Lefebvre A.H., Ballal D.R. Gas Turbine Combustion: Alternative Fuels and Emissions
Подождите немного. Документ загружается.


40 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
Following the same procedure as that employed for weak mixtures leads,
for n = 2 and m = 1, to
m
VP TERT
A
c
c
215
2
1
α
φ
η
η
.
exp
,
(
)
−
(
)
(2.6)
while for n = 1.75 and m = 0.75, we have
m
VP TERT
A
c
c
175
075
125
175
1
.
.
.
.
exp
.α
φ
η
η
(
)
−
(
)
(2.7)
The manner in which the heat-release rate varies with the fraction of the
fuel burned η
c
is illustrated in Figure 2.1. At low levels of η
c
, heat-release
rates are low because the temperature is low. As combustion proceeds,
the temperature rises, thereby increasing the rate of heat release until a max-
imum is reached at a level of η
c
that varies between 0.7 and 0.9, depending
on the equivalence ratio of the mixture and its initial temperature. Beyond
this point, any further increase in reaction rate, resulting from the continu-
ing rise in temperature, is more than offset by the reduction caused by the
fall in concentration of the oxygen and unburned fuel. Thus, the heat-release
rate falls off, becoming zero at the maximum temperature, which also cor-
respond to 100% combustion efciency.
0
Limiting
load line
Heat from
reaction
Stable
operating
point
Load line
1.0
Fraction of fuel burned
Heat release rate
Figure 2.1
Mechanism of ame blowout.

Combustion Fundamentals 41
The load line in Figure 2.1 represents the amount of heat required to raise
the unburned mixture to the reaction temperature. The point at which it inter-
sects the heat-release curve represents the operating point of the combustor.
As the throughput is increased, the slope of this line increases until, nally, it
no longer intersects the heat-release curve, and the ame blows out.
2.6 Laminar Premixed Flames
The burning velocity of a ame, i.e., the rate at which a plane combustion
wave will propagate through a gaseous ammable mixture, is determined
partly by the rate of chemical reaction in the thin ame zone, and by heat
and mass transfer from the ame to the unburned gas. The key processes
involved have been described by Gaydon and Wolfhard [6]. Conductive and
radiative heating of the unburned gas serve to initiate reaction by a thermal
mechanism, while back diffusion of active species from the ame zone can
initiate reaction by a diffusion mechanism. The burning velocity of a ame
is therefore affected by ame radiation and hence by ame temperature, by
local gas properties such as viscosity and diffusion coefcient, and by the
imposed variables of pressure, temperature, and AFR. The burning veloc-
ity may be dened as the velocity with which a plane ame front moves in
a direction normal to its surface through the adjacent unburned gas. It is a
fundamental property of a combustible mixture and is important practically,
both in the stabilization of ames and in determining rates of heat release.
It is found in practice that for any fuel, the burning velocity has a repro-
ducible constant value when the imposed variables are xed. It is also of
interest to note that the burning velocities of stoichiometric mixtures of
many hydrocarbon fuels burning with air approach a single common value
of about 0.43 m/s at normal atmospheric temperature and pressure. This
is probably because most complex fuels are largely pyrolyzed to methane,
other one- or two-carbon-atom hydrocarbons, and hydrogen before entering
into the ame reaction zone. Hence, the gas composition entering the ame
zone is substantially independent of the original fuel.
2.6.1 Factors influencing Laminar Flame Speed
The most important factors governing the laminar burning velocity are
equivalence ratio (fuel/air ratio), temperature, and pressure.
2.6.1.1 Equivalence Ratio
The variation of ame speed with mixture strength roughly follows that
of ame temperature. In almost all cases, the maximum value occurs at an

42 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
equivalence ratio of between 1.05 and 1.10. Notable exceptions to this general
rule are hydrogen and carbon monoxide. Their laminar burning velocities
reach a maximum at an equivalence ratio of around 2.
2.6.1.2 Initial Temperature
Dugger and Heimel [7] investigated the effect of initial mixture tempera-
ture on maximum burning velocity for mixtures with air of methane, pro-
pane, and ethylene, over temperatures ranging from 141 to 617 K. Their
results showed that ame speed increases with an increase in tempera-
ture. The experimental data were correlated by the following empirical
equations:
Methane10
Propane
L
6
o
L
:..
:.
.
ST
S
=+×
=
−
00816
0
211
110 342
010259
20
+×
=+×
−
−
.
:. .
.
10
Ethylene 10
6
o
L
T
S
66
o
T
174.
2.6.1.3 Pressure
Flame theory suggests that pressure is an important parameter whose effect
may be related to the reaction order by an expression of the form
SP
n
L
α
−
(
)
22/
.
Thus, for a bimolecular reaction (n = 2), burning velocity should be indepen-
dent of pressure. For the slow-burning fuels (S
L
< 0.6 m/s) employed in gas
turbines, such as natural gas and vaporized kerosine, the observed pressure
dependence can be expressed as a simple law
SP
x
L
α
−
,
where x varies from 0.1 to 0.5 [8–10].
2.7 Laminar Diffusion Flames
For laminar ames in premixed systems, chemical reaction rates are rate
controlling. Even with non-premixed systems, if the mixing occurs rapidly
compared with the chemical reactions, combustion rates may be considered

Combustion Fundamentals 43
solely in terms of homogeneous processes. However, there are some systems
in which mixing is slow compared with chemical reaction rates, so that
mixing time controls the burning rate. This is true for so-called “diffusion
ames,” in which the fuel and oxidant come together in a reaction zone
through molecular and turbulent diffusion. The fuel may be in the form of a
gaseous jet or a liquid or solid surface. Thus, there are two categories within
diffusion-controlled combustion, according to the initial physical state of the
fuel and/or oxidant. If both the fuel and the oxidant are initially gaseous,
then the ame is referred to as a diffusion ame. If both the fuel and oxidant
are initially in different physical states, i.e., liquid and gas or solid and gas,
although the system is still diffusion controlled, the process is usually called
heterogeneous combustion. Examples in this category include hydrocarbon-
droplet and coal combustion.
2.8 Turbulent Premixed Flames
Although it has long been recognized that ame speeds can be apprecia-
bly increased by turbulence, as evidenced by the very high burning rates
achieved in both piston and gas turbine engines, the manner and extent of
this inuence are still not fully resolved. The rst contribution to the under-
standing of turbulent ames was made by Damkohler [11], who visualized a
turbulent ame as being essentially the same in structure as a laminar ame.
He attributed the observed increase in burning rate to the effect of turbu-
lence in wrinkling the ame front, thereby increasing its specic surface area
and hence also its ability to consume fresh mixture. Damkohler proposed
the following equation for large-scale turbulence:
SSu
TL
=+
′
,
(2.8)
where S
T
is the turbulent ame speed, S
l
is the laminar burning velocity, and
u′ is the RMS value of uctuating velocity.
In due course, several more theories embodying the wrinkled-ame con-
cept emerged, differing from Damkohler’s theory and from each other mainly
in the methods employed to relate turbulence properties to the increase in
specic surface of the ame. Schelkin’s [12] approach led to a relationship of
the form
SS B
u
S
TL
L
=+
′
1
2
05.
,
(2.9)

44 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
in which B is a constant of the order of unity. At high velocities, Equations 2.8
and 2.9 become
Su
T
=
′
.
(2.10)
Ballal and Lefebvre [13] carried out a series of experiments on enclosed,
premixed turbulent ames. A number of different turbulence-promoting
grids were located, in turn, at the upstream end of the test section, to create
in the combustion zone conditions in which the separate effects of turbu-
lence intensity and scale on burning velocity and ame structure could be
determined. The scales encountered in turbulent ow range in size from the
Kolmogoroff scale η, which represents the size of the smallest eddies in the
ow, to the integral scale L, which represents the size of the largest eddies.
For conditions of low turbulence (u′< 2S
L
), it was found that turbulence
does not roughen the ame, which retains a smooth laminar appearance.
However, burning velocity is increased owing to the effect of turbulence
in wrinkling the ame, thereby extending its surface area, as rst noted by
Damkohler [11]. The ratio of turbulent to laminar ame speeds is given by
S
S
uL
S
T
LLL
=+
′
22
1003..
δ
(2.11)
At very high levels of turbulence, the turbulent eddies are too small to
wrinkle the ame. Nevertheless, high burning rates are achieved, owing to
the very large total area of ame surface created at the interfaces between
the multitudinous small eddies and the combustion products in which they
are enveloped. In this region, the concept of a continuous, coherent ame
surface is no longer realistic, and the combustion zone may be regarded as
a fairly thick matrix of burned gases interspersed with eddies of unburned
mixture.
The ratio of turbulent to laminar ame speeds in this region of high tur-
bulence is given by
S
S
u
S
T
L
L
L
=
′
05..
δ
η
(2.12)
The structure of the ame at the two extremes of low and high turbu-
lence intensity is illustrated in Figure 2.2. The top photograph of this gure
shows that when turbulence is low, the ame surface comprises an agglom-
eration of round swellings that gradually grow in size as the ame expands
downstream. The laceration and disruption of the ame surface at condi-
tions of high turbulence intensity are illustrated in the bottom photograph
of Figure 2.2.

Combustion Fundamentals 45
2.9 Flame Propagation in Heterogeneous Mixtures
of Fuel Drops, Fuel Vapor, and Air
Comparatively few studies have been made of ame propagation through het-
erogeneous fuel–air mixtures, the earliest published work in this area being
the classic treatise of Burgoyne and Cohen [15]. Subsequent studies include
those of Cekalin [16], Mizutani and Nishimoto [17], Mizutani and Nakajima
[18], Polymeropoulos and Das [19], Ballal and Lefebvre [20], Polymeropoulos
[21], and Myers and Lefebvre [22]. The paucity of literature on this subject is
not altogether surprising in view of the formidable experimental difculties
involved. Foremost among these is the creation of a uniform and reproduc-
ible multidroplet mist. Allied to this is the problem of accurate measurement
of mean drop size, drop-size distribution, overall equivalence ratio, and con-
centration of fuel vapor. Another difcult task is the measurement of the rate
of ame propagation through the mixture, where serious errors can arise
due to the upward buoyancy of the burned gases and the downward settling
velocity of the fuel drops relative to the ame. These effects are especially
signicant for slow-burning mixtures because they are of the same order of
magnitude as the laminar ame speed.
(a)
(b)
Figure 2.2
Stoichiometric propane-air ames under conditions of low and high turbulence. (a) Upper
photograph, u′ = 3.1 m/s. (b) Lower photograph, u′ = 30.5 m/s. (Reprinted from Lefebvre, A.H.
and Reid, R., Combustion and Flame, 10(4), 355–66, 1966. With permission from Elsevier, Inc.)

46 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
Ballal and Lefebvre [20] proposed a model for ame propagation through
quiescent combustible mixtures in which the fuel is present in the form of
a multidroplet mist or vapor, or both. The basis of the model is that, under
normal steady-state conditions, the rate of ame propagation through a fuel
mist is always such that the quench time of the reaction zone is just equal to
the sum of the evaporation and chemical reaction times. This model yields
the following expression for ame speed
S
CfD
CBS
=
−
(
)
+
(
)
+
α
ρ
ρ
α
g
F
g
g
L
ln 1
3
3
32
2
2
2
2
1
8
−−05.
,
(2.13)
where α
g
is the thermal diffusivity of fully vaporized fuel–air mixture, and
f is the fraction of fuel initially present as vapor. C
2
and C
3
are drop-size dis-
tribution parameters; C
2
= D
20
/D
32
and C
3
= D
30
/D
32
. Unless the distribution
of drop sizes in the spray is known, values of C
2
and C
3
must be determined
experimentally. Suitable values of C
2
and C
3
for pressure-swirl and airblast
atomizers are 0.41 and 0.56, respectively [20].
In the above expression, the mass transfer number B provides a measure of
the volatility of the fuel. Replacing B with the evaporation constant λ allows
Equation 2.13 to be simplied to
S
CfD
CS
=
−
(
)
+
−
3
2
32
2
2
2
05
1
1
αλ
gL
.
.
(2.14)
For a monodisperse spray of fuel drops and air, this equation reduces to
S
D
S
=+
−
2
2
05
1
αλ
AL
.
.
(2.15)
In the above equations, S
L
is expressed essentially as the sum of two
terms. The rst characterizes the evaporation rate, and thus depends on
fuel volatility, mean drop size, and vapor fraction. The second characterizes
the chemical reaction rate. When the evaporation time is longer than the
chemical reaction time, ame speed is enhanced by increases in gas den-
sity, fuel volatility, vapor concentration, and reduction in mean drop size. If
conditions are such that chemical reaction rates are limiting to ame speed,
the latter reverts to the normal burning velocity for the fully evaporated
mixture. However, if the reaction time is small in comparison to the time
required for evaporation, the equations predict that ame speed is inversely
proportional to mean drop size. This theoretical nding is fully conrmed
in the experimental investigation of ame propagation in heterogeneous
Combustion Fundamentals 47
mixtures of fuel drops and air carried out by Myers and Lefebvre [22].
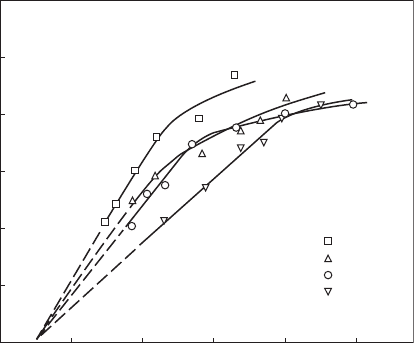
Figures 2.3 and 2.4 are typical of the results obtained. Figure 2.3 shows
ame speed plotted against the reciprocal of mean drop size for several dif-
ferent fuel/air ratios. It demonstrates, over wide ranges of mean drop size,
a straight-line relationship between S
T
and SMD
–1
, indicating that evapora-
tion rates are controlling the ame speed. In theory, the straight portion
of the lines drawn in Figure 2.3 should pass through the origin, i.e., ame
speed should become zero for innite fuel drop size. In practice, the lines
tend to intercept the abscissa at a nite value of SMD. Thus, although the
theory suggests that ame speed should reduce gradually to zero, corre-
sponding to innitely large drops, the results for JP 7 and other hydrocar-
bon fuels indicate that, in practice, there is always a maximum mean drop
size above which ame propagation is impossible. For the fuels and test
conditions examined by Myers and Lefebvre, this practical limit on mean
drop size is around 400 µm.
Generally, it is found that ame speed increases with reduction in mean
drop size until a critical value is reached. For drop sizes smaller than the
critical value, which is around 60–70 µm for kerosine-type fuels, the curves
atten out, indicating that for nely atomized sprays, ame speeds are much
less dependent on evaporation rates, and are governed primarily by chemi-
cal reaction rates.
The inuence of mean drop size on ame speeds is shown more directly
in Figure 2.4. This gure also illustrates the benecial effect on ame speed
Fuel/air ratio
0.08
0.06
0.04
0.02
Mainstream velocity = 24 m/s
Fuel = JP 7
6
5
4
3
S
T
, m/s
2
1
0
0 0.005 0.010
SMD (µm)
–1
0.015 0.020 0.025
Figure 2.3
Inuence of fuel/air ratio and mean drop size on ame speed for a mainstream velocity of
24 m/s. (Reprinted from Myers, G.D. and Lefebvre, A.H., Combustion and Flame, 66(2), 193–210,
1986. Copyright by The Combustion Institute.)

48 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
of an increase in ow velocity. This benet derives mainly from the increase
in turbulence intensity that accompanies an increase in ow velocity. It is
well established that turbulence promotes ame speeds in gaseous fuel–
air mixtures (see previous section). With heterogeneous mixtures, it has
the added advantage of enhancing fuel evaporation rates. The net effect is
that ame speeds increase with an increase in ow velocity, as illustrated in
Figure 2.4.
Equations 2.13 through 2.15 apply strictly to ame propagation through
slow-moving or quiescent fuel mists of the type studied by Ballal and
Lefebvre [20]. However, they are still valid for turbulent mixtures of fuel
drops and air, provided that S
L
is replaced by S
T
, and λ
eff
which takes
account of the role of turbulence in enhancing evaporation rates, is used
instead of λ.
30
0
1
2
3
S
T
, m/s
4
5
6
7
Mainstream
air velocity
Fuel/air ratio = 0.020
Fuel = JP 7
33 m/s
24 "
18 "
10.4 "
40 50 60 70
SMD, µm
80 90 100 110
Figure 2.4
Inuence of mainstream velocity and mean drop size on ame speed. (Reprinted from
Myers, G.D. and Lefebvre, A.H., Combustion and Flame, 66(2), 193–210, 1986. Copyright by The
Combustion Institute.)
Combustion Fundamentals 49
2.10 Droplet and Spray Evaporation
The evaporation of fuel droplets in a spray involves simultaneous heat and
mass transfer processes, in which the heat for evaporation is transferred to
the drop surface by conduction and convection from the surrounding air
or gas, and vapor is transferred by convection and diffusion back into the
gas stream. The overall rate of evaporation depends on the pressure, tem-
perature, and transport properties of the gas; the temperature, volatility, and
diameter of the drops in the spray; and the velocity of the drops relative to
that of the surrounding gas.
If a single-component fuel drop is suddenly immersed in gas at high tem-
perature, it starts to heat up exactly like any other cold body when placed in
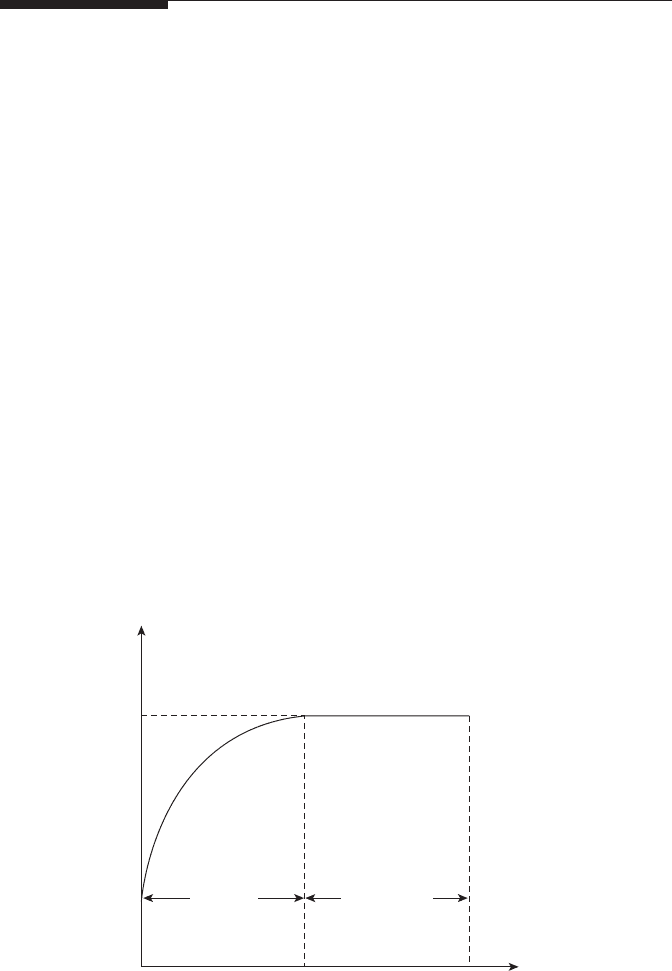
a hot environment [23]. Figure 2.5 shows how the temperature of a fuel drop
varies during its lifetime. Starting from its initial value T
F
, the fuel tempera-
ture increases until eventually it reaches its steady-state value, T
st
. This point
denotes the end of the heat-up period, and from then on the drop tempera-
ture remains constant at T
st
, until evaporation is complete. Thus, the total
drop evaporation time can be subdivided into two main components, one for
the heat-up period and another for the steady-state phase.
During the rst phase of the evaporation process, almost all of the heat
supplied to the drop serves merely to raise its temperature. Little or no mass
transfer from the drop occurs during this stage, which corresponds to the
horizontal portions of the curves drawn in Figure 2.6. As the fuel temper-
ature rises, fuel vapor is formed at the drop surface and part of the heat
T
T
st
T
F
0
0 t
hu
t
e
Time
Heatup
period
Steady-state
period
Figure 2.5
Variation of fuel temperature during drop lifetime.
