Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

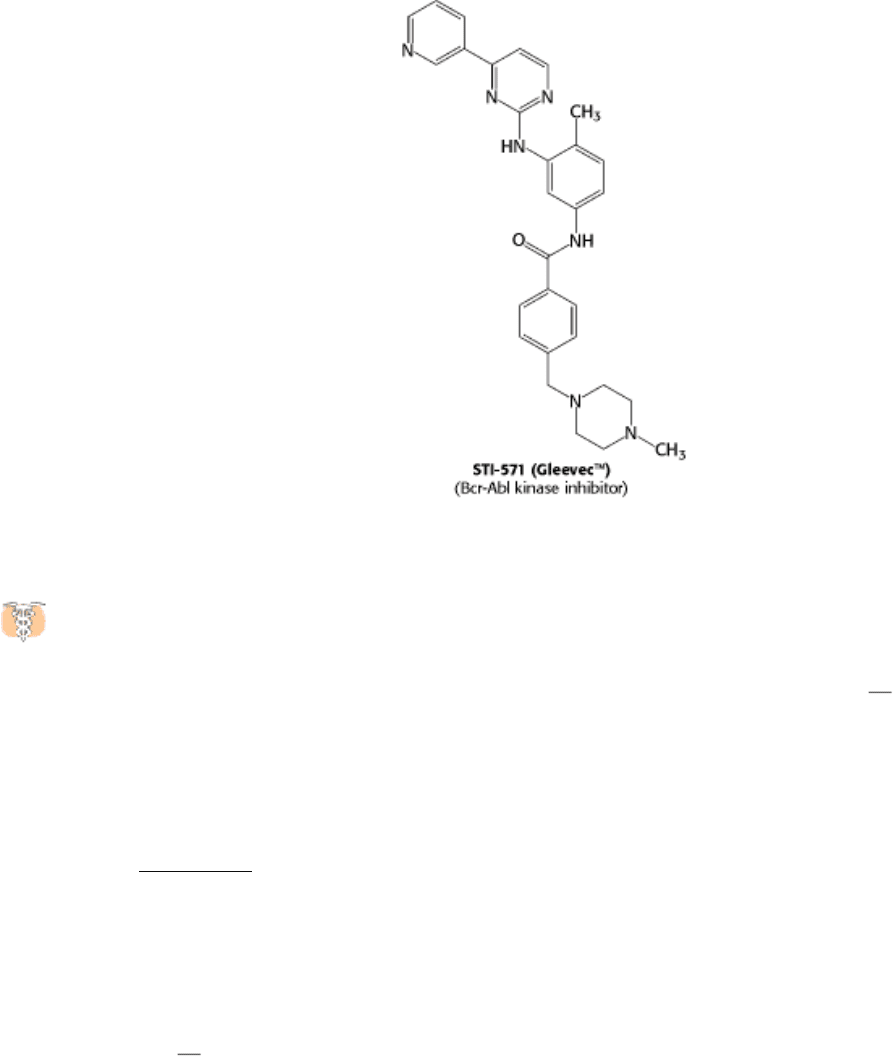
Bcr-Abl that consists primarily of sequences for the c-Abl kinase. However, the bcr-abl gene is not regulated
appropriately; it is expressed at higher levels than the gene encoding the normal c-Abl kinase. In addition, the Bcr-Abl
protein may have regulatory properties that are subtly different from those of the c-Abl kinase itself. Thus, leukemia
cells express a unique target for chemotherapy. Recent clinical trials of a specific inhibitor of the Bcr-Abl kinase have
shown dramatic results; more than 90% of patients responded well to the treatment. This approach to cancer
chemotherapy is fundamentally distinct from most approaches, which target cancer cells solely on the basis of their rapid
growth, leading to side effects because normal rapidly growing cells also are affected. Thus, our understanding of signal-
transduction pathways is leading to conceptually new disease treatments.
15.5.2. Cholera and Whooping Cough Are Due to Altered G-Protein Activity
We consider here some pathologies of the G-protein-dependent signal pathways. Let us first consider the
mechanism of action of the cholera toxin, secreted by the intestinal bacterium Vibrio cholera. Cholera is an acute
diarrheal disease that can be life threatening. It causes voluminous secretion of electrolytes and fluids from the intestines
of infected persons. The cholera toxin, choleragen, is a protein composed of two functional units a B subunit that
binds to G
M
1
gangliosides of the intestinal epithelium and a catalytic A subunit that enters the cell. The A subunit
catalyzes the covalent modification of a G
α
s
protein: the α subunit is modified by the attachment of an ADP-ribose to an
arginine residue. This modification stabilizes the GTP-bound form of G
α
s
, trapping the molecule in the active
conformation. The active G protein, in turn, continuously activates protein kinase A. PKA opens a chloride channel (a
CFTR channel; Section 13.3) and inhibits the Na
+
-H
+
exchanger by phosphorylation. The net result of the
phosphorylation of these channels is an excessive loss of NaCl and the loss of large amounts of water into the intestine.
Patients suffering from cholera for 4 to 6 days may pass as much as twice their body weight in fluid. Treatment consists
of rehydration with a glucose-electrolyte solution.
Whereas cholera is a result of a G protein trapped in the active conformation, causing the signal-transduction pathway to
be perpetually stimulated, pertussis, or whooping cough, is a result of the opposite situation. Pertussis toxin also adds an
ADP-ribose moiety,
in this case, to a G
α
i
protein, a G
α
protein that inhibits adenyl cyclase, closes Ca
2+
channels, and
opens K
+
channels. The effect of this modification, however, is to lower the G protein's affinity for GTP, effectively
trapping it in the "off" conformation. The pulmonary symptoms have not yet been traced to a particular target of the G
α
i
protein. Pertussis toxin is secreted by Bordetella pertussis, the bacterium responsible for whooping cough.

Cholera and pertussis are but two examples of diseases caused by defects in G proteins. Table 15.4 lists others. In light
of the fact that G proteins relay signals for more than 500 receptors, it is likely that this list will continue to grow.
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism 15.5. Defects in Signaling Pathways Can Lead to Cancer and Other Diseases
Figure 15.35. Src Structure.
(A) Cellular Src includes an SH3 domain, an SH2 domain, a protein kinase domain, and a
carboxyl-terminal tail that includes a key tyrosine residue. (B) Structure of c-Src in an inactivated form with the
key tyrosine residue phosphorylated. The phosphotyrosine residue is bound in the SH2 domain; the linker between
the SH2 domain and the protein kinase domain is bound by the SH3 domain. These interactions hold the kinase domain
in an inactive conformation.
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism 15.5. Defects in Signaling Pathways Can Lead to Cancer and Other Diseases
Figure 15.36. Activation Pathways for c-Src. Inactive c-Src can be activated by one of at least three distinct pathways:
(1) displacement of the SH2 domain, (2) dephosphorylation, or (3) displacement of the SH3 domain.

II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism 15.5. Defects in Signaling Pathways Can Lead to Cancer and Other Diseases
Figure 15.37. Formation of the Bcr-Abl Gene by Translocation. In chronic myologenous leukemia, parts of
chromosomes 9 and 22 are reciprocally exchanged, causing the bcr and abl genes to fuse. The protein kinase encoded by
the bcr-abl gene is expressed at higher levels in cells having this translocation than is the c-abl gene in normal cells.
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism 15.5. Defects in Signaling Pathways Can Lead to Cancer and Other Diseases
Table 15.4. Diseases of heterotrimeric G proteins
Disease
Excessive signaling
Cholera
Cancer (adenoma) of pituitary and thyroid
Cancer (adenoma) of adrenal and ovary
Essential hypertension
Deficient signaling
Night blindness
Pseudohypoparathyroidism type Ib
Pertussis
Source: After Z. Farfel, H. R. Bourne, and T. Iiri. N. Engl. J. Med. 340(1999):1012.
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism
15.6. Recurring Features of Signal-Transduction Pathways Reveal Evolutionary
Relationships
Many features of signal-transduction pathways are ancient. For example, cAMP signals the need for energy in
prokaryotes as well as eukaryotes, although the mechanisms for detecting cAMP are different. Similarly, the GTP-
binding proteins the G
α
subunits of the hetero-trimeric G proteins and the members of the Ras family are part of an
ancient superfamily of evolutionarily related proteins. Other members of this superfamily are proteins that cycle between
ATP- and ADP-bound forms; these proteins function in ATP synthesis (Section 18.4.5) and in generating molecular
motion (Chapter 34). The superfamily also includes proteins taking part in protein synthesis (Section 29.4.2). The key
feature of these proteins is that they undergo significant conformational changes on binding nucleoside triphosphates and
hydrolyzing them to nucleoside diphosphates. These proteins can thus function as molecular "on-off" switches. A
domain with this ability must have arisen early in evolution and been adapted to meet a range of biochemical needs since.
Other proteins crucial to signal-transduction pathways arose much later. For example, the eukaryotic protein kinases are
one of the largest protein families in all eukaryotes and yet appear to be absent in prokaryotes. The evolution of the
eukaryotic protein kinase domain appears to have been an important biochemical step in the appearance of eukaryotes
and the subsequent development of multicellular organisms.
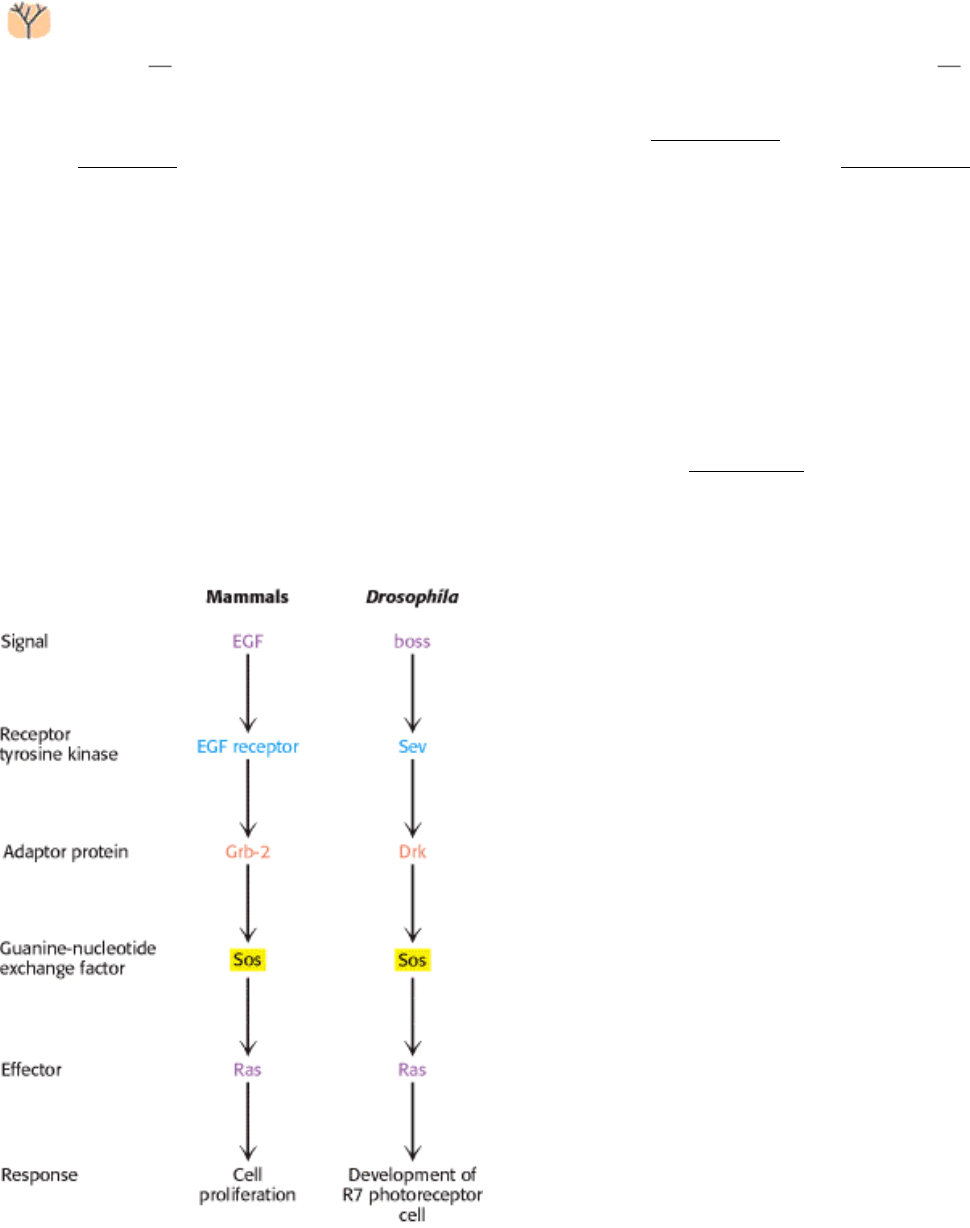
Entire signaling pathways have been conserved between organisms. For example, a key pathway in eye development in
Drosophila is completely analogous to the EGF pathways in human beings (Figure 15.38). Thus, the wiring of this
growth-control pathway is at least 800 million years old.
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism 15.6. Recurring Features of Signal-Transduction Pathways Reveal Evolutionary Relationships
Figure 15.38. Pathway Conservation. A pathway homologous to the mammalian EGF signal-transduction pathway
functions in Drosophila to control the development of a specific photoreceptor cell in the eye.
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism
Summary
The highly specific binding of signal molecules, many of which are hormones and growth factors, to receptor molecules
initiates the signal-transduction cascade. Secondary messengers carry the signal inside the cell and often use protein
phosphorylation as a signaling device.
Seven-Transmembrane-Helix Receptors Change Conformation in Response to Ligand
Binding and Activate G Proteins
Seven-transmembrane-helix receptors operate in conjunction with heterotrimeric G proteins. The binding of hormone to
a 7TM receptor triggers the exchange of GTP for GDP bound to the α subunit of the G protein. G
α
proteins can transmit
information in a number of ways. G
α
s
-GTP activates adenylate cyclase, an integral membrane protein that catalyzes the
synthesis of cAMP. Cyclic AMP then activates protein kinase A by binding to its regulatory subunit, thus unleashing its
catalytic chains. PKA, a multifunctional kinase, alters the activity of many target proteins by phosphorylating serine and
threonine residues.
The Hydrolysis of Phosphatidyl Inositol Bisphosphate by Phospholipase C Generates
Two Messengers
The phosphoinositide cascade is mediated by 7TM receptors and G
α
q
proteins. The receptor-triggered activation of
phospholipase C generates two intracellular messengers by hydrolysis of phosphatidyl inositol 4,5-bisphosphate. Inositol
trisphosphate opens calcium channels in the endoplasmic and sarcoplasmic reticulum membranes, leading to an elevated
level of Ca
2+
in the cytosol. Diacylglycerol activates protein kinase C, which phosphorylates serine and threonine
residues in target proteins.
Calcium Ion Is a Ubiquitous Cytosolic Messenger
Calcium ion acts by binding to calmodulin and other calcium sensors. Calmodulin contains four calcium-binding
modules called EF hands that recur in other proteins. Ca
2+
-calmodulin activates target proteins by binding to positively
charged amphipathic helices.
Some Receptors Dimerize in Response to Ligand Binding and Signal by Cross-
Phosphorylation
Some ligands induce dimerization of the receptors to which they bind. Such a receptor contains an extracellular domain
that binds the ligand, a transmembrane region, and a cytosolic domain that either binds or contains a protein kinase. The
growth-hormone receptor participates in an example of this type of signal-transduction pathway. Dimerization of the
receptor activates Janus kinase 2, a protein kinase associated with the intracellular part of the receptor. The kinase, in
turn, phosphorylates and activates a transcription factor called STAT5.
Intrinsic tyrosine kinases are covalently incorporated in the intracellular domains of some receptors, such as epidermal
growth factor receptor and the insulin receptor. When such receptor tyrosine kinases dimerize, cross-phosphorylation
occurs. The phosphorylated tyrosines in activated receptor tyrosine kinases serve as docking sites for SH2 domains
present in numerous signaling proteins and permit further propagation of the signal. A prominent component of such
pathways is the small GTPase Ras. The Ras protein, like the G
α
subunit, cycles between an inactive form bound to GDP
and an active form bound to GTP.
Defects in Signaling Pathways Can Lead to Cancer and Other Diseases
If the genes encoding components of the signal-transduction pathways are altered by mutation, pathological conditions,
most notably cancer, may result. In their mutated form, these genes are called oncogenes. The normal counterparts are
called proto-oncogenes and function in pathways that control cell growth and replication. Mutated versions of ras are
frequently found in human cancers.
Recurring Features of Signal-Transduction Pathways Reveal Evolutionary
Relationships
Many aspects of signal transduction are ancient and appear throughout the kingdoms of life. G proteins of various classes
are employed as molecular switches in a host of biochemical processes.
Key Terms
ligand
primary messenger
second messenger
protein kinase
protein phosphatase
seven-transmembrane-helix (7TM) receptor
G protein
adenylate cyclase
G
α
G
β
γ
G-protein-coupled receptor (GPCR)
desensitization (adaptation)
protein kinase A (PKA)
phosphoinositide cascade
phospholipase C
protein kinase C (PKC)
pseudosubstrate sequence
calmodulin (CaM)
EF hand
calmodulin-dependent protein (CaM) kinase
SH2 domain
receptor tyrosine kinase (RTK)
SH3 domain
small G protein
oncogene
proto-oncogene
II. Transducing and Storing Energy 15. Signal-Transduction Pathways: An Introduction to Information Metabolism
Problems
1.
Levels of amplification. At which stages in the signaling pathway from epinephrine to cAMP does a significant
amount of amplification occur? Answer the same question for the signaling pathways from human growth hormone
to STAT5 and from EGF to Ras.
See answer
2.
Active mutants. Some protein kinases are inactive unless they are phosphorylated on key serine or threonine
residues. In some cases, active enzymes can be generated by mutating these serine or threonine residues to
glutamate. Propose an explanation.
See answer
3.
In the pocket. SH2 domains bind phosphotyrosine residues in deep pockets on their surfaces. Would you expect SH2
domains to bind phosphoserine or phosphothreonine with high affinity? Why or why not?
See answer
4.
Inhibitory constraints. Many proteins in signal-transduction pathways are activated by the removal of an inhibitory
constraint. Give two examples of this recurring mechanism.
See answer
5.
Pseudosubstrate mutant. A mutated form of a protein kinase C isozyme that has three changes in amino acids in the
pseudosubstrate sequence is isolated. What properties would you expect this mutated form to have?
See answer

6.
Antibodies mimicking hormones. Antibodies have two identical antigen-binding sites. Remarkably, antibodies to the
extracellular parts of growth-factor receptors often lead to the same cellular effects as does exposure to growth
factors. Explain this observation.
See answer
7.
Facile exchange. A mutated form of the α subunit of the heterotrimeric G protein has been identified; this form
readily exchanges nucleotides even in the absence of an activated receptor. What effect would you expect this
mutated α subunit to have on its signaling pathway?
See answer
8.
Blocking one side. Human growth hormone binds simultaneously to two growth-hormone receptors through two
different surfaces. Suppose sequence changes in a mutated form of growth hormone disrupt one interaction surface
but do not affect the other. What properties would such a mutated hormone have? Why might it be useful?
See answer
9.
Diffusion rates. Normally, rates of diffusion vary inversely with molecular weights; so smaller molecules diffuse
faster than do larger ones. In cells, however, calcium ion diffuses more slowly than does cAMP. Propose a possible
explanation.
See answer
10.
Awash with glucose. Glucose is mobilized for ATP generation in muscle in response to epinephrine, which
activates G
α
s
. Cyclic AMP phosphodiesterase is an enzyme that converts cAMP into AMP. How would inhibitors
of cAMP phosphodiesterase affect glucose mobilization in muscle?
See answer
Chapter Integration Problem
11.
Nerve growth factor pathway. Nerve growth factor (NGF) binds to a protein tyrosine kinase receptor. The amount
of diacylglycerol in the plasma membrane increases in cells expressing this receptor when treated with NGF.
Propose a simple signaling pathway and identify the isoform of any participating enzymes. Would you expect the
concentrations of any other common second messengers to increase on NGF treatment?
See answer
Mechanism Problem
12.
Distant relatives. The structure of adenylate cyclase is similar to the structures of some types of DNA polymerases,
suggesting that these enzymes derived from a common ancestor. Compare the reactions catalyzed by these two
enzymes. In what ways are they similar?
See answer
Data Interpretation Problems
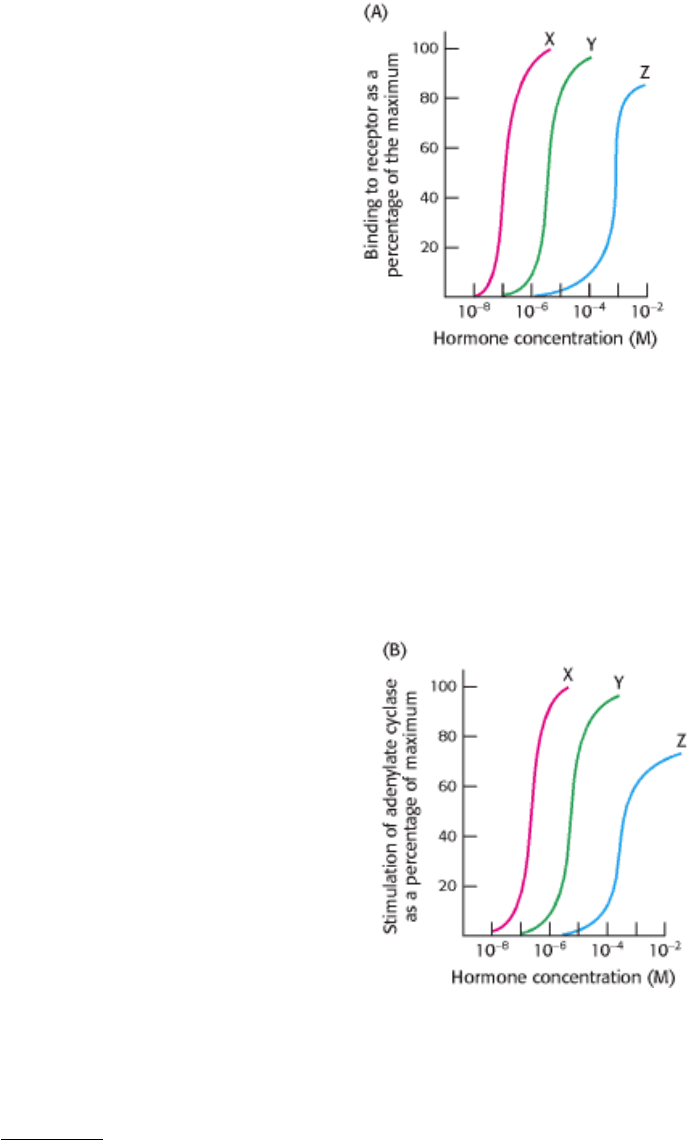
13.
Establishing specificity. You wish to determine the hormone-binding specificity of a newly identified membrane
receptor. Three different hormones, X, Y, and Z, were mixed with the receptor in separate experiments, and the
percentage of binding capacity of the receptor was determined as a function of hormone concentration, as shown in
graph A.
(a) What concentrations of each hormone yield 50% maximal binding?
(b) Which hormone shows the highest binding affinity for the receptor?
You next wish to determine whether the hormone-receptor complex stimulates the adenylate cyclase cascade. To do
so, you measure adenylate cyclase activity as a function of hormone concentration, as shown in graph B.
(c) What is the relation between the binding affinity of the hormone-receptor complex and the ability of the
hormone to enhance adenylate cyclase activity? What can you conclude about the mechanism of action of the
hormone-receptor complex?
(d) Suggest experiments that would determine whether a G
α
s
protein is a component of the signal-transduction
pathway.
See answer

14.
Binding issues. A scientist wishes to determine the number of receptors specific for a ligand X, which he has in
both radioactive and nonradioactive form. In one experiment, he adds increasing amounts of the radioactive X and
measures how much of it is bound to the cells. The result is shown as total activity in the adjoining graph. Next, he
performs the same experiment, except that he includes a several hundredfold excess of nonradioactive X. This
result is shown as nonspecific binding. The difference between the two curves is the specific binding.
(a) Why is the total binding not an accurate representation of the number of receptors on the cell surface?
(b) What is the purpose of performing the experiment in the presence of excess nonradioactive ligand?
(c) What is the significance of the fact that specific binding attains a plateau?
See answer
15.
Counting receptors. With the use of experiments such as those described in problems 13 and 14, it is possible to
calculate the number of receptors in the cell membrane. Suppose that the specific activity of the ligand is 10
12
cpm
per millimole and that the maximal specific binding is 10
4
cpm per milligram of membrane protein. There are 10
10
cells per milligram of membrane protein. Assume that one ligand binds per receptor. Calculate the number of
receptor molecules present per cell.
See answer
Media Problem
16.
Grubby binding. The Structural Insights module on SH2 domains describes some of the determinants of
SH2 specificity and the ways in which SH2-phosphotyrosine binding can affect protein function. Given that
the Src kinase SH2 domain binds Src phosphotyrosine 527, what effect do you think mutation of Glu 529 to Asn
would have on the protein kinase activity of Src? Suppose you now obtained a second mutation within Src that
reversed the effect of the first. Can you predict what that second mutation might be?
