Lefebvre A.H., Ballal D.R. Gas Turbine Combustion: Alternative Fuels and Emissions
Подождите немного. Документ загружается.

50 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
transferred to the drop is now used to furnish the heat of vaporization of the
fuel. Eventually, the drop attains its steady-state temperature and the heat
supplied to the drop is used solely as heat of vaporization. This condition
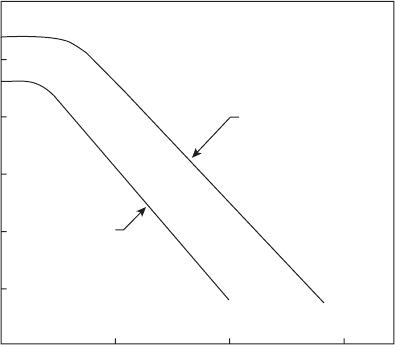
corresponds to the straight lines drawn in Figure 2.6.
2.10.1 Heat-up Period
Figure 2.6 shows the relationship between (drop diameter)
2
and evaporation
time for droplets of kerosine and JP 4 fuels. Inspection of this gure reveals
that the slope of the D
2
/t graph is almost zero in the rst stage of the evapora-
tion process and then gradually increases with time until the drop attains
its steady-state temperature, after which the value of D
2
/t remains fairly
constant throughout the remainder of the drop lifetime. The vaporization
curves drawn in Figure 2.6 are based on measurements carried out in air at
2000 K temperature and normal atmospheric pressure. At this pressure, the
heat-up period constitutes only a very small portion of the total evaporation
time, as indicated in Figure 2.6. However, for many fuels at high ambient
pressures and temperatures, the heat-up period is much longer; so much so
that drops formed from multicomponent fuels containing several different
petroleum compounds may not experience steady-state evaporation during
their lifetime. The practical signicance of these observations is that actual
drop and spray evaporation rates can be appreciably lower than the experi-
mental values quoted in the literature, most of which were measured during
steady-state evaporation at normal atmospheric pressure.
0
3.2
2.8
2.4
2.0
(Drop diameter)
2
, mm
2
1.6
1.2
0.8
12
Time, s
JP 4
Kerosine
3
Figure 2.6
Evaporation rate curves for kerosine and JP 4.

Combustion Fundamentals 51
2.10.2 evaporation Constant
One of the rst theoretical approaches to the problem of droplet evaporation
was made by Godsave [24], who derived the rate of evaporation of a single
drop as
mD
FF
=
(
)
πρλ4,
(2.16)
where
λ=
(
)
dD dt
2
.
(2.17)
Note that λ corresponds to the slope of the lines drawn in Figure 2.6.
The average rate of evaporation during the drop lifetime is obtained from
Equation 2.16 as
mD
Fo
F
=
(
)
πρλ6.
(2.18)
The drop lifetime is also readily obtained by assuming λ is constant and
integrating Equation 2.17 gives
tD
eo
=
2
λ.
(2.19)
Following Spalding, the evaporation rate of a single drop can also be
expressed in terms of a mass transfer number B [25,26]. We have
mDkc B
Fp
g
ln 1+=
(
)
(
)
2π ,
(2.20)
whereas drop lifetime is given by
tDkc B
eFop
g
n1+=
(
)
(
)
ρ
2
81.
(2.21)
A drawback to using B instead of λ for calculating m
F
and t
e
is that the accu-
racy of the results is very dependent on the choice of values of k and c
p
[27].
Values of λ may be used to determine the transfer number B (and vice
versa). Equating (2.16) and (2.20) gives
λρ=
(
)
(
)
8 kc B
p
g
F
ln 1+ .
(2.22)

52 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
2.10.3 Convective effects
In most continuous ow combustors, the fuel is sprayed into air or gas
owing at high velocity. Where relative motion exists between the droplets
and the surrounding gas, the rate of evaporation is enhanced by forced con-
vection. This effect can be accommodated by multiplying the evaporation
rate calculated for quiescent conditions by the correction factor
1022
05
+ .,
.
Re
D
where Re
D
, the drop Reynolds number, is typically around 5.
2.10.4 effective evaporation Constant
From a practical viewpoint, it would be very convenient if Godsave’s evapo-
ration constant, which corresponds to steady-state evaporation in quiescent
air, could be modied to take into account both the adverse effect of the
heat-up period and the benecial effect of forced convection. To accomplish
this, Chin and Lefebvre [28] dened an effective evaporation constant as
λ
effoe
= Dt
2
,
(2.23)
where t
e
is the total time required to evaporate the fuel drop, including both
convective and transient heat-up effects, and D
o
is the initial drop diameter.
Calculated values of λ
eff
for an ambient air pressure of 100 kPa are plotted
in Figure 2.7. Similar graphs for higher levels of pressure may be found in
Chapter 5. Figure 2.7 shows plots of λ
eff
vs. T
bn
, the normal boiling point, for
various values of UD
o
at three levels of ambient temperature, namely, 500,
1200, and 2000 K. While recognizing that no single fuel property can fully
describe the evaporation characteristics of any given fuel, the normal boiling
point has much to commend it for this purpose, because it is directly related
to fuel volatility and vapor pressure. It also has the virtue of being quoted in
fuel specications. Figure 2.7 shows that λ
eff
increases with increases in ambi-
ent temperature, velocity, and drop size, and diminishes with an increase in
normal boiling temperature.
For any given conditions of pressure, temperature, and relative velocity, the
lifetime of a fuel drop of any given size is obtained from Equation 2.23 as
tD
eoeff
=
2
λ ,
(2.24)
while the average rate of fuel evaporation is given by
mD
FFeffo
=
(
)
πρλ6.
(2.25)

Combustion Fundamentals 53
The velocity term U in Figure 2.7 denotes the relative velocity between
the drop and the surrounding gas. Small droplets rapidly attain the same
velocity as the surrounding gas, after which they are susceptible only to
the uctuating component of velocity, u′. For gas turbine combustors, where
the value of u′ is usually high enough to affect evaporation rates, U in
Figure 2.7 should be replaced with u′.
420 440 460 480
T
bn
, K
T
∞
= 500 K
T
∞
= 1200 K
T
∞
= 2000 K
UD
0
,
(m/s)(µm)
10000
5000
2000
1000
500
200
100
0
10000
5000
2000
1000
500
200
100
0
10000
5000
2000
1000
500
200
100
0
P = 100 kPa
1.6
1.4
1.2
1.0
0.8
0.6
1.0
0.8
0.6
λ
eff
, mm
2
/s
0.4
0.2
0.24
0.20
0.16
0.12
0.08
0.04
500 520 540 560
Figure 2.7
Variation of effective evaporation constant with normal boiling point at normal atmospheric
pressure. (From Chin, J.S. and Lefebvre, A.H., Proceedings of the 20th Automotive Technology
Development Contractor Coordination Meeting, 325–31 1982.)

54 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
The concept of an effective value of evaporation constant concept has
many useful practical applications. For example, Equation 2.29 may be used
to calculate fuel spray evaporation rates in a combustion zone, whereas
Equation 2.24 greatly facilitates calculations on the length of duct required
for complete evaporation of the fuel spray when injected into a ducted air-
stream. The drop diameter selected for insertion into Equation 2.24 should,
of course, be that of the largest drop in the spray.
2.10.5 Spray evaporation
For a volume of air, V, containing n fuel drops of Sauter mean diameter D
o
,
the average rate of fuel evaporation can be expressed as
mnD
FFeffo
=
(
)
πρλ6.
(2.26)
The fuel/air ratio in the volume is obtained as
qnDV=
(
)
πρ ρ6
3
Fo A
,
(2.27)
which may be rewritten as
nVDq=
(
)
(
)
(
)
6
3
πρρ
AF o
.
(2.28)
Substituting for n from Equation 2.28 into Equation 2.26 yields
mVqD
FAeffo
=ρ λ
2
.
(2.29)
This equation gives the average rate of evaporation of a fuel spray.
More detailed information on single drop and spray evaporation, includ-
ing the effects of evaporation on drop-size distributions in sprays, may be
found in Lefebvre [27].
2.10.6 Some recent Developments
Progress in the modeling of droplet vaporization up to 1994 has been
reviewed by Peng and Aggarwal [29]. Their review includes the methodolo-
gies currently available for representing droplet motion and vaporization his-
tory in two-phase ow computations. More recent work includes a numerical
study of two-component droplet evaporation by Stengele et al. [30]. This work
is of special interest for gas turbine applications because it features gas tem-
peratures of 800 and 2000 K and a range of pressures from 1 to 40 bars.
As a further advance on the λ
eff
concept [28], Chin has developed more
sophisticated models for the evaporation of multicomponent fuel drops
Combustion Fundamentals 55
that employ variable nite mass and thermal diffusivity. This work has
culminated in a practical engineering calculation method for commercial
gas turbine fuels. The procedures employed by Chin are too detailed for
inclusion here, thus for further information, reference should be made to the
original publications [31–33].
2.11 Ignition Theory
Most ignition theories are based on the idea that the transient ignition source,
usually an electric spark, must supply sufcient energy to the combustible
mixture to create a volume of hot gas that just satises the necessary and suf-
cient condition for propagation, namely, that the rate of heat generation just
exceeds the rate of heat loss.
The work of Lewis et al. [34–36] did much to clarify and improve knowledge
of spark ignition in quiescent mixtures. The rst major contribution to ignition
theory for owing mixtures was made by Swett [37], who studied the inu-
ence on ignition energy of variations in pressure, velocity, equivalence ratio,
and turbulence. Swett’s theory is based on the ideas that (1) only a portion
of the discharge length is important in the ignition process and (2) heat loss
by thermal conduction is negligible compared with heat loss by eddy diffu-
sion. Both of these ideas were fully conrmed in subsequent experiments
carried out by Ballal and Lefebvre on ignition in owing mixtures [38,39].
Unfortunately, Swett’s treatment of turbulence is very limited and much of
his experimental data are suspect for reasons discussed in Reference [39].
2.11.1 gaseous Mixtures
Ballal and Lefebvre [38] analyzed the processes governing the rate of heat
generation in an incipient spark kernel and the rate of heat loss by thermal
conduction and turbulent diffusion. They conclude that, for the spark kernel
to survive and propagate unaided throughout a gaseous mixture, its mini-
mum dimension should always exceed the quenching distance as expressed by
d
k
cS u
q
po L
=
−
′
(
)
10
016ρ .
,
(2.30)
for low-turbulence (u′ < 2S
L
) and
d
k
cS u
q
po T
=
−
′
(
)
10
063ρ .
,
(2.31)
for highly turbulent mixtures
()
L
′
uS 2
.

56 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
The minimum ignition energy E
min
is dened as the amount of energy
needed to heat to its adiabatic ame temperature, the smallest volume of gas
whose minimum dimension is equal to the quenching distance. Clearly, the
smallest volume that satises this criterion is a sphere of diameter d
q
, so
EcTd
min
().=
po ad q
/ρπ∆ 6
3
(2.32)
Substituting d
q
from Equations 2.30 and 2.31 into Equation 2.32 leads, respec-
tively to
ET
kS u
c
min
.
[( .)]
()
,=
−
′
−
524
016
13
2
∆
L
po
ρ
(2.33)
and
ET
kS u
c
min
.
.
.=
−
′
(
)
(
)
−
524
063
1
3
2
∆
T
po
ρ
(2.34)
The result of plotting measured values of E
min
that were obtained over
wide ranges of pressure, velocity, turbulence intensity, and mixture compo-
sition for both methane and propane fuels against values of d
q
as calculated
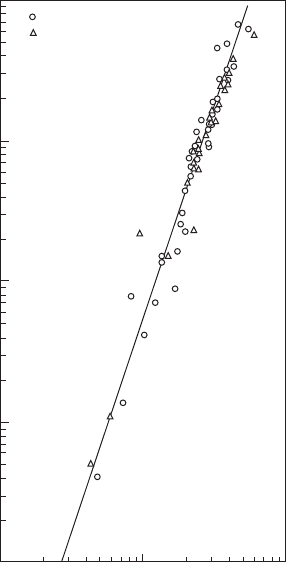
from Equations 2.30 and 2.31 is shown in Figure 2.8. The straight line drawn
through the data points has a slope of 3.0, thus conrming the cubic relation-
ship between E
min
and d
q
, as expressed in Equation 2.32.
The theory predicts that d
q
(and hence E
min
) increases with an increase in
turbulence intensity; this is borne out by tests performed on propane–air
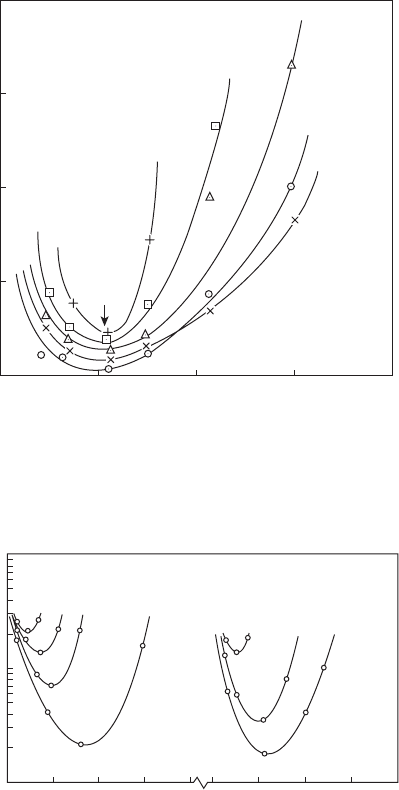
mixtures, the results of which are shown in Figure 2.9. The effect of pres-
sure on d
q
is illustrated in Figure 2.10. Inspection of the data points indicates
that quenching distance is roughly inversely proportional to pressure, as
predicted by Equation 2.30. This corresponds to a pressure dependence for
minimum energy of E
min
αP
–2
.
Figure 2.10 also illustrates the benecial effect (from an ignition stand-
point) of replacing some or all of the nitrogen in the air with oxygen.
2.11.2 Heterogeneous Mixtures
All the evidence obtained in the studies of Subba Rao, Rao, and Lefebvre
[40,41] on the ignition of owing mixtures of fuel drops and air (see
Chapter 5) serves to suggest that passage of the spark creates a kernel in
which high gas temperatures are attained, partly from the energy supplied
in the spark, but also from the heat liberated by the evaporation and rapid
combustion of the smallest fuel drops. This initial high temperature then
Combustion Fundamentals 57
falls as heat is lost by diffusion to the fresh mixture in contact with the
outside surface of the kernel, and to the remaining fuel drops undergoing
evaporation within the kernel. The key factor governing ignition is whether
these droplets can evaporate and generate heat quickly enough to counter
the heat loss from the kernel to its surroundings before it has shrunk below
its minimum critical size, which corresponds to the quenching distance for
the mixture.
When ignition is successful, combustion of the fuel vapor continues to pro-
duce heat that diffuses outward from the kernel to raise the temperature
and initiate combustion in the surrounding unburned mixture. The ame
then spreads rapidly to all regions where the air and fuel are in combustible
proportions.
Analysis of all the experimental data obtained in these investigations
[40,41] led to the conclusion that the sole criterion for the successful ignition
of mixtures of fuel drops and air is a sufciency of fuel vapor in the ignition
0.1
0.01
0.1
1
10
100
110
Quenching distance, mm
Minimum ignition energy, mJ
Slope = 3
Methane
Propane
φ = 1
P = 8.1, 18, 35.4 kPa
Figure 2.8
Relationship between E
min
and d
q
for both quiescent and owing mixtures. ϕ = 1.0. (From Ballal,
D.R. and Lefebvre, A.H., Proceedings of the Royal Society, London Series A, 357 (1689), 163–81, 1977.)
58 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
zone. Ignition will automatically ensue if passage of the spark creates suf-
cient thermal energy to produce the requisite amount of fuel vapor. The
basic argument is that over wide ranges of operating conditions, the chemi-
cal reaction time is so short in comparison with the time required to produce
an adequate amount of fuel vapor in the ignition zone that for all practical
0.5
80
Minimum ignition energy, mJ
60
40
20
0
1.0
22%
15%
8%
5%
1%
2.5%
Tu rbulence
intensity
Pressure = 0.17 atmos
Velocity = 6 m/s
Fuel = propane
1.5
Equivalence ratio
2.0 2.5
Figure 2.9
Inuence of turbulence intensity on minimum ignition energy for propane–air mixtures.
(From Ballal, D.R. and Lefebvre, A.H., Fifteenth Symposium (International) on Combustion, The
Combustion Institute, Pittsburgh, PA, 1473–81, 1975.)
0
0.1
1.0
10.0
Pressure = 8 kPa Pressure = 35 kPa
% O
2
21 35
50
100
21
35 50
% O
2
Quenching distance, mm
10 20 30
Propane, percent volume
40 010203040
Figure 2.10
Inuence of pressure and oxygen concentration on quenching distance for propane.
Velocity = 15.3 m/s. (From Ballal, D.R. and Lefebvre, A.H., Proceedings of the Royal Society, London
Series A, 357 (1689), 163–81, 1977.)

Combustion Fundamentals 59
purposes it can be neglected [42]. This is in marked contrast to the ignition
process in homogeneous mixtures, which is totally dominated by chemical
reaction rates.
These considerations led to the development by Ballal and Lefebvre [42,43]
of a theoretical model for the prediction of quenching distance and minimum
ignition energy in liquid fuel sprays. The model is based on the assumption
that chemical reaction rates are innitely fast and that the onset of ignition is
limited solely by the rate of fuel evaporation.
The process of ignition is envisaged as occurring in the following man-
ner. Passage of the spark creates a small, roughly spherical, volume of air
(henceforth referred to as the spark kernel) whose temperature is sufciently
high to initiate rapid evaporation of the fuel drops contained within the vol-
ume. Reaction rates and mixing times are assumed innitely fast, so any
fuel vapor created within the spark kernel is instantly transformed into com-
bustion products at the stoichiometric ame temperature. If the rate of heat
release by combustion exceeds the rate of heat loss by thermal conduction
at the surface of the inamed volume, then the spark kernel grows in size
to ll the entire combustion volume. If, however, the rate of heat release is
lower than the rate of heat loss, the temperature within the spark kernel falls
steadily until fuel evaporation ceases altogether.
Thus, of crucial importance is the spark-kernel size for which the rate of
heat loss at the kernel’s surface is just balanced by the rate of heat release, due
to the instantaneous combustion of fuel vapor, throughout its volume. As
with homogeneous mixtures, this concept leads to the denition of quenching
distance as the critical size that the inamed volume must attain to propagate
unaided; the amount of energy required from an external source to attain
this critical size is termed the minimum ignition energy.
Analysis of the relevant heat-transfer and evaporation processes [42,43]
yields the following expression for the quenching distance of quiescent or
slow-moving multidroplet mists:
d
D
B
q
F
Ast
ln 1+
=
(
)
ρ
2
05
ρφ
.
.
(2.35)
It should be noted that this equation was derived through consideration of
the basic mechanisms of heat generation within the kernel and heat loss from
its surface, and it contains no experimental or arbitrary constants. It is valid
for monodisperse sprays only. However, for polydisperse sprays of the type
provided by most practical atomizing devices, it can be shown [44] that the
quenching distance is given by
d
CD
CB
q
F
Ast
ln 1+
=
(
)
3
3
32
2
2
05
ρ
ρφ
.
.
(2.36)
