Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


II. Transducing and Storing Energy 17. The Citric Acid Cycle
17.3. The Citric Acid Cycle Is a Source of Biosynthetic Precursors
Thus far, discussion has focused on the citric acid cycle as the major degradative pathway for the generation of ATP. As
a major metabolic hub of the cell, the citric acid cycle also provides intermediates for biosyntheses (Figure 17.19). For
example, most of the carbon atoms in porphyrins come from succinyl CoA. Many of the amino acids are derived from α -
ketoglutarate and oxaloacetate. These biosynthetic processes will be discussed in subsequent chapters.
17.3.1. The Citric Acid Cycle Must Be Capable of Being Rapidly Replenished
The important point now is that citric acid cycle intermediates must be replenished if any are drawn off for biosyntheses.
Suppose that much oxaloacetate is converted into amino acids for protein synthesis and, subsequently, the energy needs
of the cell rise. The citric acid cycle will operate to a reduced extent unless new oxaloacetate is formed, because acetyl
CoA cannot enter the cycle unless it condenses with oxaloacetate. Even though oxaloacetate is recycled, a minimal level
must be maintained to allow the cycle to function.
How is oxaloacetate replenished? Mammals lack the enzymes for the net conversion of acetyl CoA into oxaloacetate or
any other citric acid cycle intermediate. Rather, oxaloacetate is formed by the carboxylation of pyruvate, in a reaction
catalyzed by the biotin-dependent enzyme pyruvate carboxylase.
Recall that this enzyme plays a crucial role in gluconeogenesis (Section 16.3.2). It is active only in the presence of acetyl
CoA, which signifies the need for more oxaloacetate. If the energy charge is high, oxaloacetate is converted into glucose.
If the energy charge is low, oxaloacetate replenishes the citric acid cycle. The synthesis of oxaloacetate by the
carboxylation of pyruvate is an example of an anaplerotic reaction (of Greek origin, meaning to "fill up"), a reaction that
leads to the net synthesis, or replenishment, of pathway components. Note that, because the citric acid cycle is a cycle, it
can be replenished by the generation of any of the intermediates.
17.3.2. The Disruption of Pyruvate Metabolism Is the Cause of Beriberi and Poisoning
by Mercury and Arsenic
Beriberi, a neurologic and cardiovascular disorder, is caused by a dietary deficiency of thiamine (also called
vitamin B
1
). The disease has been and continues to be a serious health problem in the Far East because rice, the
major food, has a rather low content of thiamine. This deficiency is partly ameliorated if the whole rice grain is soaked in
water before milling
some of the thiamine in the husk then leaches into the rice kernel. The problem is exacerbated if
the rice is polished, because only the outer layer contains significant amounts of thiamine. Beriberi is also occasionally
seen in alcoholics who are severely malnourished and thus thiamine deficient. The disease is characterized by neurologic
and cardiac symptoms. Damage to the peripheral nervous system is expressed as pain in the limbs, weakness of the
musculature, and distorted skin sensation. The heart may be enlarged and the cardiac output inadequate.

Beriberi-
A vitamin-deficiency disease first described in 1630 by Jacob
Bonitus, a Dutch physician working in Java:
"A certain very troublesome affliction, which attacks men, is called
by the inhabitants Beriberi (which means sheep). I believe those,
whom this same disease attacks, with their knees shaking and the
legs raised up, walk like sheep. It is a kind of paralysis, or rather
Tremor: for it penetrates the motion and sensation of the hands and
feet indeed sometimes of the whole body."
Which biochemical processes might be affected by a deficiency of thiamine? Thiamine pyrophosphate is the prosthetic
group of three important enzymes: pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and transketolase.
Transketolase functions in the pentose phosphate pathway, which will be discussed in Chapter 20. The common feature
of enzymatic reactions utilizing TPP is the transfer of an activated aldehyde unit. In beriberi, the levels of pyruvate and
α-ketoglutarate in the blood are higher than normal. The increase in the level of pyruvate in the blood is especially
pronounced after the ingestion of glucose. A related finding is that the activities of the pyruvate and α-ketoglutarate
dehydrogenase complexes in vivo are abnormally low. The low transketolase activity of red cells in beriberi is an easily
measured and reliable diagnostic indicator of the disease.
Why does TPP deficiency lead primarily to neurological disorders? The nervous system relies essentially on glucose as
its only fuel. In contrast, most other tissues can use fats as a source of fuel for the citric acid cycle. The product of
aerobic glycolysis, pyruvate, can enter the citric acid cycle only through the pyruvate dehydrogenase complex.
Symptoms similar to those of beriberi arise if an organism is exposed to mercury or arsenite (AsO
3
3-
). Both elements
have a high affinity for neighboring sulfhydryls, such as those in the reduced dihydrolipoyl groups of the dihydrolipoyl
dehydrogenase component of the pyruvate dehydrogenase complex (Figure 17.20). The binding of mercury or arsenite to
the dihydrolipoyl groups inhibits the complex and leads to central nervous system pathologies. The proverbial phrase
"mad as a hatter" refers to the strange behavior of poisoned hat makers who used mercury nitrate to soften and shape
animal furs. This form of mercury is absorbed through the skin. Similar problems afflicted the early photographers, who
used vaporized mercury to create daguerreotypes.
[The Granger Collection.]
Treatment for these poisons is the administration of sulfhydryl reagents with adjacent sulfhydryl groups to compete with
the dihydrolipoyl residues for binding with the metal ion, which is then excreted in the urine. Indeed, 2,3-
dimercaptopropanol (see Figure 17.20) was developed after World War I as an antidote to lewisite, an arsenic-based
chemical weapon. This compound was initially called BAL, for British anti-lewisite.
17.3.3. Speculations on the Evolutionary History of the Citric Acid Cycle
How did the citric acid cycle come into being? Although definitive answers are elusive, it is nevertheless
instructive to speculate how this complicated central hub of metabolism developed. We can perhaps begin to
comprehend how evolution might work at the level of biochemical pathways.
The manuscript proposing the citric acid cycle was submitted for
publication to Nature but was rejected. It was subsequently
published in Enzymologia. Dr. Krebs proudly displayed the rejection
letter throughout his career as encouragement for young scientists.
"June 1937
The editor of NATURE presents his compliments to Dr. H. A. Krebs
and regrets that as he has already sufficient letters to fill the
correspondence columns of NATURE for seven or eight weeks, it is
undesirable to accept further letters at the present time on account of
the time delay which must occur in their publication.
If Dr. Krebs does not mind much delay the editor is prepared to keep
the letter until the congestion is relieved in the hope of making use of
it.
He returns it now, in case Dr. Krebs prefers to submit it for early
publication to another periodical."
It is most likely that the citric acid cycle was assembled from preexisting reaction pathways. As noted earlier, many of
the intermediates formed in the citric acid cycle are used in biosynthetic pathways to generate amino acids and
porphyrins. Thus, compounds such as pyruvate, α-ketoglutarate, and oxaloacetate were likely present early in evolution
for biosynthetic purposes. The oxidative decarboxylation of these α-ketoacids is quite favorable thermodynamically. The
elegant modular structures of the pyruvate and α-ketoglutarate dehydrogenase complexes reveal how three reactions
(decarboxylation, oxidation, and thioester formation) can be linked to harness the free energy associated with
decarboxylation to drive the synthesis of both acyl CoA derivatives and NADH. These reactions almost certainly formed
the core of processes that preceded the citric acid cycle evolutionarily. Interestingly, α-ketoglutarate can be directly
converted into oxaloacetate by transamination of the respective amino acids by aspartate aminotransferase, another key
biosynthetic enzyme. Thus, cycles comprising smaller numbers of intermediates could have existed before the present
form evolved to harvest the electrons from pyruvate or other compounds more efficiently.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.3. The Citric Acid Cycle Is a Source of Biosynthetic Precursors
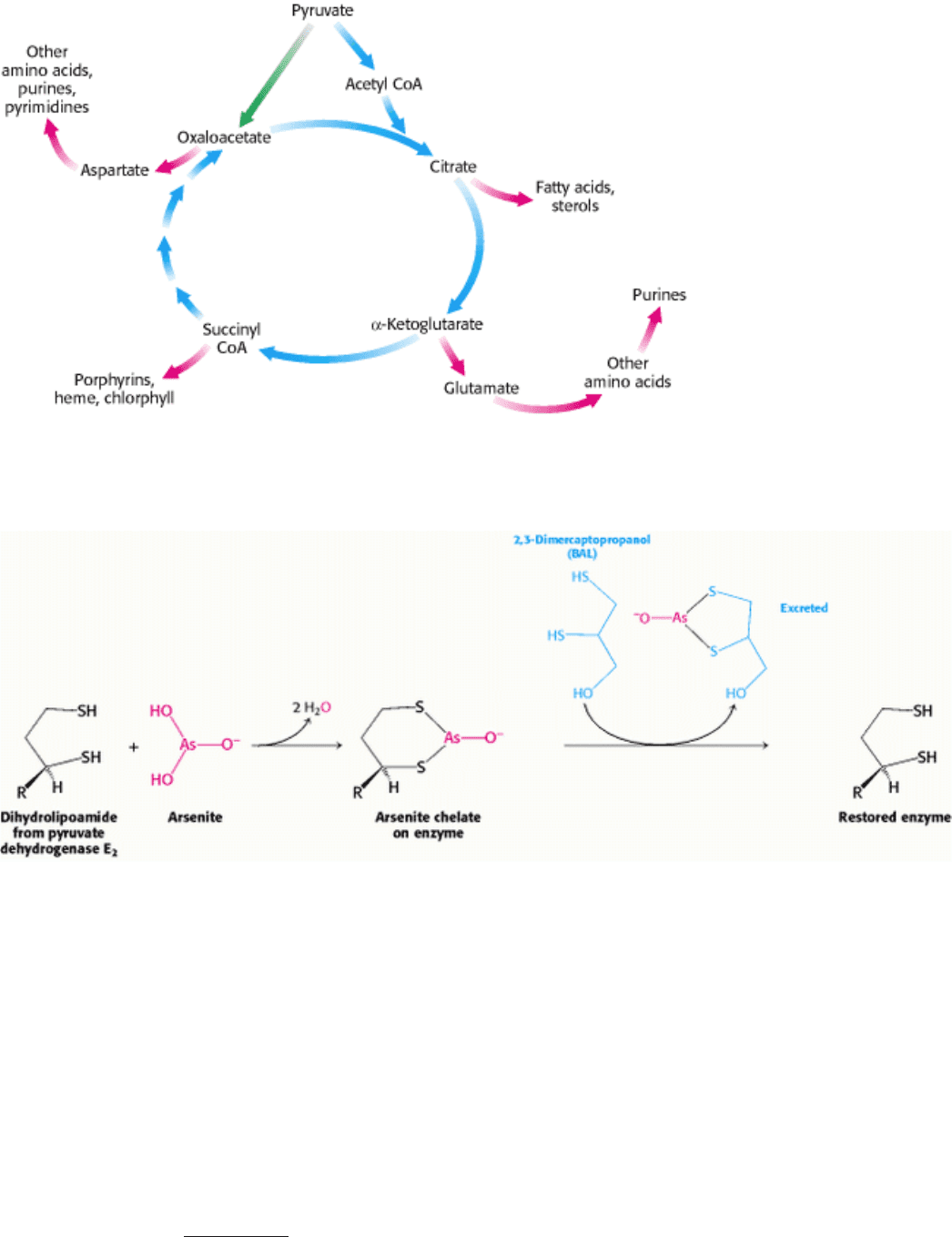
Figure 17.19. Biosynthetic Roles of the Citric Acid Cycle. Intermediates drawn off for biosyntheses (shown by red
arrows) are replenished by the formation of oxaloacetate from pyruvate.
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.3. The Citric Acid Cycle Is a Source of Biosynthetic Precursors
Figure 17.20. Arsenite Poisoning. Arsenite inhibits the pyruvate dehydrogenase complex by inactivating the
dihydrolipoamide component of the transacetylase. Some sulfhydryl reagents, such as 2,3-dimercaptoethanol, relieve the
inhibition by forming a complex with the arsenite that can be excreted.
II. Transducing and Storing Energy 17. The Citric Acid Cycle
17.4. The Glyoxylate Cycle Enables Plants and Bacteria to Grow on Acetate
Many bacteria and plants are able to subsist on acetate or other compounds that yield acetyl CoA. They make use of a
metabolic pathway absent in most other organisms that converts two-carbon acetyl units into four-carbon units
(succinate) for energy production and biosyntheses. This reaction sequence, called the glyoxylate cycle, bypasses the two
decarboxylation steps of the citric acid cycle. Another key difference is that two molecules of acetyl CoA enter per turn
of the glyoxylate cycle, compared with one in the citric acid cycle.
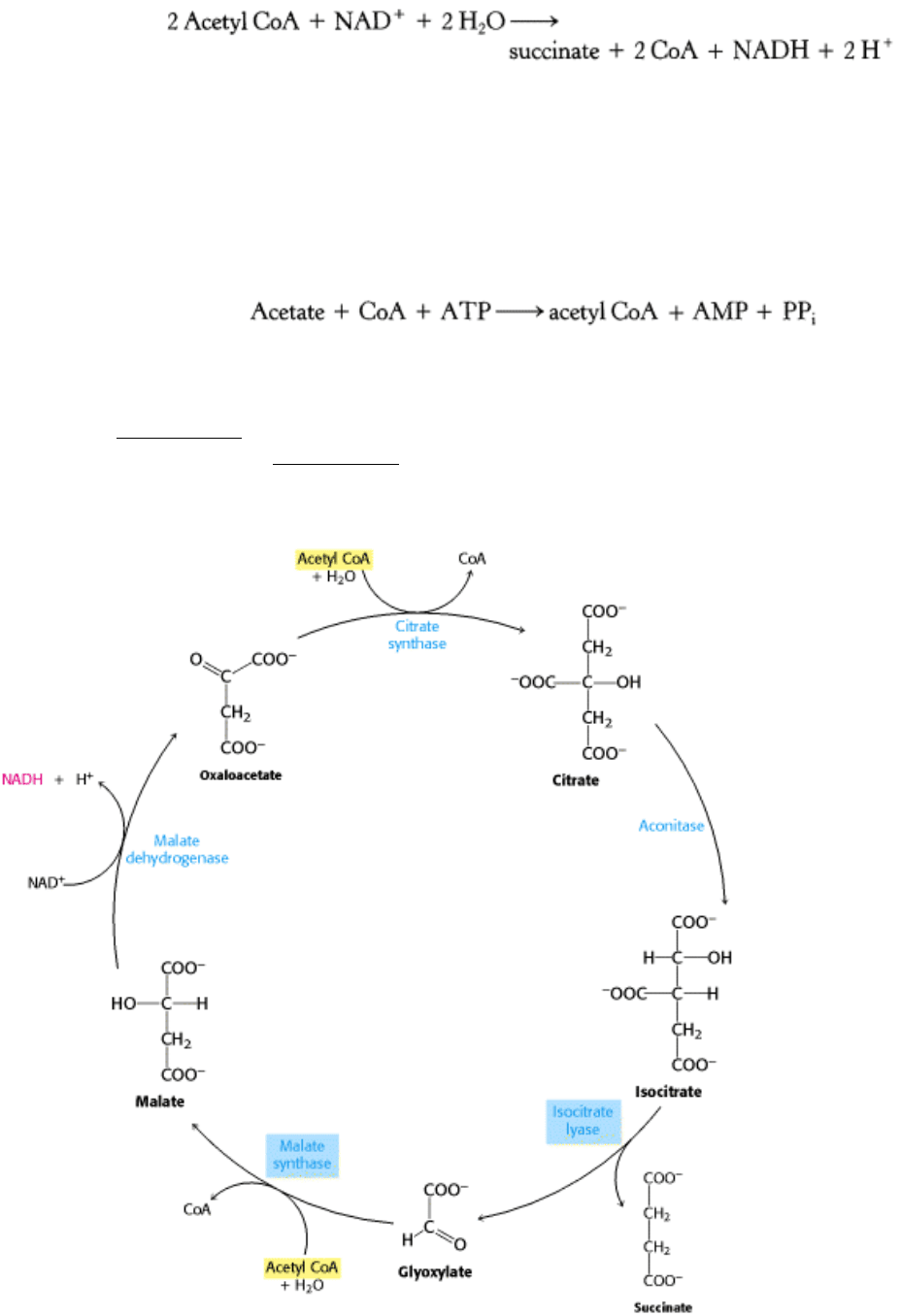
The glyoxylate cycle (Figure 17.21), like the citric acid cycle, begins with the condensation of acetyl CoA and
oxaloacetate to form citrate, which is then isomerized to isocitrate. Instead of being decarboxylated, isocitrate is cleaved
by isocitrate lyase into succinate and glyoxylate. The subsequent steps regenerate oxaloacetate from glyoxylate. Acetyl
CoA condenses with glyoxylate to form malate in a reaction catalyzed by malate synthase, which resembles citrate
synthase. Finally, malate is oxidized to oxaloacetate, as in the citric acid cycle. The sum of these reactions is:
In plants, these reactions take place in organelles called glyoxysomes. Succinate, released midcycle, can be converted
into carbohydrates by a combination of the citric acid cycle and gluconeogenesis. Thus, organisms with the glyoxylate
cycle gain a metabolic versatility.
Bacteria and plants can synthesize acetyl CoA from acetate and CoA by an ATP-driven reaction that is catalyzed by
acetyl CoA synthetase.
Pyrophosphate is then hydrolyzed to orthophosphate, and so the equivalents of two compounds having high phosphoryl
transfer potential are consumed in the activation of acetate. We will return to this type of activation reaction in fatty acid
degradation (Section 22.2.2), where it is used to form fatty acyl CoA, and in protein synthesis, where it is used to link
amino acids to transfer RNAs (Section 29.2.1).
II. Transducing and Storing Energy 17. The Citric Acid Cycle 17.4. The Glyoxylate Cycle Enables Plants and Bacteria to Grow on Acetate
Figure 17.21. The Glyoxylate Pathway. The glyoxylate cycle allows plants and some microorganisms to grow on
acetate because the cycle bypasses the decarboxylation steps of the citric acid cycle. The enzymes that permit the
conversion of acetate into succinate-isocitrate lyase and malate synthase-are boxed in blue.
II. Transducing and Storing Energy 17. The Citric Acid Cycle
Summary
The citric acid cycle is the final common pathway for the oxidation of fuel molecules. It also serves as a source of
building blocks for biosyntheses. Most fuel molecules enter the cycle as acetyl CoA. The link between glycolysis and the
citric acid cycle is the oxidative decarboxylation of pyruvate to form acetyl CoA. In eukaryotes, this reaction and those
of the cycle take place inside mitochondria, in contrast with glycolysis, which takes place in the cytosol.
The Citric Acid Cycle Oxidizes Two-Carbon Units
The cycle starts with the condensation of oxaloacetate (C
4
) and acetyl CoA (C
2
) to give citrate (C
6
), which is isomerized
to isocitrate (C
6
). Oxidative decarboxylation of this intermediate gives α-ketoglutarate (C
5
). The second molecule of
carbon dioxide comes off in the next reaction, in which α-ketoglutarate is oxidatively decarboxylated to succinyl CoA
(C
4
). The thioester bond of succinyl CoA is cleaved by inorthophosphate to yield succinate, and a high phosphoryl
transfer potential compound in the form of GTP is concomitantly generated. Succinate is oxidized to fumarate (C
4
),
which is then hydrated to form malate (C
4
). Finally, malate is oxidized to regenerate oxaloacetate (C
4
). Thus, two carbon
atoms from acetyl CoA enter the cycle, and two carbon atoms leave the cycle as CO
2
in the successive decarboxylations
catalyzed by isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. In the four oxidation-reduction reactions in
the cycle, three pairs of electrons are transferred to NAD
+
and one pair to FAD. These reduced electron carriers are
subsequently oxidized by the electron-transport chain to generate approximately 9 molecules of ATP. In addition, 1
molecule of a compound having a high phosphoryl transfer potential is directly formed in the citric acid cycle. Hence, a
total of 10 molecules of compounds having high phosphoryl transfer potential are generated for each two-carbon
fragment that is completely oxidized to H
2
O and CO
2
.
Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled
The citric acid cycle operates only under aerobic conditions because it requires a supply of NAD
+
and FAD. The
irreversible formation of acetyl CoA from pyruvate is an important regulatory point for the entry of glucose-derived
pyruvate into the citric acid cycle. The activity of the pyruvate dehydrogenase complex is stringently controlled by
reversible phosphorylation. The electron acceptors are regenerated when NADH and FADH
2
transfer their electrons to
O
2
through the electron-transport chain, with the concomitant production of ATP. Consequently, the rate of the citric
acid cycle depends on the need for ATP. In eukaryotes, the regulation of two enzymes in the cycle also is important for
control. A high energy charge diminishes the activities of isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.
These mechanisms complement each other in reducing the rate of formation of acetyl CoA when the energy charge of
the cell is high and when biosynthetic intermediates are abundant.
The Citric Acid Cycle Is a Source of Biosynthetic Precursors
When the cell has adequate energy available, the citric acid cycle can also provide a source of building blocks for a host
of important biomolecules, such as nucleotide bases, proteins, and heme groups. This use depletes the cycle of
intermediates. When the cycle again needs to metabolize fuel, anaplerotic reactions replenish the cycle intermediates.
The Glyoxylate Cycle Enables Plants and Bacteria to Grow on Acetate
The glyoxylate cycle enhances the metabolic versatility of many plants and bacteria. This cycle, which uses some of the
reactions of the citric acid cycle, enables these organisms to subsist on acetate because it bypasses the two
decarboxylation steps of the citric acid cycle.
Key Terms
citric acid (tricarboxylic acid, TCA; Krebs) cycle
oxidative phosphorylation
acetyl CoA
pyruvate dehydrogenase complex
flavoprotein
citrate synthase
iron-sulfur (nonheme iron) protein
isocitrate dehydrogenase
α-ketoglutarate dehydrogenase
metabolon
anaplerotic reaction
beriberi
glyoxylate cycle
isocitrate lyase
malate synthase
glyoxysome
II. Transducing and Storing Energy 17. The Citric Acid Cycle
Problems
1.
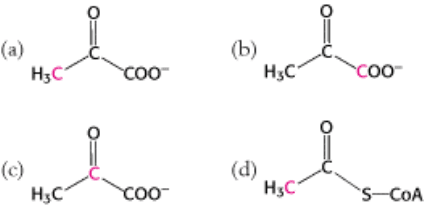
Flow of carbon atoms. What is the fate of the radioactive label when each of the following compounds is added to a
cell extract containing the enzymes and cofactors of the glycolytic pathway, the citric acid cycle, and the pyruvate
dehydrogenase complex? (The
14
C label is printed in red.)
(e) Glucose 6-phosphate labeled at C-1.
See answer
2.
(a) Which enzymes are required to get net synthesis of oxaloacetate from acetyl CoA?
(b) Write a balanced equation for the net synthesis.
(c) Do mammalian cells contain the requisite enzymes?
See answer
3.
Driving force. What is the ∆ G° for the complete oxidation of the acetyl unit of acetyl CoA by the citric acid cycle?
See answer
4.
Acting catalytically. The citric acid cycle itself, which is composed of enzymatically catalyzed steps, can be thought
of essentially as the product of a supramolecular enzyme. Explain.
See answer
5.
Probing stereospecificity. A sample of deuterated reduced NAD was prepared by incubating H
3
C-CD
2
-OH and NAD
+
with alcohol dehydrogenase. This reduced coenzyme was added to a solution of 1,3-BPG and glyceraldehyde 3-
phosphate dehydrogenase. The NAD
+
formed by this second reaction contained one atom of deuterium, whereas
glyceraldehyde 3-phosphate, the other product, contained none. What does this experiment reveal about the
stereospecificity of glyceraldehyde 3-phosphate dehydrogenase?
See answer
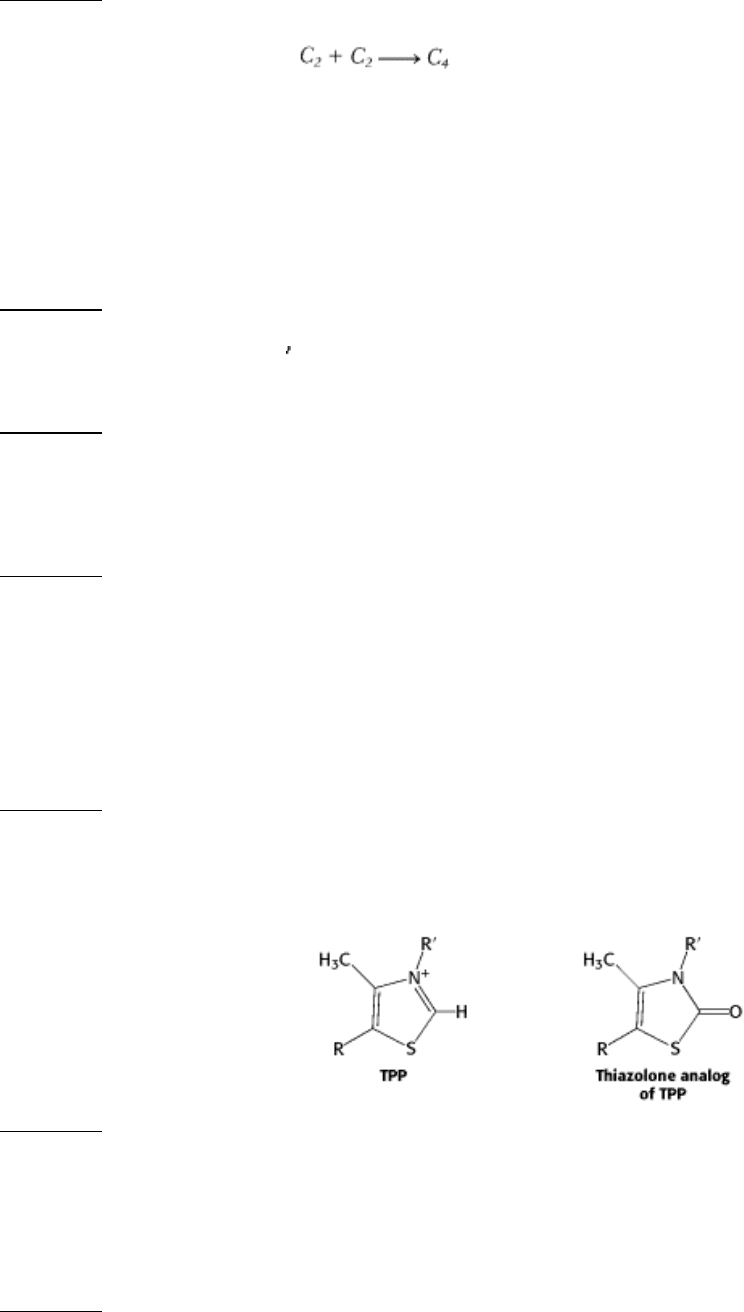
6.
A potent inhibitor. Thiamine thiazolone pyrophosphate binds to pyruvate dehydrogenase about 20,000 times as
strongly as does thiamine pyrophosphate, and it competitively inhibits the enzyme. Why?
See answer
7.
Lactic acidosis. Patients in shock will often suffer from lactic acidosis due to a deficiency of O
2
. Why does a lack of
O
2
lead to lactic acid accumulation? One treatment for shock is to administer dichloroacetate, which inhibits the
kinase associated with the pyruvate dehydrogenase complex. What is the biochemical rationale for this treatment?
See answer
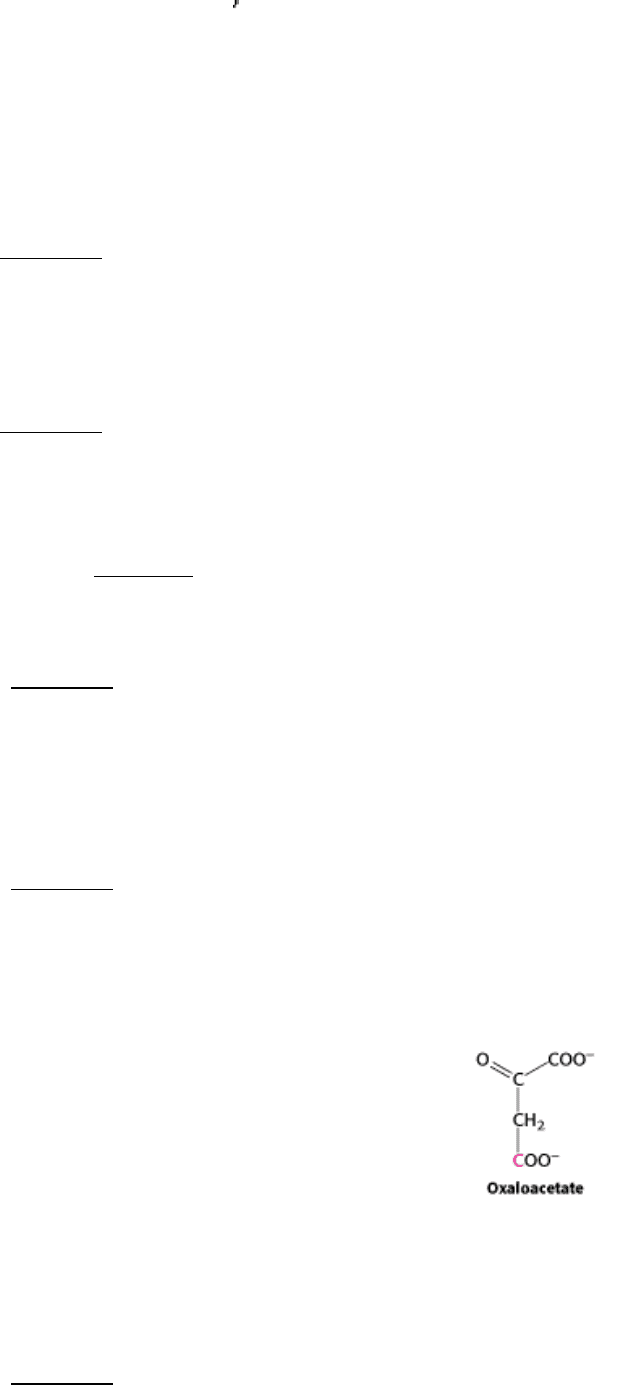
8.
Coupling reactions. The oxidation of malate by NAD
+
to form oxaloacetate is a highly endergonic reaction under
standard conditions [∆ G°
+ 7 kcal mol
-1
(+ 29 kJ mol
-1
)]. The reaction proceeds readily under physiological
conditions.
(a) Why?
(b) Assuming an [NAD
+
]/[NADH] ratio of 8 and a pH of 7, what is the lowest [malate]/[oxaloacetate] ratio at which
oxaloacetate can be formed from malate?
See answer
9.
Synthesizing α-ketoglutarate. It is possible, with the use of the reactions and enzymes discussed in this chapter, to
convert pyruvate into α-ketoglutarate without depleting any of the citric acid cycle components. Write a balanced
reaction scheme for this conversion, showing cofactors and identifying the required enzymes.
See answer
Chapter Integration Problem
10.
Fats into glucose? Fats are usually metabolized into acetyl CoA and then further processed through the citric acid
cycle. In Chapter 16, we learned that glucose could be synthesized from oxaloacetate, a citric acid cycle
intermediate. Why, then, after a long bout of exercise depletes our carbohydrate stores, do we need to replenish
those stores by eating carbohydrates? Why do we not simply replace them by converting fats into carbohydrates?
See answer
Mechanism Problems
11.
Theme and variation. Propose a reaction mechanism for the condensation of acetyl CoA and glyoxylate in the
glyoxylate cycle of plants and bacteria.
See answer
12.
Symmetry problems. In experiments carried out in 1941 to investigate the citric acid cycle, oxaloacetate labeled
with
14
C in the carboxyl carbon atom farthest from the keto group was introduced to an active preparation of
mitochondria.
Analysis of the α-ketoglutarate formed showed that none of the radioactive label had been lost. Decarboxylation of
α-ketoglutarate then yielded succinate devoid of radioactivity. All the label was in the released CO
2
. Why were the
early investigators of the citric acid cycle surprised that all the label emerged in the CO
2
?
See answer

13.
Symmetric molecules reacting asymmetrically. The interpretation of the experiments described in problem 12 was
that citrate (or any other symmetric compound) cannot be an intermediate in the formation of α-ketoglutarate,
because of the asymmetric fate of the label. This view seemed compelling until Alexander Ogston incisively
pointed out in 1948 that "it is possible that an asymmetric enzyme which attacks a symmetrical compound can
distinguish between its identical groups." For simplicity, consider a molecule in which two hydrogen atoms, a
group X, and a different group Y are bonded to a tetrahedral carbon atom as a model for citrate. Explain how a
symmetric molecule can react with an enzyme in an asymmetric way.
See answer
Data Interpretation
14.
A little goes a long way. As will become clearer in Chapter 18, the activity of the citric acid cycle can be monitored
by monitoring the amount of O
2
consumed. The greater the rate of O
2
consumption, the faster the rate of the cycle.
Hans Krebs used this assay to investigate the cycle in 1937. He used as his experimental system minced pigeon
breast muscle, which is rich in mitochondria. In one set of experiments, Krebs measured the O
2
consumption in the
presence of carbohydrate only and in the presence of carbohydrate and citrate. The results are shown in the
following table.
(a) How much O
2
would be absorbed if the added citrate were completely oxidized to H
2
O and CO
2
?
(b) Based on your answer to part a, what do the results given in the table suggest?
See answer
15.
Arsenite poisoning. The effect of arsenite on the experimental system of problem 14 was then examined.
Experimental data (not presented here) showed that the amount of citrate present did not change in the course of the
experiment in the absence of arsenite. However, if arsenite was added to the system, different results were obtained,
as shown in the following table.
(a) What is the effect of arsenite on the disappearance of citrate?
(b) How is the arsenite's action altered by the addition of more citrate?
(c) What do these data suggest about the site of action of arsenite?
See answer
16.
Isocitrate lyase and tuberculosis. The bacterium Mycobacterium tuberculosis, the cause of tuberculosis, can invade
the lungs and persist in a latent state for years. During this time, the bacteria reside in granulomas nodular scars
containing bacteria and host-cell debris in the center and surrounded by immune cells. The granulomas are lipid-
rich, oxygen-poor environments. How these bacteria manage to persist is something of a mystery. The results of
recent research suggest that the glyoxylate cycle is required for the persistence. The following data show the
amount of bacteria [presented as colony-forming units (cfu)] in mice lungs in the weeks after an infection.
In graph A, the black circles represent the results for wild-type bacteria and the red circles represent the results for
bacteria from which the gene for isocitrate lyase was deleted.
