Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

The efficiency of the citric acid cycle may be enhanced by the arrangement of the constituent enzymes. Evidence is
accumulating that the enzymes are physically associated with one another to facilitate substrate channeling between
active sites. The word metabolon has been suggested as the name for such multienzyme complexes.
As will be discussed in Chapter 18, the electron-transport chain oxidizes the NADH and FADH
2
formed in the citric acid
cycle. The transfer of electrons from these carriers to O
2
, the ultimate electron acceptor, leads to the generation of a
proton gradient across the inner mitochondrial membrane. This proton-motive force then powers the generation of ATP;
the net stoichiometry is about 2.5 ATP per NADH, and 1.5 ATP per FADH
2
. Consequently, 9 high-transfer-potential
phosphoryl groups are generated when the electron-transport chain oxidizes 3 molecules of NADH and 1 molecule of
FADH
2
, and 1 high-transfer-potential phosphoryl group per acetyl unit is directly formed in the citric acid cycle. Thus, 1
acetate unit generates approximately 10 molecules of ATP. In dramatic contrast, only 2 molecules of ATP are generated
per molecule of glucose (which generates 2 molecules of acetyl CoA) by anaerobic glycolysis.
Recall that molecular oxygen does not participate directly in the citric acid cycle. However, the cycle operates only
under aerobic conditions because NAD
+
and FAD can be regenerated in the mitochondrion only by the transfer of
electrons to molecular oxygen. Glycolysis has both an aerobic and an anaerobic mode, whereas the citric acid cycle is
strictly aerobic. Glycolysis can proceed under anaerobic conditions because NAD
+
is regenerated in the conversion of
pyruvate into lactate.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
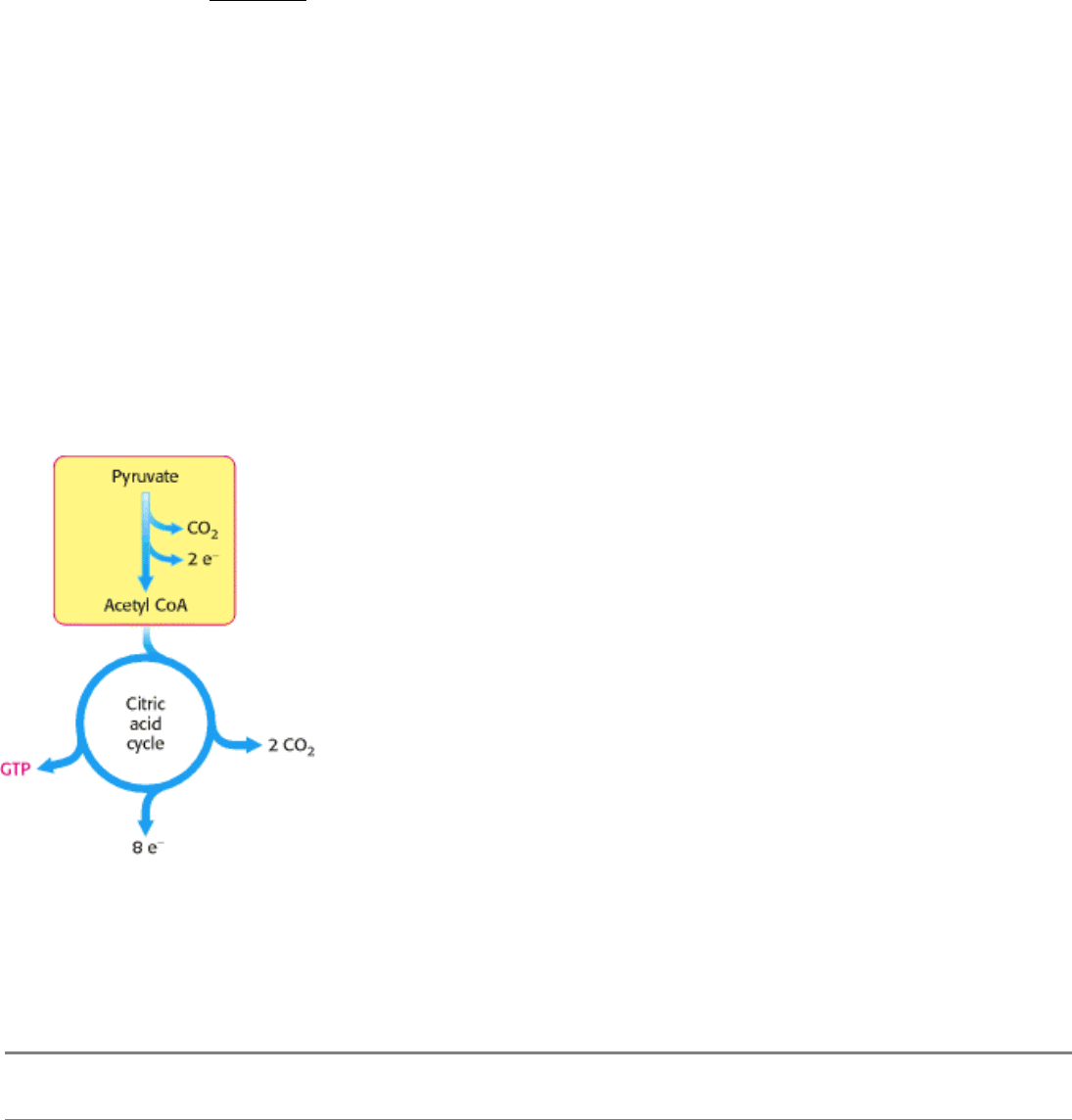
Figure 17.4. The Link between Glycolysis and the Citric Acid Cycle. Pyruvate produced by glycolysis is converted
into acetyl CoA, the fuel of the citric acid cycle.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Table 17.1. Pyruvate dehydrogenase complex of E. coli
Enzyme Abbreviation Number of chains Prosthetic group Reaction catalyzed
Pyruvate dehydrogenase
component
E
1
24 TPP Oxidative decarboxylation of
pyruvate
Dihydrolipoyl transacetylase E
2
24 Lipoamide Transfer of the acetyl group to CoA
Dihydrolipoyl dehydrogenase E
3
12 FAD Regeneration of the oxidized form
of lipoamide
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
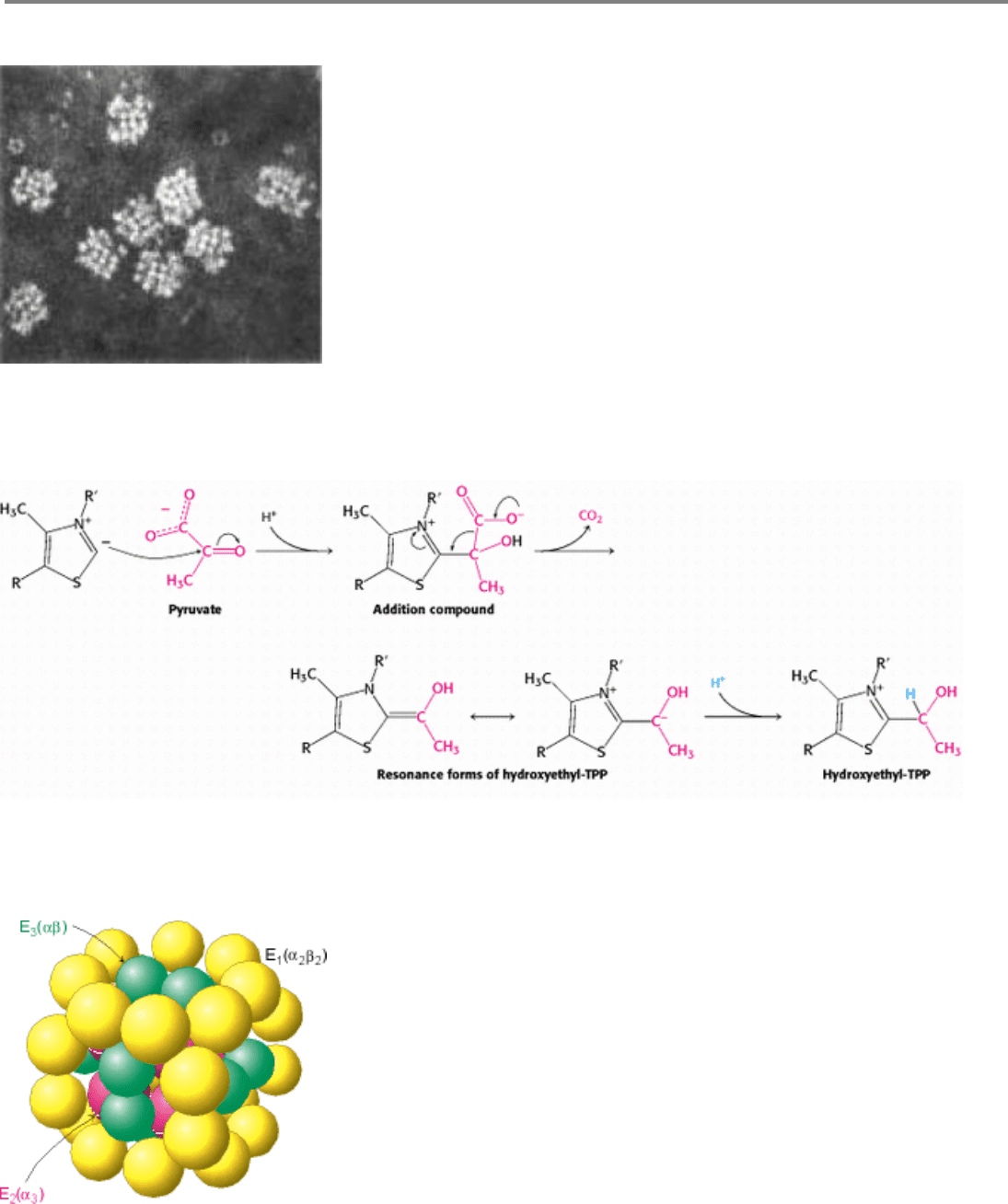
Figure 17.5. Electron Micrograph of the Pyruvate Dehydrogenase Complex From E. coli. [Courtesy of Dr. Lester
Reed.]
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Figure 17.6. Mechanism of the Decarboxylation Reaction of E
1
, THe Pyruvate Dehydrogenase Component of the
Pyruvate Dehydrogenese Complex.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Figure 17.7. Schematic Representation of the Pyruvate Dehydrogenase Complex. The transacetylase core (E
2
) is
shown in red, the pyruvate dehydrogenase component (E
1
) in yellow, and the dihydrolipoyl dehydrogenase (E
3
) in green.
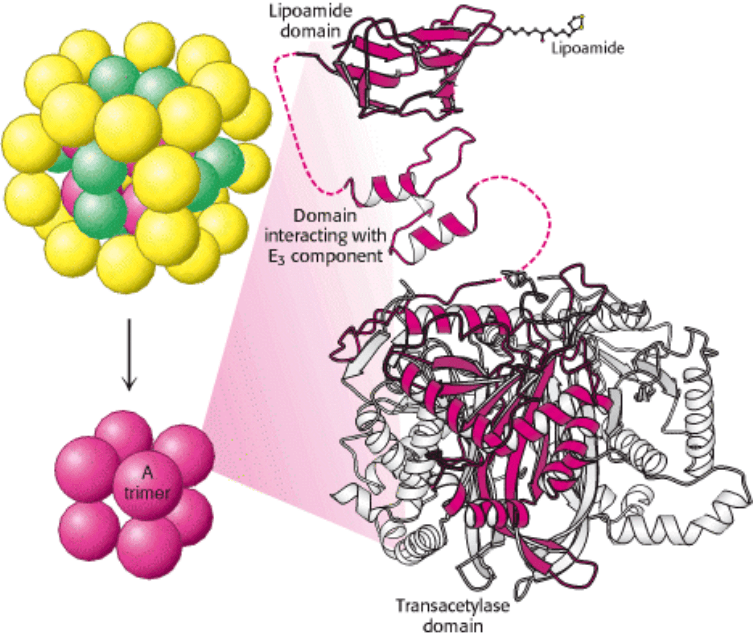
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Figure 17.8. Structure of the Transacetylase (E
2
) core. Each red ball represents a trimer of three E
2
subunits. Each
subunit consists of three domains: a lipoamide-binding domain, a small domain for interaction with E
3
, and a large
transacetylase catalytic domain. All three subunits of the transacetylase domain are shown in the ribbon representation,
with one depicted in red.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
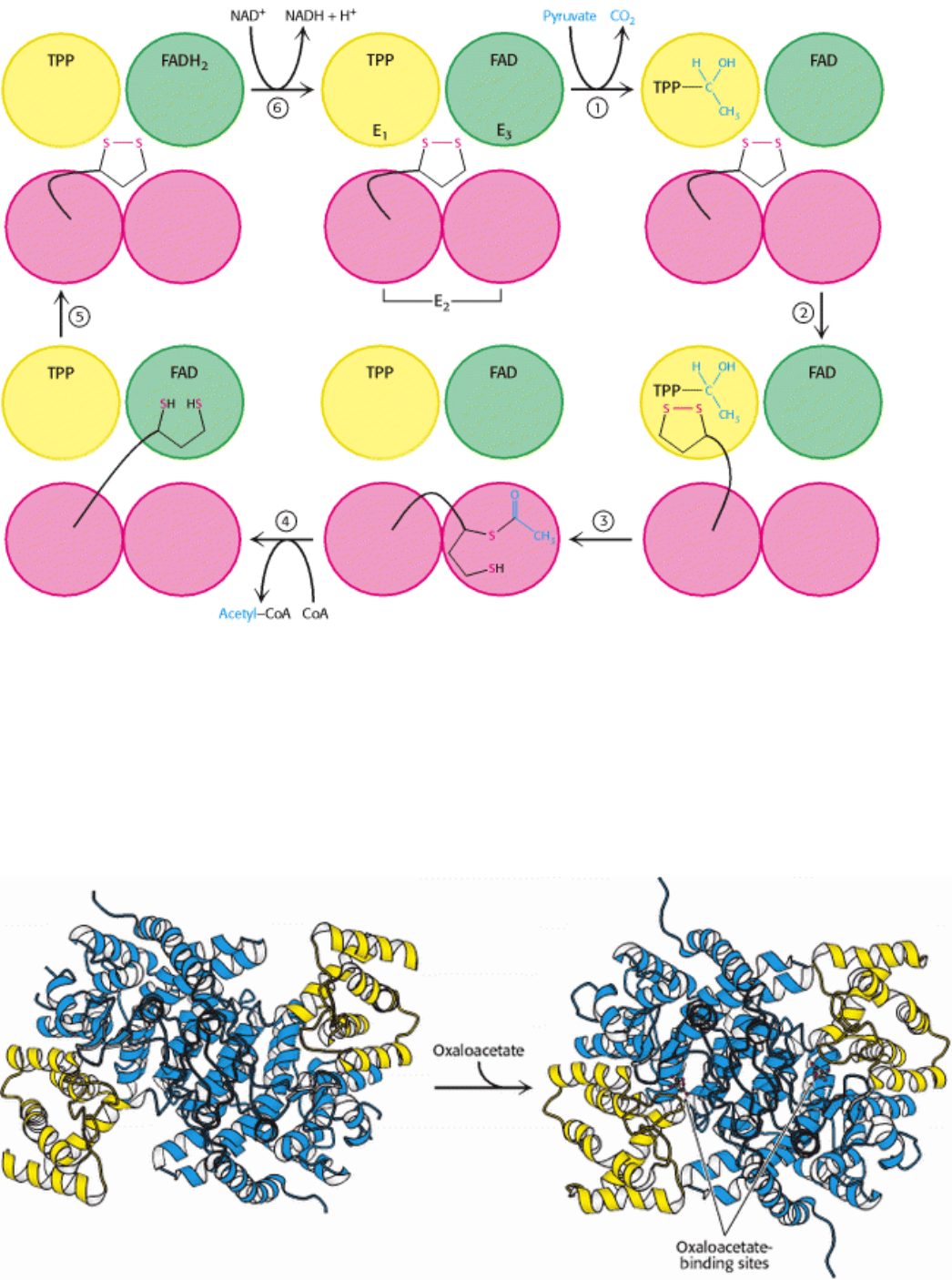
Figure 17.9. Reactions of the Pyruvate Dehydrogenase Complex. At the top (center), the enzyme (represented by a
yellow, a blue, and two red spheres) is unmodified and ready for a catalytic cycle. (1) Pyruvate is decarboxylated to form
the hydroxyethyl TPP. (2) The dihydrolipoyl arm of E
2
moves into the active site of E
1
. (3) E
1
catalyzes the transfer of
the two-carbon group to the dihydrolipoyl group to form the acetyl-lipoyl complex. (4) E
2
catalyzes the transfer of the
acetyl moiety to CoA to form the product acetyl CoA. The disulfhydryl lipoyl arm then swings to the active site of E
3
. E
3
catalyzes (5) the reduction of the lipoic acid and (6) the transfer of the protons and electrons to NAD
+
to complete the
reaction cycle.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Figure 17.10. Conformational Changes in Citrate Synthase on Binding Oxaloacetate. The small domain of each
subunit of the homodimer is shown in yellow; the large domain is shown in blue. (Left) Open form of enzyme
alone. (Right) Closed form of the liganded enzyme.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
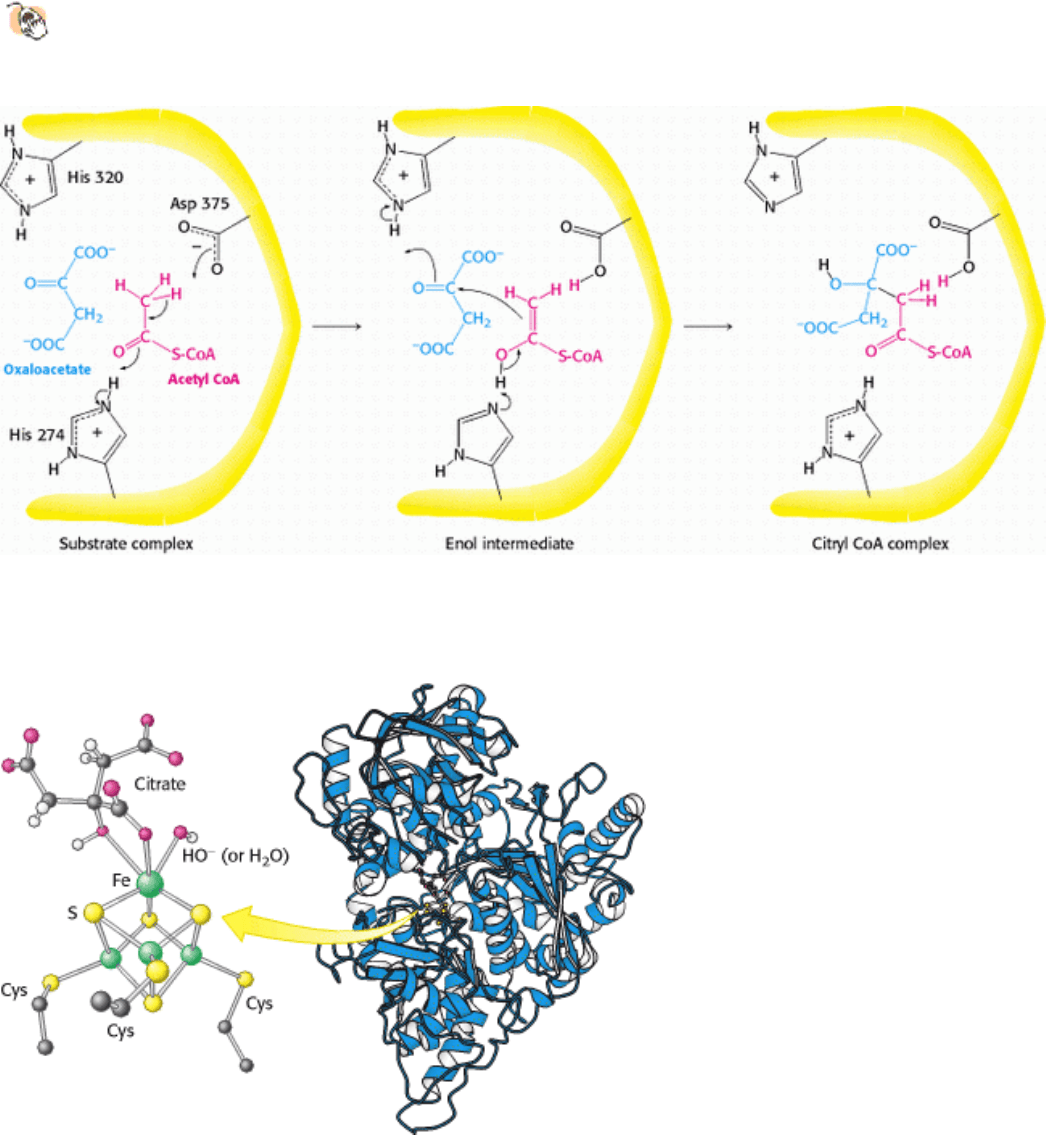
Figure 17.11. Mechanism of Synthesis of Citryl CoA by Citrate Synthase. The condensation of oxaloacetate and
acetyl CoA proceeds through an enol intermediate. The subsequent hydrolysis of citryl CoA yields citrate and CoA.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Figure 17.12. Binding of Citrate to the Iron-Sulfur Complex of Aconitase. A 4Fe-4S iron-sulfur cluster is a
component of the active site of aconitase. One of the iron atoms of the cluster binds to the carboxylate and hydroxyl
groups of citrate.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
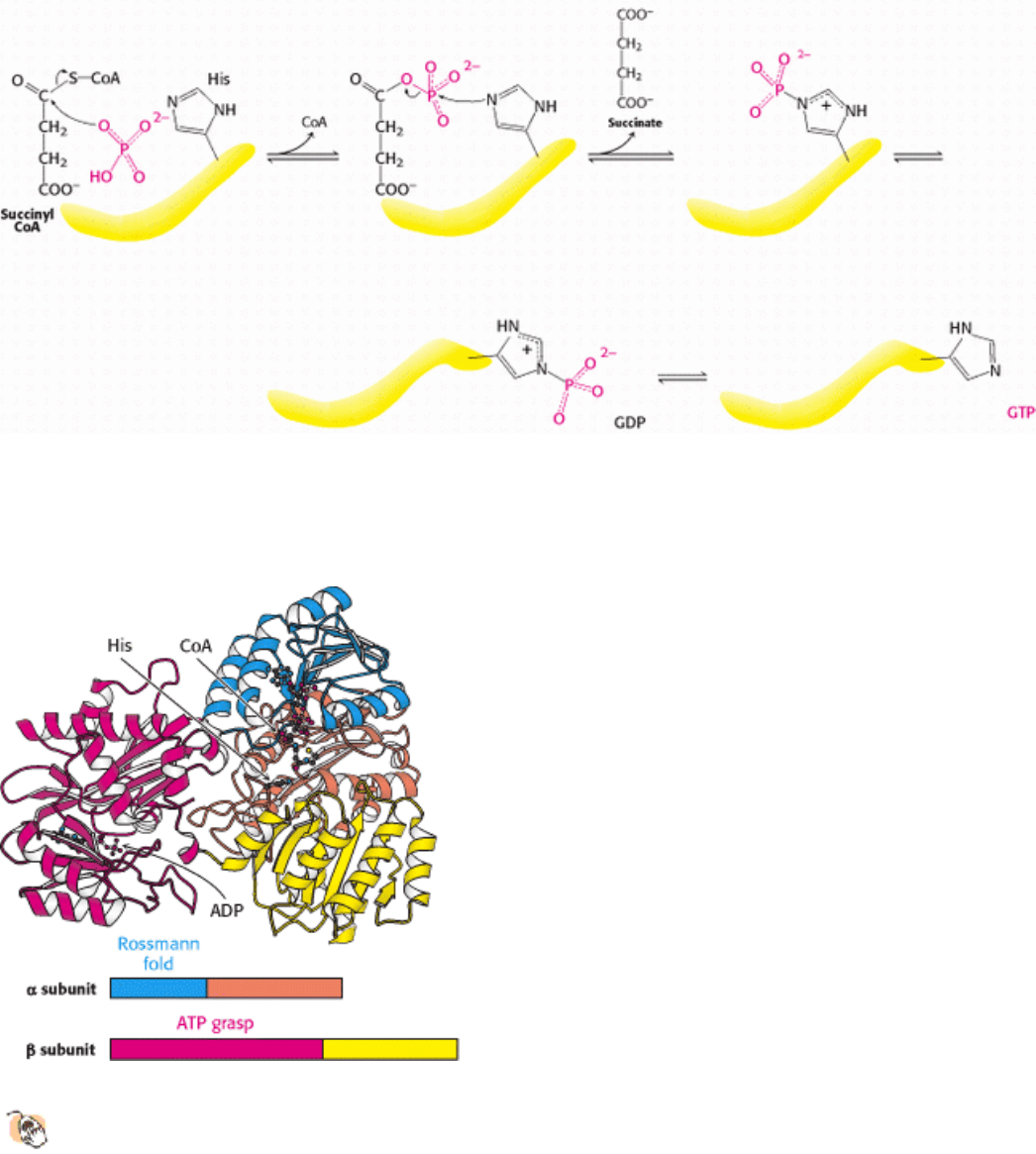
Figure 17.13. Reaction Mechanism of Succinyl CoA Synthetase. The formation of GTP at the expense of succinyl
CoA is an example of substrate-level phosphorylation. The reaction proceeds through a phosphorylated enzyme
intermediate.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Figure 17.14. Structure of Succinyl CoA Synthetase.
The enzyme is composed of two subunits. The α subunit
contains a Rossmann fold that binds the ADP component of CoA, and the β subunit contains a nucleotide-
activating region called the ATP-grasp domain. The ATP-grasp domain is shown here binding a molecule of ADP.
The histidine residue picks up the phosphoryl group from near the CoA and swings over to transfer it to the nucleotide
bound in the ATP-grasp domain.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
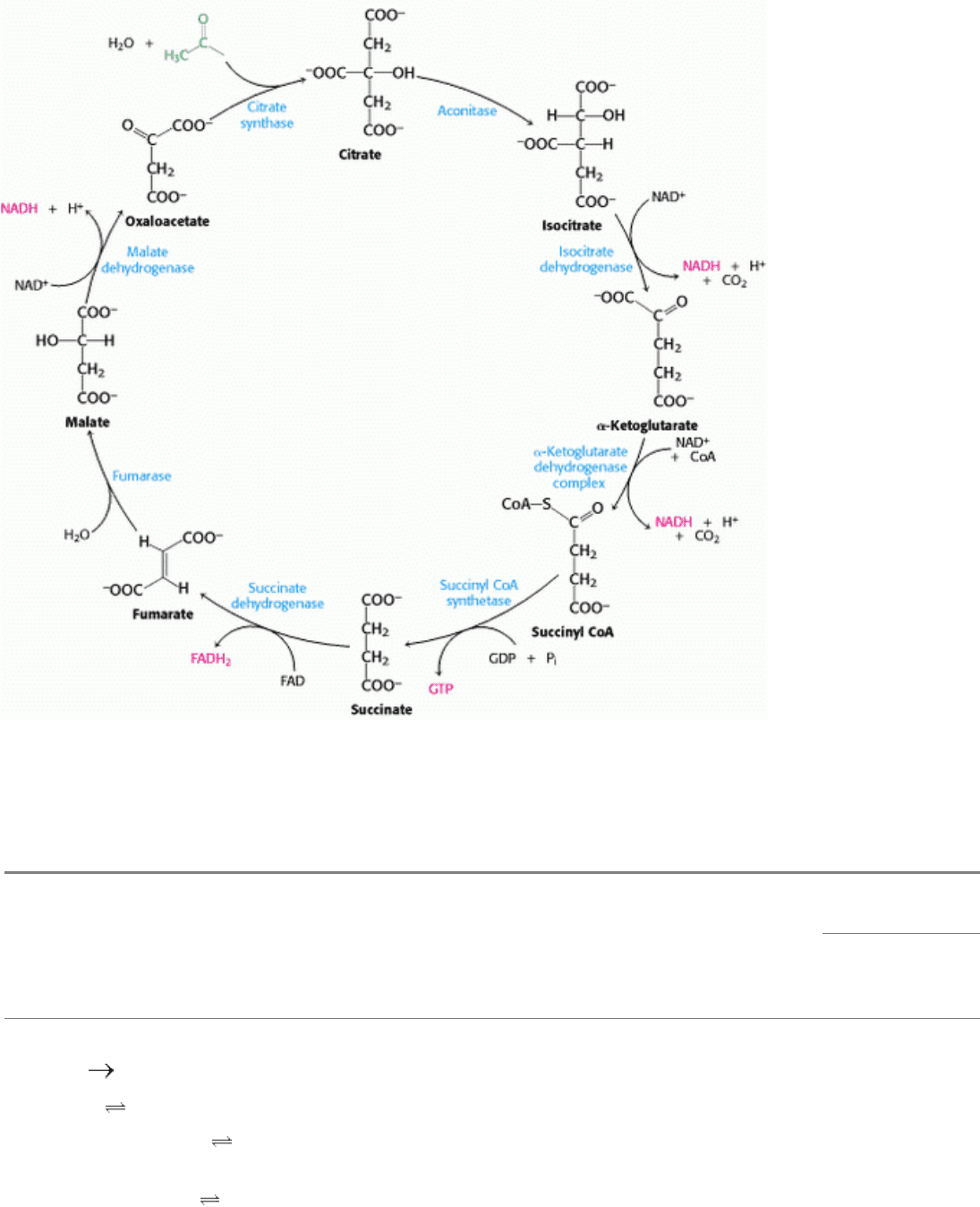
Figure 17.15. The Citric Acid Cycle.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.1. The Citric Acid Cycle Oxidizes Two-Carbon Units
Table 17.2. Citric acid cycle
DG°
Step Reaction Enzyme Prosthetic group Type*
kcal mol
-
1
kJ mol
-
1
1 Acetyl CoA + oxaloacetate +
H
2
O citrate + CoA + H
+
Citrate synthase a -7.5 -31.4
2a Citrate
cis-aconitate + H
2
O Aconitase Fe-S b +2.0 +8.4
2b cis-Aconitate+ H
2
O
isocitrate
Aconitase Fe-S c -0.5 -2.1
3
Isocitrate + NAD
+
α-
ketoglutarate + CO
2
+ NADH
Isocitrate dehydrogenase d + e -2.0 -8.4

4
α-Ketoglutarate + NAD
+
+
CoA succinyl CoA + CO
2
+ NADH
α-Ketoglutarate dehydrogenase
complex
Lipoic acid, FAD,
TPP
d + e -7.2 -30.1
5 Succinyl CoA + P
i
+ GDP
succinate + GTP + CoA
Succinyl CoA synthetase f -0.8 -3.3
6 Succinate + FAD (enzyme-
bound)
fumarate +
FADH
2
(enzyme-bound)
Succinate dehydrogenase FAD, Fe-S e ˜0 0
7 Fumarate + H
2
O l-malate Furmarase c -0.9 -3.8
8
l-Malate + NAD
+
oxaloacetate + NADH + H
+
Malate dehydrogenase e +7.1 +29.7
*
Reaction type: (a) condensation; (b) dehydration; (c) hydration; (d) decarboxylation; (e) oxidation; (f) substrate-level
phosphorylation.
II. Transducing and Storing Energy 17. The Citric Acid Cycle
17.2. Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled
The citric acid cycle is the final common pathway for the aerobic oxidation of fuel molecules. Moreover, as we will see
shortly (Section 17.3) and repeatedly elsewhere in our study of biochemistry, the cycle is an important source of building
blocks for a host of important biomolecules. As befits its role as the metabolic hub of the cell, entry into the cycle and
the rate of the cycle itself are controlled at several stages.
17.2.1. The Pyruvate Dehydrogenase Complex Is Regulated Allosterically and by
Reversible Phosphorylation
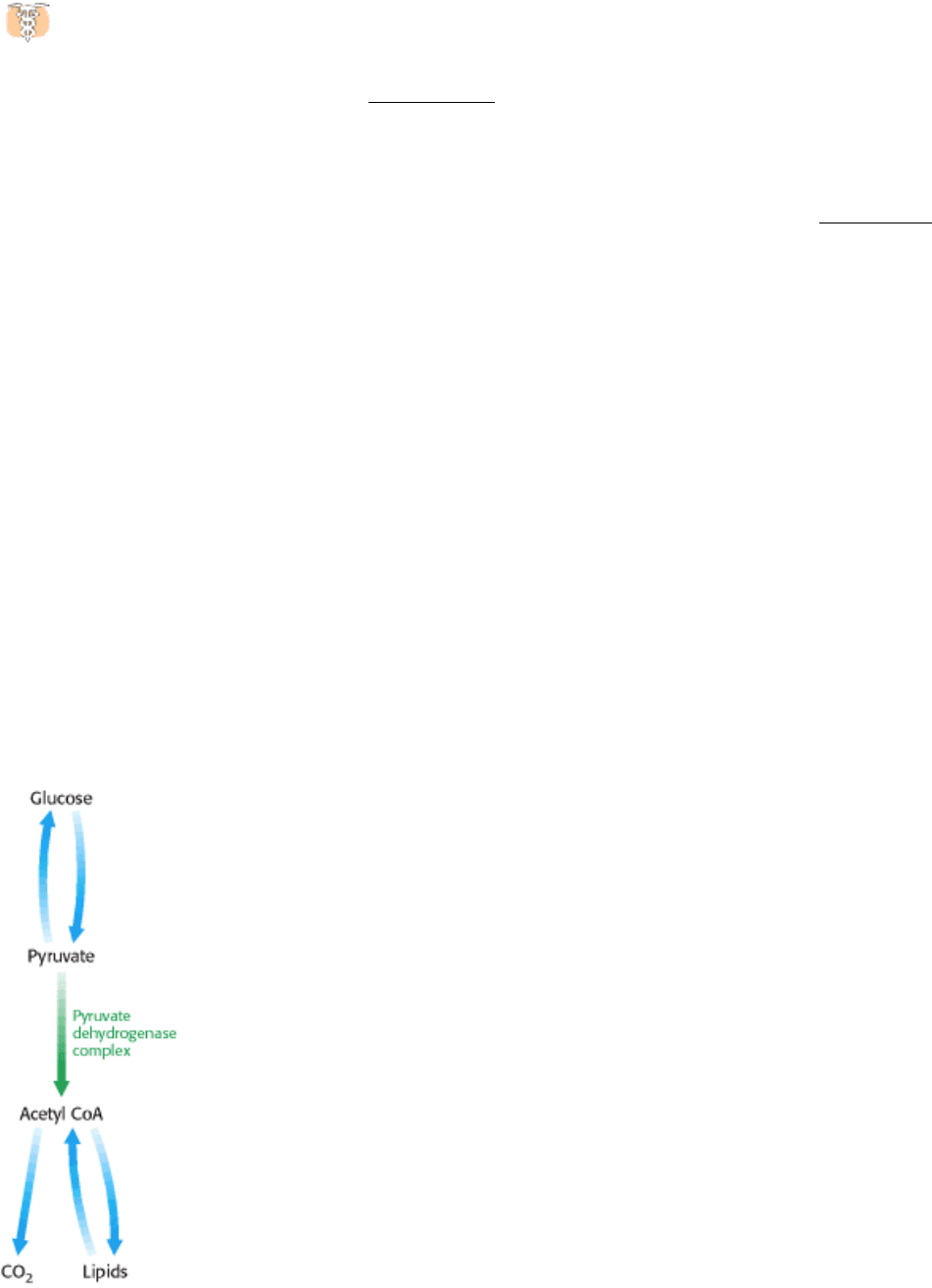
As we saw earlier, glucose can be formed from pyruvate (Section 16.3). However, the formation of acetyl CoA from
pyruvate is an irreversible step in animals and thus they are unable to convert acetyl CoA back into glucose. The
oxidative decarboxylation of pyruvate to acetyl CoA commits the carbon atoms of glucose to two principal fates:
oxidation to CO
2
by the citric acid cycle, with the concomitant generation of energy, or incorporation into lipid (Figure
17.16). As expected of an enzyme at a critical branch point in metabolism, the activity of the pyruvate dehydrogenase
complex is stringently controlled by several means (Figure 17.17). High concentrations of reaction products of the
complex inhibit the reaction: acetyl CoA inhibits the transacetylase component (E
2
), whereas NADH inhibits the
dihydrolipoyl dehydrogenase (E
3
). However, the key means of regulation in eukaryotes is covalent modification of the
pyruvate dehydrogenase component. Phos-phorylation of the pyruvate dehydrogenase component (E
1
) by a specific
kinase switches off the activity of the complex. Deactivation is reversed by the action of a specific phosphatase. The site
of phosphorylation is the transacetylase component (E
2
), again highlighting the structural and mechanistic importance of
this core. Increasing the NADH/NAD
+
, acetyl CoA/CoA, or ATP/ADP ratio promotes phosphorylation and, hence,
deactivation of the complex. In other words, high concentrations of immediate (acetyl CoA and NADH) and ultimate
(ATP) products inhibit the activity. Thus, pyruvate dehydrogenase is switched off when the energy charge is high and
biosynthetic intermediates are abundant. On the other hand, pyruvate as well as ADP (a signal of low energy charge)
activate the dehydrogenase by inhibiting the kinase.
In contrast, α
1
-adrenergic agonists and hormones such as vasopressin stimulate pyruvate dehydrogenase by triggering a
rise in the cytosolic Ca
2+
level (Section 15.3.2), which in turn elevates the mitochondrial Ca
2+
level. The rise in
mitochondrial Ca
2+
activates the pyruvate dehydrogenase complex by stimulating the phosphatase. Insulin also
accelerates the conversion of pyruvate into acetyl CoA by stimulating the dephosphorylation of the complex. In turn,
glucose is funneled into pyruvate.
The importance of this covalent control is illustrated in people with a phosphatase deficiency. Because pyruvate
dehydrogenase is always phosphorylated and thus inactive, glucose is processed to lactic acid. This condition
results in unremitting lactic acidosis (high blood levels of lactic acid), which leads to the malfunctioning of many tissues,
most notably the central nervous system (Section 17.3.2).
17.2.2. The Citric Acid Cycle Is Controlled at Several Points
The rate of the citric acid cycle is precisely adjusted to meet an animal cell's needs for ATP (Figure 17.18). The primary
control points are the allosteric enzymes isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.
Isocitrate dehydrogenase is allosterically stimulated by ADP, which enhances the enzyme's affinity for substrates. The
binding of isocitrate, NAD
+
, Mg
2+
, and ADP is mutually cooperative. In contrast, NADH inhibits iso-citrate
dehydrogenase by directly displacing NAD
+
. ATP, too, is inhibitory. It is important to note that several steps in the cycle
require NAD
+
or FAD, which are abundant only when the energy charge is low.
A second control site in the citric acid cycle is α-ketoglutarate dehydrogenase. Some aspects of this enzyme's control are
like those of the pyruvate dehydrogenase complex, as might be expected from the homology of the two enzymes. α-
Ketoglutarate dehydrogenase is inhibited by succinyl CoA and NADH, the products of the reaction that it catalyzes. In
addition, α-ketoglutarate dehydrogenase is inhibited by a high energy charge. Thus, the rate of the cycle is reduced when
the cell has a high level of ATP.
In many bacteria, the funneling of two-carbon fragments into the cycle also is controlled. The synthesis of citrate from
oxaloacetate and acetyl CoA carbon units is an important control point in these organisms. ATP is an allosteric inhibitor
of citrate synthase. The effect of ATP is to increase the value of K
M
for acetyl CoA. Thus, as the level of ATP increases,
less of this enzyme is saturated with acetyl CoA and so less citrate is formed.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.2. Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled
Figure 17.16. From Glucose to Acetyl CoA. The synthesis of acetyl CoA by the pyruvate dehydrogenase complex is a
key irreversible step in the metabolism of glucose.
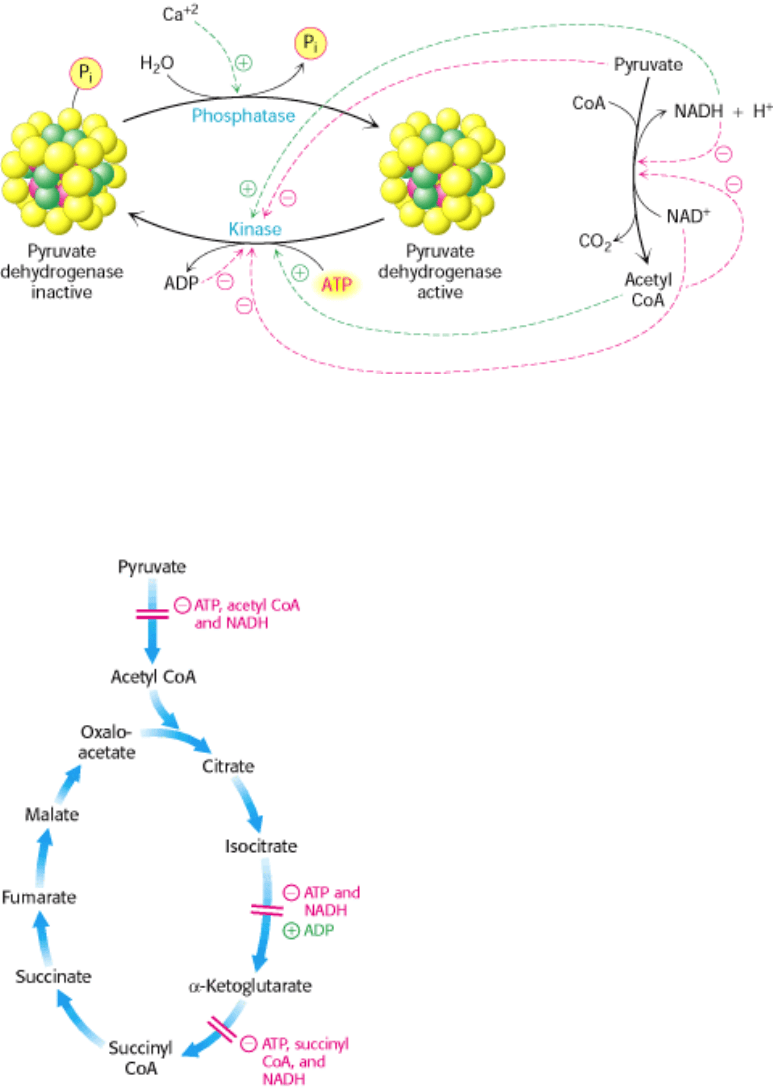
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.2. Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled
Figure 17.17. Regulation of the Pyruvate Dehydrogenase Complex. The complex is inhibited by its immediate
products, NADH and acetyl CoA. The pyruvate dehydrogenase component is also regulated by covalent modification. A
specific kinase phosphorylates and inactivates pyruvate dehydrogenase, and a phosphatase actives the dehydrogenase by
removing the phosphoryl. The kinase and the phosphatase also are highly regulated enzymes.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.2. Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled
Figure 17.18. Control of the Citric Acid Cycle. The citric acid cycle is regulated primarily by the concentration of
ATP and NADH. The key control points are the enzymes isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.
