
adenosine triphosphate (2 ATP).
8.2.1.3.2
Transition Reaction
In the transition reaction, the two pyruvate
molecules are converted to two molecules of
acetyl coenzyme A, two molecules of carbon
dioxide (2 CO2), and two molecules of reduced
nicotinamide adenine dinucleotide (2 NADH).
8.2.1.3.3
Citric Acid (Krebs) Cycle
The Krebs cycle takes two molecules of acetyl
coenzyme A from the transition cycle and con-
verts them into four molecules of carbon
dioxide (4 CO2), six molecules of reduced
nicotinamide adenine dinucleotide (6 NADH),
two molecules of reduced flavin adenine dinu-
cleotide (2 FADH2), and two molecules of
adenosine triphosphate (2 ATP).
8.2.1.3.4 Summary of the Oxidation of
Glucose by Intermediary Metabolism
The products of intermediary metabolism for
the oxidation of one molecule of glucose are 6
molecules of carbon dioxide (6 CO2), 10 mole-
cules of reduced nicotinamide adenine dinu-
cleotide (10 NADH), 2 molecules of reduced
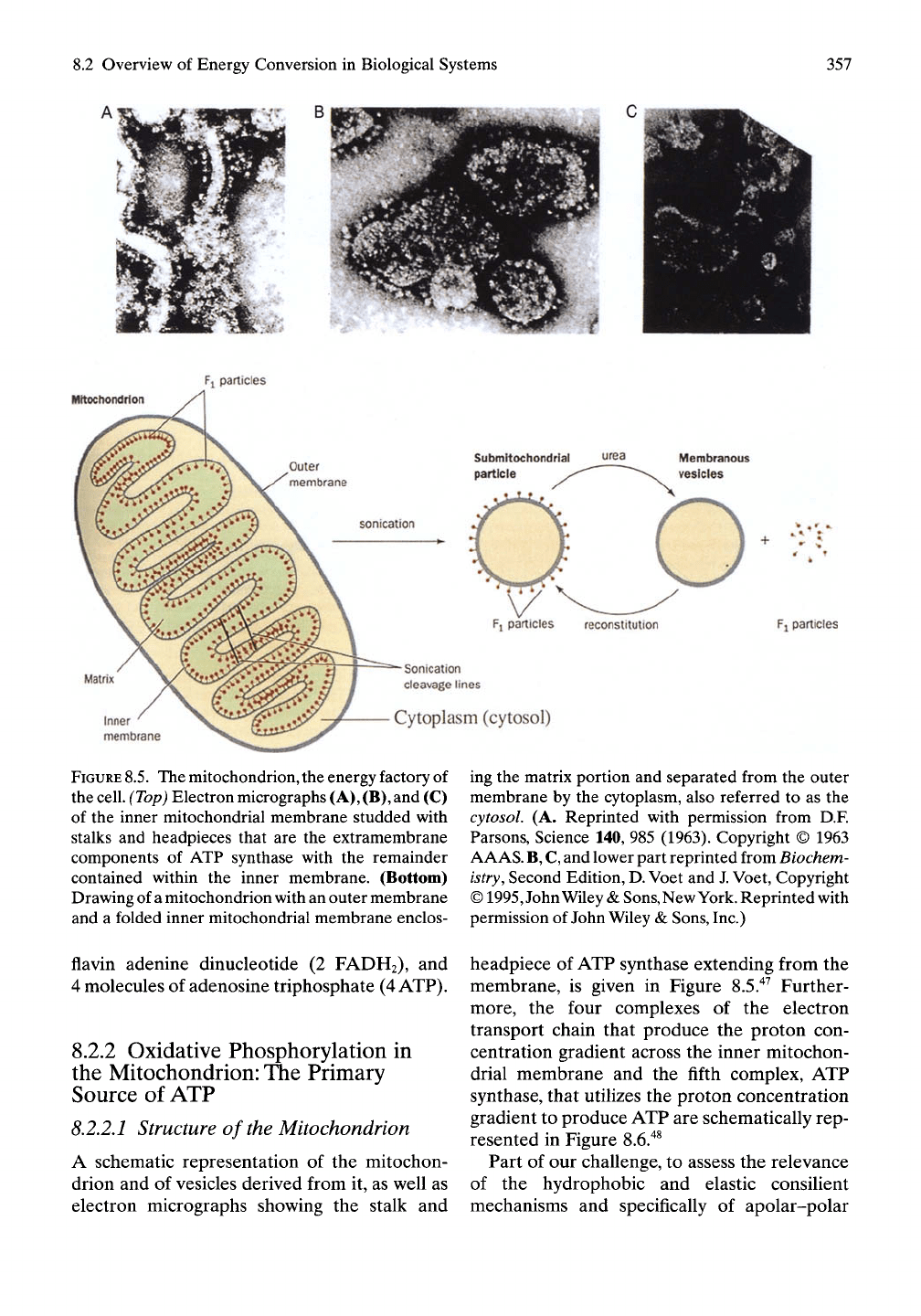
>.2 Overview of Energy Conversion in Biological Systems
A lev
js^mmmt'^r B
357
Fj particles
Mitochondrion
Fi particles
Inner
rDembrane
Cytoplasm (cytosol)
FIGURE
8.5.
The mitochondrion, the energy factory
of
the
cell.
(Top)
Electron micrographs
(A),
(B),
and (C)
of the inner mitochondrial membrane studded with
stalks and headpieces that are the extramembrane
components of ATP synthase with the remainder
contained within the inner membrane. (Bottom)
Drawing of
a
mitochondrion with an outer membrane
and a folded inner mitochondrial membrane enclos-
flavin adenine dinucleotide (2 FADH2), and
4 molecules of adenosine triphosphate (4 ATP).
8.2.2 Oxidative Phosphorylation in
the Mitochondrion: TTie Primary
Source of ATP
8.22,1
Structure
of
the Mitochondrion
A schematic representation of the mitochon-
drion and of vesicles derived from it, as well as
electron micrographs showing the stalk and
ing the matrix portion and separated from the outer
membrane by the cytoplasm, also referred to as the
cytosol. (A. Reprinted with permission from D.F.
Parsons, Science 140, 985 (1963). Copyright © 1963
AAAS.
B,
C,
and lower part reprinted from Biochem-
istry,
Second Edition,
D.
Voet and
J.
Voet, Copyright
©
1995,
John Wiley
&
Sons,New
York.
Reprinted with
permission of John Wiley &
Sons,
Inc.)
headpiece of ATP synthase extending from the
membrane, is given in Figure 8.5."^^ Further-
more, the four complexes of the electron
transport chain that produce the proton con-
centration gradient across the inner mitochon-
drial membrane and the fifth complex, ATP
synthase, that utilizes the proton concentration
gradient to produce ATP are schematically rep-
resented in Figure 8.6."^^
Part of our challenge, to assess the relevance
of the hydrophobic and elastic consilient
mechanisms and specifically of apolar-polar
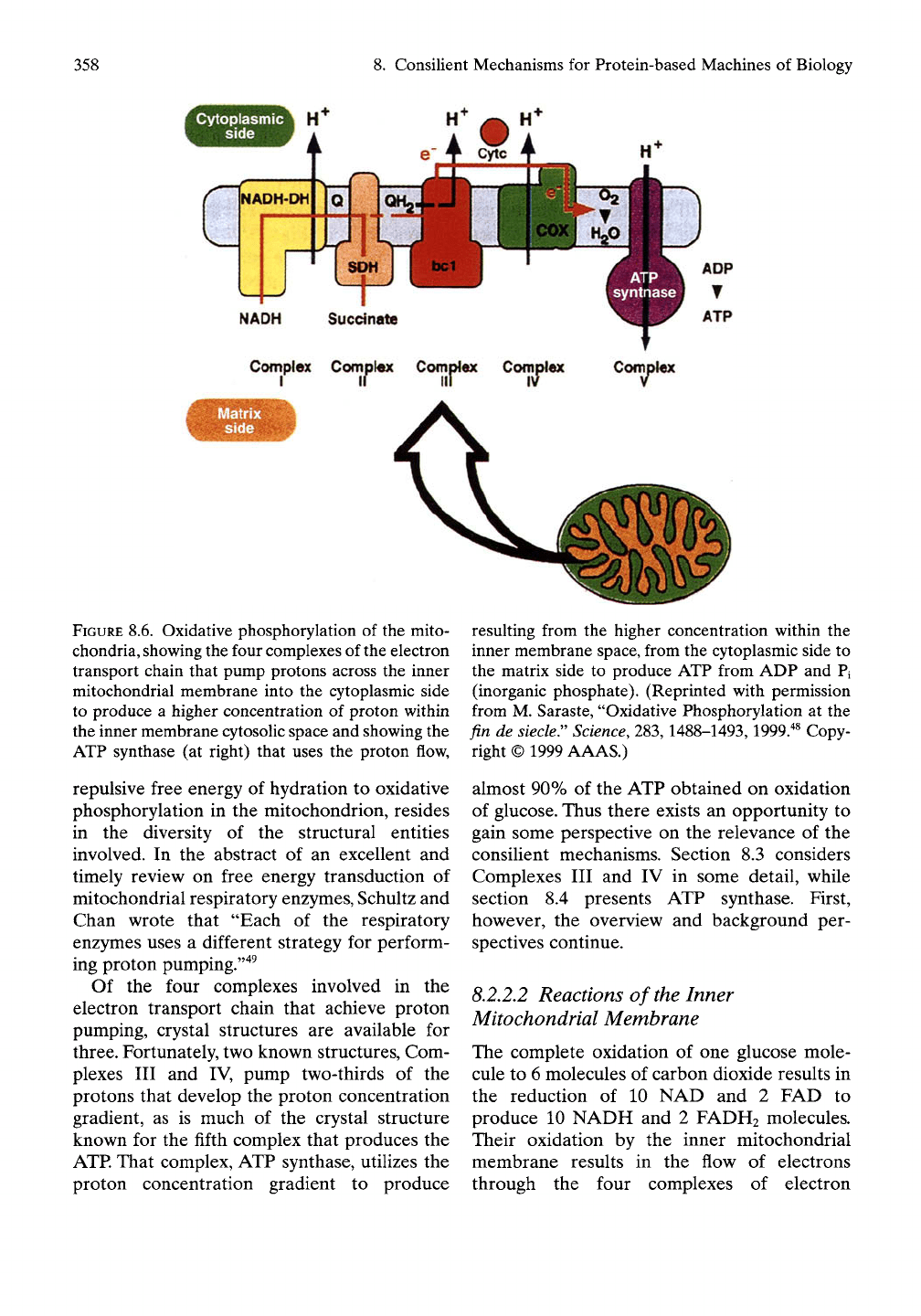
358
8. Consilient Mechanisms for Protein-based Machines of Biology
Cytoplasmic
side
ADP
ATP
Complex Comolex Complex Complex Complex
FIGURE 8.6. Oxidative phosphorylation of the mito-
chondria, showing the four complexes of the electron
transport chain that pump protons across the inner
mitochondrial membrane into the cytoplasmic side
to produce a higher concentration of proton within
the inner membrane cytosolic space and showing the
ATP synthase (at right) that uses the proton flow,
repulsive free energy of hydration to oxidative
phosphorylation in the mitochondrion, resides
in the diversity of the structural entities
involved. In the abstract of an excellent and
timely review on free energy transduction of
mitochondrial respiratory enzymes, Schultz and
Chan wrote that "Each of the respiratory
enzymes uses a different strategy for perform-
ing proton pumping.'"^^
Of the four complexes involved in the
electron transport chain that achieve proton
pumping, crystal structures are available for
three. Fortunately, two known structures. Com-
plexes III and IV, pump two-thirds of the
protons that develop the proton concentration
gradient, as is much of the crystal structure
known for the fifth complex that produces the
ATP.
That complex, ATP synthase, utilizes the
proton concentration gradient to produce
resulting from the higher concentration within the
inner membrane space, from the cytoplasmic side to
the matrix side to produce ATP from ADP and Pi
(inorganic phosphate). (Reprinted with permission
from M. Saraste, "Oxidative Phosphorylation at the
fin de siecler
Science,
283,1488-1493,1999.^^ Copy-
right © 1999 AAAS.)
almost 90% of the ATP obtained on oxidation
of glucose. Thus there exists an opportunity to
gain some perspective on the relevance of the
consilient mechanisms. Section 8.3 considers
Complexes III and IV in some detail, while
section 8.4 presents ATP synthase. First,
however, the overview and background per-
spectives continue.
8.2.2.2 Reactions of the Inner
Mitochondrial Membrane
The complete oxidation of one glucose mole-
cule to 6 molecules of carbon dioxide results in
the reduction of 10 NAD and 2 FAD to
produce 10 NADH and 2 FADH2 molecules.
Their oxidation by the inner mitochondrial
membrane results in the flow of electrons
through the four complexes of electron

8.2 Overview of Energy Conversion in Biological Systems
359
transport chain that pump protons into the
intermembrane space and reduces oxygen
molecules to water. The return of the protons
through the fifth complex, ATP synthase, results
in the production of 32 molecules of ATP. The
chemical energy obtained on oxidation of the
nucleotides, reduced on oxidation of one mole-
cule of glucose, ultimately appears in the for-
mation of 32 molecules of ATP.
This brings the total number of ATP mole-
cules formed from ADP and Pi to 36 in the
process of the oxidation of a single glucose
molecule. The set of reactions in the mitochon-
drion that produces 32 of the 36 ATP molecules
are collectively called oxidative phosphoryla-
tion.
This occurs in two steps. Step 1 utilizes a
series of four protein-based machines. Com-
plexes I, II, III, and IV, collectively referred to
as the electron transport chain.
ThQSQ
complexes
oxidize the 10 NADH and 2 FADH2 molecules
and pump protons across the inner mitochon-
drial membrane to produce a proton concen-
tration gradient. Step 2 involves the most
important energy converting protein-based
machine of biology (ATP synthase) that utilizes
the concentration gradient of protons across
the inner membrane of the mitochondria to
produce ATP from ADP and Pj.
8.2.2.3 Step 1: Oxidation of NADH and
FADH2, Via the Electron Transport Chain,
Pumps Protons into the Intermembrane
Space and Results in the Reduction of
Oxygen to Water
Step 1 involves four enzyme complexes with
reactions much as given by Schultz and Chan"*^:
Complex I: Oxidizes one molecule of NADH,
and, by an as yet unknown mechanism,
pumps 4 protons into the intermembrane
(cytosolic) space and reduces ubiquininone
(coenzyme Q) to produce ubiquinol, QH2.
Overall equation:
NADH
+
5H^ (matrix) + Q ^
NAD^
+
QH2 + 4H^ (cytosol) (8.8)
Complex II: FAD oxidizes succinate to pro-
duce fumarate and FADH2, which reduces
ubiquininone to produce ubiquinol, QH2.
Overall equation:
Succinate + Q ^ Fumarate
+
QH2 (8.9)
Complex III: The cytochrome bci complex
oxidizes 2 molecules of ubiquinol, pumps
4 protons into the intermembrane (cytosolic)
space, and reduces two molecules of
cytochrome c. Overall equation:
2QH2 + 2cyt c^^ + Q + 2H^ (matrix) -^
2Q-F2cyt c'-^ +QH2 +4HMcytosol) (8.10)
Complex IV: Cytochrome c oxidase oxidizes
cytochrome c pumps 2 to 4 protons into the
intermembrane (cytosolic) space and reduces
oxygen to water. Overall equation:
4cyt
c^-"
+
O2
+
4H"'
(scalar) +
4H"'
(matrix) ->
4cyt c'^ + 2H2O
-h
4H^ (cytosol)
(8.11)
Complexes III and IV are considered in some
structural detail in section 8.3.
Based on the above outline of key elements
of the four complexes, 10 NADH molecules
would contribute about 100 protons and 2
FADH2 molecules would contribute another
12 protons. As indicated below, production of
32 ATPs by ATP synthase would utilize an
equivalent number, nominally 106 protons.
8.2.2.4 Step 2: Proton Driven
Phosphorylation Via ATP Synthase
The result of the oxidation of the reduced coen-
zyme molecules, 10 NADH and 2FADH2, is the
development of a proton concentration gradi-
ent across the inner mitochondrial membrane.
The return of the protons back across the inner
mitochondrial membrane results in the produc-
tion of 32 ATP molecules from 32 molecules of
ADP plus 32 molecules of Pi by means of the
ATP
synthase.
The understanding of this source
of energy for ATP synthesis derives from the
now well accepted, but once highly controver-
sial, Mitchell chemiosmotic hypothesis.^^
8.2.3 Question: Why Do Ions Form
and Why do Ions Pair?
Despite this issue having been addressed in
several contexts above, it is again stated sue-

360
8. Consilient Mechanisms for Protein-based Machines of Biology
cinctly here to emphasize the importance of the
perspective.
8.2.3.1 When There Is Adequate Water,
Ions Dissolve, That Is, They Prefer to Be
Fully Hydrated
Ions dissolve from salts, or whenever otherwise
ion paired, due to lowering of the ion
(self-
energy) free energy on achieving full hydration.
When there is inadequate hydration, the
driving force is for charge neutralization.
Neutralization of a negatively charged group
could occur by protonation or by pairing
with a cation, and neutralization of a positively
charged group would occur by loss of a proton
or by pairing with an anion. In either case the
neutralizing ion could be either an inorganic or
an organic ion, as in the case of the charged side
chains of amino acid residues.
8.2.3.2 AGap Provides the Driving Force
for Ion Pairing, Which Is an Expression of
Limited Availability of Water for
Hydration of the Ion
When a clustering of hydrophobic groups
occurs, there develops a competition for hydra-
tion between charged and hydrophobic groups
that raises the free energy of the charged species.
This results in an apolar-polar repulsive free
energy of hydration (AGap) that is seen as a shift
in pKa. Functional groups with a shift in pKa are
at an increase in free energy and would lower
that free energy by ion pairing. Ion pairing
within or between protein subunits is the result
of this AGap and is caused by the proximity of
hydrophobic groups. As concerns the protein-
based machine, it is the sequence-imposed coex-
istence of polar and apolar groups along a
protein chain that gives rise to association of
hydrophobic domains, and ion-pair formation
becomes an integral part of the energetics for
hydrophobic association. An apolar-polar
repulsive free energy of hydration also appUes
to oxidized and reduced states of prosthetic
groups and cofactors and to their interactions.
8.2.4 Question: Can Free Energy
Transduction Be Localized at Some
Crucial Part of the Enzymatic Cycle?
By the hydrophobic consilient mechanism of
energy transduction, the energizing of a protein
(an enzyme) results from AGap, the change in
apolar-polar repulsive free energy of hydration
due to molecules or inorganic ions interacting
with the enzyme or protein of interest.^^
Depending on the changes in the state of the
molecules or inorganic ions interacting with
the protein, the protein may even be energized
further, as on the splitting of ATP to form the
most energized state, ADP plus Pi. There then
occur stepwise decreases in the energized state
that generally results most dramatically on
release of Pi. The energized state abates as the
apolar-polar repulsions continue to relax. As
the cycle progresses, the enzyme becomes fully
de-energized only on return to its initial state.
Therefore, the answer to the posed question is
no;
the free energy transduction cannot be local-
ized at any particular part of the cycle, because
different amounts of energy reside within the
enzyme at different steps of the cycle, that
is,
due
primarily to apolar-polar repulsion, the ener-
gized state of the enzyme is different at each step
of the cycle.
8.2.5 A Glimpse of Selected Energy
Conversions Going Forward
This section provides in a relatively limited
detail a standard overview of energy conver-
sion. For all but the photosynthetic organisms,
however, energy conversion can be represented
in terms of three stages. The first stage is the
development of a proton concentration gradi-
ent across the inner membrane of the mito-
chondrion. The second stage is the use of that
concentration gradient to produce ATP, the
representative biological energy coin, again
centered on the inner membrane of the mito-
chondrion. The third stage is the use of ATP and
its equivalent nucleotide NTPs by ATPases or
in general by NTPases.

8.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
361
In subsequent sections one representative
protein-based machine is emphasized for each
stage. For proton pumping across the inner
mitochondrial membrane discussed in section
8.3,
Complex III (ubiquinonexytochrome c oxi-
doreductase), also called the cytochrome bci
complex, provides an amazing example of
both aspects of the consilient mechanism,
those controlling hydrophobic association, the
apolar-polar repulsive free energy of hydration
(AGap), and the development of elastic force
due to a decrease in internal chain dynamics
between two fixed points in a protein chain
segment. The singular choice of ATP synthase
also provides an opportunity in section 8.4 to
emphasize the use of AGap to energize ADP
plus Pi to form ATP. Among the myriad of
ATPases, the myosin II motor of muscle con-
traction, a linear contractile motor, discussed in
section 8.5 and as anticipated in Chapter 7, pro-
vides a remarkably apparent example of AGap,
whereby binding ATP disrupts hydrophobic
association of the amino-terminal domain with
the head of the lever arm, and loss of phosphate
re-establishes the hydrophobic association to
provide the powerstroke.
8.3 The Electron Transport
Chain: Protein Machines as
Redox-driven Proton Pumps
8.3.1 Introductory Comments
8.3.1.1 Structural Status of the Four
Protein Machines (Complexes 1,11, III,
and IV) of the Electron Transport Chain
8.3.1.1.1
Current Status of Structural Details
Available for the Four Complexes of the
Electron Transport Chain
Complex I is a very large protein machine with
about 43 subunits. Because of this the structural
information has only been achieved to date to
22 A resolution.^^'^^ The resolutions for Com-
plexes II (2.9 A), III (2.3 to 2.9 A), and IV (2.3
to 3.0 A) are nearly an order of magnitude
better and provide details at a level that allows
consideration of mechanism.
8.3.1.1.2
General Sources for More
Background Information
Two excellent reviews are available for the
protein-based machines of oxidative phospho-
rylation from which more background infor-
mation may be obtained. One is a clear and
succinct statement by Saraste,"^^ and the other,
by Schultz and Chan,"^^ is an excellent, more
extensive account of the state of information on
the structures, strategies, and thermodynamics
of the five protein-based machines of oxidative
phosphorylation. Both of these reviews may be
sought for additional insight and detail. In addi-
tion, the fifth edition of Biochemistry by Berg,
Tymozcko, and Stryer"*^ provides an outstanding
account of energy conversion in the mitochon-
drion. Our objective in section 8.3 is to consider
the structures and functions of the electron-
transport/proton-pumping complexes in terms
of the consilient mechanism. We begin by a
summary statement of simple hypotheses
whereby the apolar-polar repulsive free energy
of hydration, AGap, would play the pivotal role
of proton gatekeeper that is based on a struc-
tural analysis of Complex III and whereby
elastic force generation plays a pivotal role in
domain movement for selected electron trans-
fer. Due to both time constraints and limita-
tions in structural data, the other complexes are
not considered in as much detail.
8.3.1.2 Relevance of the Consilient
Mechanisms to the Coupling of Electron
Transport to Proton Gating/Pumping
8.3.1.2.1
Hypothesis 1: AGap as the
Gatekeeper for Transmembrane Proton
Pumping by the Electron Transport Chain
By the operative component,
AGap,
of the Gibbs
free energy of hydrophobic association, AGHA,
polar groups compete with hydrophobic groups
for hydration; the result is that the formation of
charged groups disrupts hydrophobic associa-
tion. Recognition of two aspects of the com-
petition becomes central to understanding
mechanism. One is that hydrophobic associa-
tion occurs whenever too much hydrophobic
hydration begins to form during an opening
fluctuation. Tlie second is the formation of a

362
8. Consilient Mechanisms for Protein-based Machines of Biology
charged group, whether positive or negative, that
recruits emergent hydrophobic hydration for its
own charged hydration and thereby effects
hydrophobic dissociation and the opening of an
aqueous channel.
Complex III, ubiquinonexytochrome c oxi-
doreductase, provides this example remarkably
well. Our proposal states that the oxidation of
ubiquinol, QH2, at the Qo site on the inter-
membrane side of the inner mitochondrial
membrane produces a positively charged
molecule, for example QH2^. Emergence of this
positively charged species due to the electron
flow of oxidation disrupts hydrophobic associ-
ation of the hydrophobic tip of the RIP with the
Qo site and thereby allows two protons, 2H^, to
pass into the intermembrane cytoplasmic space
with the result of a molecule of ubiquinone.
Furthermore, our proposal contends that the
reduction of ubiquinone at the Qi site produces
a negatively charged molecule, for example
Q^", on the matrix side of the inner mitochon-
drial membrane. Emergence of this negatively
charged species due to the electron flow of
reduction converts a string of hydrophobically
enclosed water molecules into a water-filled
channel for the entrance of two protons, 2H^,
from the matrix space to produce ubiquinol. In
this way by AGap, the formation of a positively
charged species opens the gate for proton
egress from the inner mitochondrial mem-
brane, and the formation of a negatively
charged species opens the gate for proton
ingress into the inner mitochondrial membrane.
Because of AGap and the structural locations of
the redox-formed positively charged and nega-
tively charged groups, the redox events and
proton transport events are tightly coupled.
Thus,
given the example of Complex III oxi-
dation of ubiquinol at the Qo site and reduction
of ubiquinone at the Qi site, we would like to
generalize the proton pumping mechanism to
the similar formation of a charged species of
whatever molecular basis at appropriate loca-
tions in the inner mitochondrial membrane.
General hypothesis of proton gating/pumping:
Proton translocation across the inner mitochon-
drial membrane occurs (1) by oxidative forma-
tion of
a
positively charged species that opens an
aqueous passageway to the cytoplasmic side of
the membrane and that becomes neutralized by
proton release to the intermembrane (cytoplas-
mic) space and (2) by the reductive formation of
a negatively charged group that opens an
aqueous passageway to the matrix side of the
membrane and that becomes neutralized by
proton uptake from the matrix space.
8.3.1.2.2
Hypothesis 2: Hydrophobic
Association Within Complex III of the
Hydrophobic (FeS) Tip of the Rieske Iron
Protein with the Hydrophobic Ubiquinol-
containing Qo Site Causes Extension and
Damping of Internal Chain Dynamics in the
Tether of the Iron Protein
Complex III exemplifies another aspect of the
consilient mechanisms. The FeS center of the
RIP resides at a very hydrophobic tip of a stylus-
hke structure with two sites for hydrophobic
association, one at the Qo site and a second at
the cytochrome Ci site. As part of our hypothe-
sis,
the relative affinity due to hydrophobic asso-
ciation at each site depends on the redox state
of the FeS center and the charge at the Qo site.
Before receipt of the electron, AGHA is more
favorable at the Qo site, whereas after receipt of
the electron and oxidation of ubiquinol, the
AGHA becomes sufficiently less favorable at the
Qo site to allow the elastic force of the stretched
tether to withdraw the FeS center from the Qo
site for translocation to the cytochrome Ci site.
On transfer of the electron to cytochrome
Ci,
the
affinity at this site decreases and the FeS center
returns to the very favorable hydrophobic
association at the Qo
site,
now occupied by a new
molecule of hydrophobic ubiquinol. The affinity
due to hydrophobic association is so favorable
at the ubiquinol-occupied Qo site that the
interconnecting chain segment between mem-
brane anchor and the globular component of the
RIP becomes stretched. We suggest that this is
yet another example of the elastic-contractile
mechanism demonstrated in the model proteins
discussed in Chapter 5 in the context of hydro-
phobic association causing extension of an inter-
connecting chain segment. Hydrophobic asso-
ciation coupled with damping of internal chain
dynamics on extension of interconnecting chain
segments results in development of elastic force.
Contraction by the muscle myosin II motor
is another example of hydrophobic association

8.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
363
on decreased charge, that is, on loss of phos-
phate, and one where damping of internal chain
motions on hydrophobic association must
surely exist as evidenced by an increase in the
elastomeric force under isometric conditions,
but the chain or chains involved have not been
as clearly identified.
8.3.2 Complex I: NADH:ubiquinone
Oxidoreductase—Oxidation of
NADH for Reduction of Ubiquinone
to Ubiquinol While Translocating Four
Protons from Matrix to Cytosol for
Every NADH Oxidized
8.3.2.1 Structural and Redox Center
Information for Complex I
The structure of Complex I of mammalian
mitochondria comprises an unusually large
complex of about 43 distinct protein subunits
with a three-dimensional structure yet to be
determined at atomic resolution. From electron
microscopy, it has been observed with an L-
shaped structure, as indicated in Figure 8.6 with
a
20
nm length in the membrane and an 8nm
protrusion into the matrix. Equation (8.5), the
overall reaction, NADH + 5 H^ (matrix) + Q ^
NAD^
+ QH2 + 4 H^ (cytosol), beUes a more
involved set of electron carriers. The process of
reducing ubiquinone, Q, to ubiquinol, QH2,
involves a substantial number of redox centers
with the coupled result of the transmembrane
transport of four protons for each NADH
molecule oxidized.
The process whereby oxidation of NADH
reduces ubiquinone to ubiquinol involves the
redox groups of flavin mononucleotide (FMN)
and six FeS centers. It appears, however, that the
membrane spanning subunits of the L-shaped
structure do not contain redox centers, but do
contain the ubiquinone. Images at 2.2 nm reso-
lution^^ demonstrate a structure more contorted
than the monolithic L-shaped block indicated
for Complex I in Figure 8.6. Accordingly, when
making suggestions of mechanism based on
AGap,
it is tempting to consider the possibility of
redox groups being sufficiently proximal to the
cytoplasmic side of the inner mitochondrial
membrane to form species such as QH^^ and the
analogous oxidized state of flavin, FMNH2^, to
provide a source of proton release to the cyto-
plasmic side of the inner mitochondrial mem-
brane. With regard to the matrix side of the
membrane, the possibility would continue with
the reduction of ubiquinone and FMN to form
negative species, such as Q^" and FMN^~, as
could happen on addition of two electrons to
each of the oxidized ubiquinone and flavin
rings,
that would open channels for protons to
enter from the matrix side of the membrane. Of
course, the problem with this analogy to the
quinol/quinone use of Complex III is the appar-
ent absence of structural information placing
flavin and ubiquinone appropriately at two sites
in the membrane component of Complex I.
8.3.2.2 Further Structural Considerations
8.3.2.2.1
Additional Detail for
L-shaped Structure
As shown in Figure 8.7 A for bovine Complex
I at 22 A resolution in ice, the monolithic block
of Figure 8.6 becomes a structure of a greater
defined shape with a bulbous matrix compo-
nent and a much more convoluted membrane
component. If the redox components, with the
exception of ubiquinone
itself,
reside in the
bulbous component, then the puzzle remains
one of how proton translocation occurs.
8.3.2.2.2
A re-arranged Horseshoe-shaped
Structure as a Candidate for the Native State
Further electron microscopic studies of an
enzymatically active Complex I show the
rearrangement from the L-shaped structure in
Figure 8.7B to the horseshoe-shaped structure
in Figure 8.7C.^^ This reversible rearrangement
due to a decrease in ionic strength reversibly
turned off (at high ionic strength) and turned
on (at low ionic strength). Specifically, raising
the concentration of NaCl to 0.2 M abolished
NADHidecylubiquinone reductase activity.
This appears to be a general salt effect on
hydrophobic association, as the same effect was
reported for KCl, LiCl, MgCl2, CaCl2, Li2S04,
and NaNOs, which is analogous to the results in
Figures 5.11 and 5.12 for elastic-contractile
model proteins. Accordingly, this gives the pos-
sibility that redox centers might occur within
the inner mitochondrial membrane.
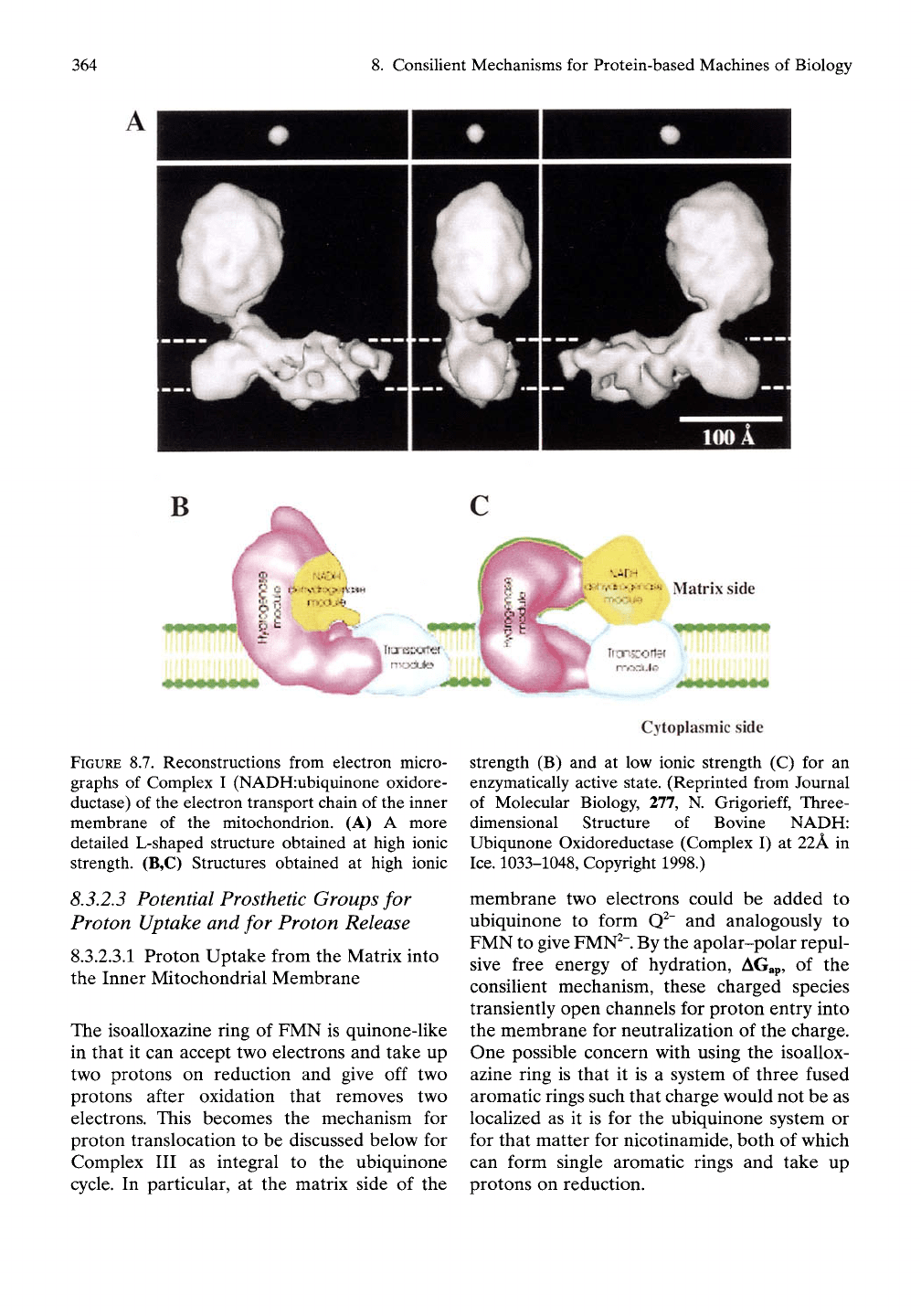
364
8. Consilient Mechanisms for Protein-based Machines of Biology
B
Matrix side
FIGURE 8.7. Reconstructions from electron micro-
graphs of Complex I (NADH:ubiquinone oxidore-
ductase) of the electron transport chain of the inner
membrane of the mitochondrion. (A) A more
detailed L-shaped structure obtained at high ionic
strength. (B,C) Structures obtained at high ionic
8,3.2.3 Potential Prosthetic Groups for
Proton Uptake and for Proton Release
8.3.2.3.1
Proton Uptake from the Matrix into
the Inner Mitochondrial Membrane
The isoalloxazine ring of FMN is quinone-like
in that it can accept two electrons and take up
two protons on reduction and give off two
protons after oxidation that removes two
electrons. This becomes the mechanism for
proton translocation to be discussed below for
Complex III as integral to the ubiquinone
cycle. In particular, at the matrix side of the
Cytoplasmic side
strength (B) and at low ionic strength (C) for an
enzymatically active state. (Reprinted from Journal
of Molecular Biology, 277, N.
Grigorieff,
Three-
dimensional Structure of Bovine NADH:
Ubiqunone Oxidoreductase (Complex I) at 22A in
Ice.
1033-1048, Copyright 1998.)
membrane two electrons could be added to
ubiquinone to form Q^" and analogously to
FMN to give FMN^". By the apolar-polar repul-
sive free energy of hydration, AGap, of the
consilient mechanism, these charged species
transiently open channels for proton entry into
the membrane for neutralization of the charge.
One possible concern with using the isoallox-
azine ring is that it is a system of three fused
aromatic rings such that charge would not be as
localized as it is for the ubiquinone system or
for that matter for nicotinamide, both of which
can form single aromatic rings and take up
protons on reduction.
