Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

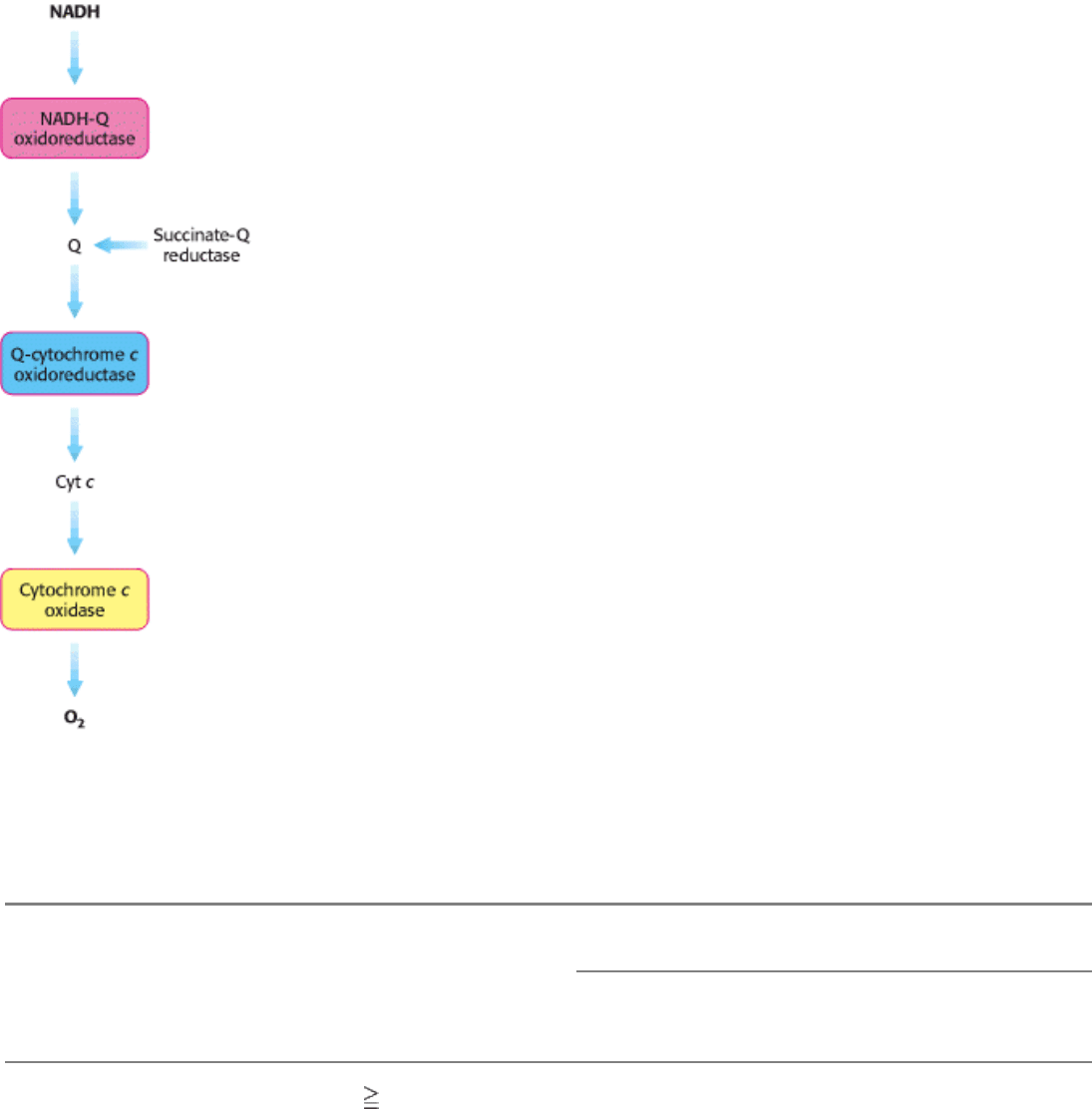
Figure 18.9. Sequence of Electron Carriers in the Respiratory Chain.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Table 18.2. Components of the mitochondrial electron-transport chain
Oxidant or reductant
Enzyme complex Mass
(kd)
Subunits Prosthetic group Matrix side Membrane core Cytosolic side
NADH-Q
oxidoreductase
880 34 FMN NADH Q
Fe-S
Succinate-Q reductase 140 4 FAD Succinate Q
Fe-S
Q-cytochrome c
oxidoreductase
250 10 Heme b
H
Q Cytochrome c
Heme b
L
Heme c
1
Fe-S
Cytochrome c oxidase 160 10 Heme a Cytochrome c
Heme a
3
Cu
A
and Cu
B
Sources: J. W. DePierre and L. Ernster, Annu. Rev. Biochem. 46(1977):215; Y. Hatefi, Annu Rev. Biochem. 54(1985);1015; and J.
E. Walker, Q. Rev. Biophys. 25(1992):253.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
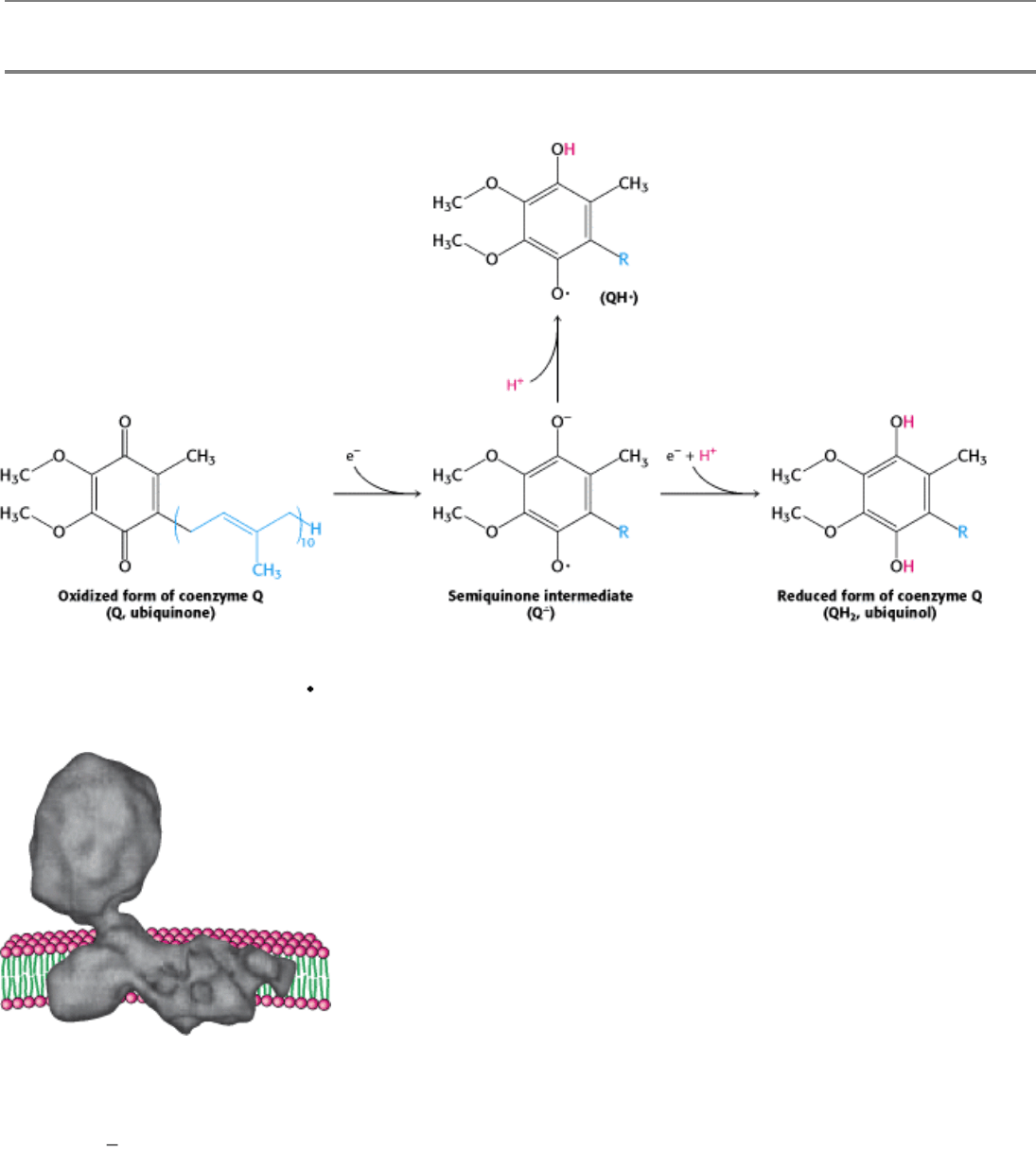
Figure 18.10. Oxidation States of Quinones. The reduction of ubiquinone (Q) to ubiquinol (QH
2
) proceeds through a
semiquinone anion intermediate (Q
-
).
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.11. Structure of NADH-Q Oxidoreductase (Complex I). The structure, determined by electron microscopy
at 22-Å resolution, consists of a membrane-spanning part and a long arm that extends into the matrix. NADH is oxidized
in the arm, and the electrons are transferred to reduce Q in the membrane. [After N. Grigorieff, J. Mol. Biol. 277
(1998):1033
1048.]
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
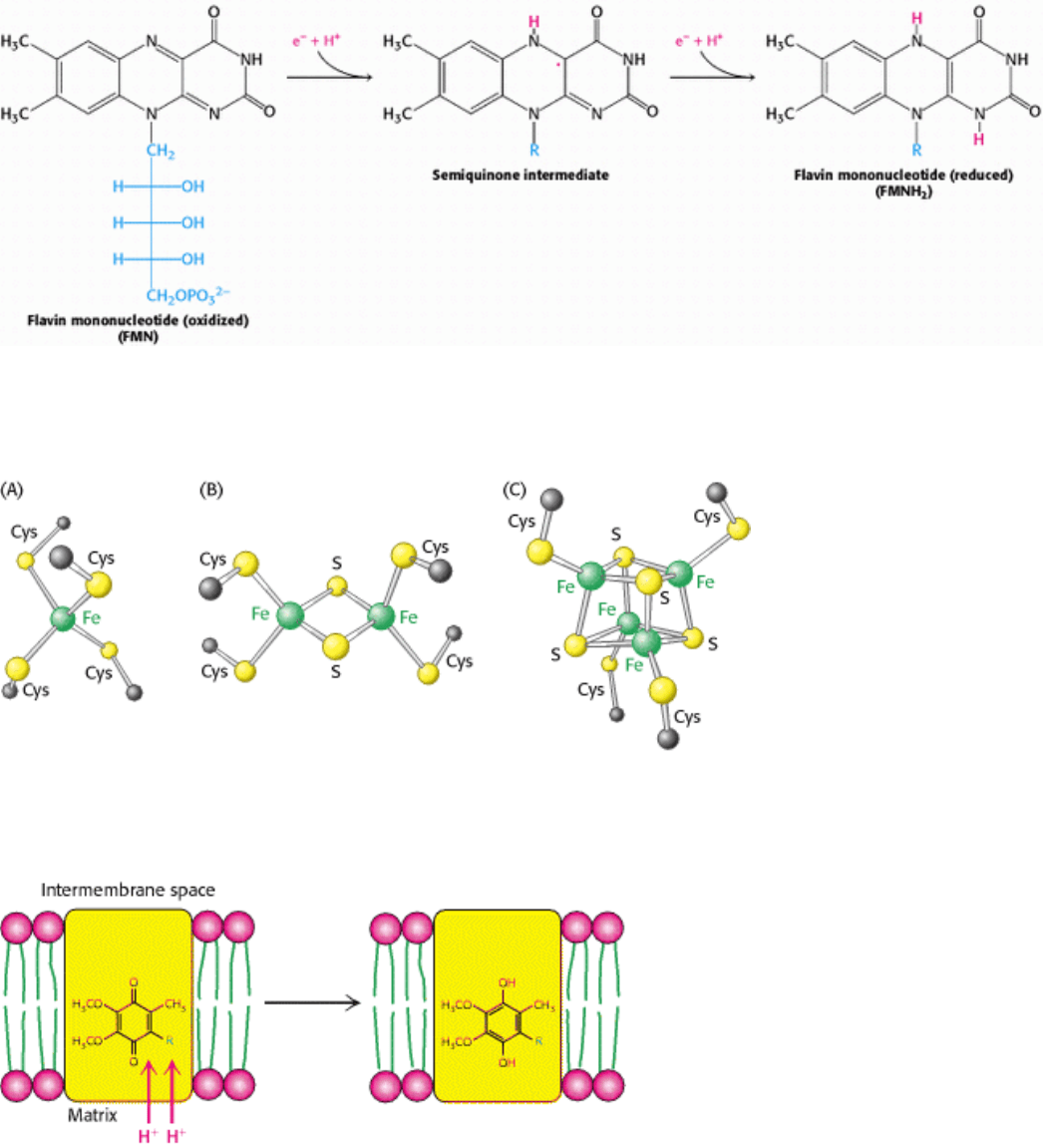
Figure 18.12. Oxidation States of Flavins. The reduction of flavin mononucleotide (FMN) to FMNH
2
proceeds
through a semiquinone intermediate.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.13. Iron-Sulfur Clusters. (A) A single iron ion bound by four cysteine residues. (B) 2Fe-2S cluster with iron
ions bridged by sulfide ions. (C) 4Fe-4S cluster. Each of these clusters can undergo oxidation-reduction reactions.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.14. Coupled Electron-Proton Transfer Reactions. The reduction of a quinone (Q) to QH
2
in an appropriate
site can result in the uptake of two protons from the mitochondrial matrix.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
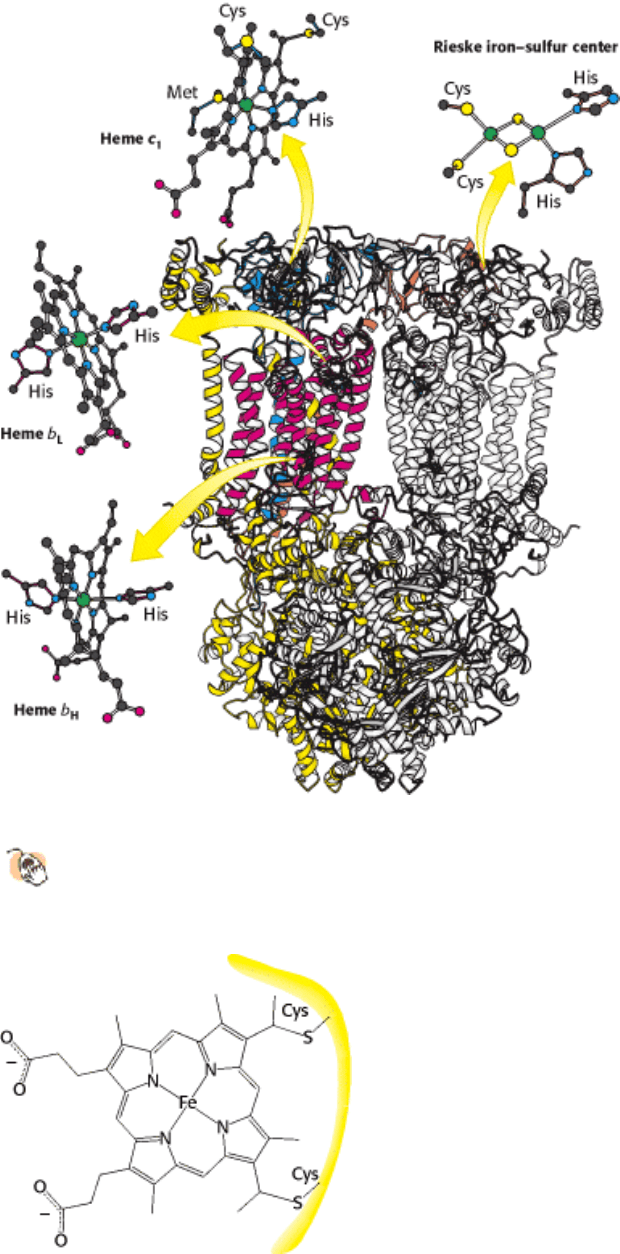
Figure 18.15. Structure of Q-Cytochrome C Oxidoreductase (Cytochrome BC
1
). This enzyme is a homodimer with
11 distinct polypeptide chains. The major prosthetic groups, three hemes and a 2Fe-2S cluster, mediate the
electron-transfer reactions between quinones in the membrane and cytochrome c in the intermembrane space.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.16. Attachment of C -Type Cytochromes. A heme group is covalently attached to a protein through
thioether linkages formed by the addition of sulfhydryl groups of cysteine residues to vinyl groups on protoporphyrin.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.17. Q Cycle. The two electrons of a bound QH
2
are transferred, one to cytochrome c and the other to a bound
Q to form the semiquinone Q
-
. The newly formed Q dissociates and is replaced by a second QH
2
, which also gives up
its electrons, one to a second molecule of cytochrome c and the other to reduce Q
-
to QH
2
. This second electron
transfer results in the uptake of two protons from the matrix. Prosthetic groups are shown in their oxidized forms in blue
and in their reduced forms in red.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
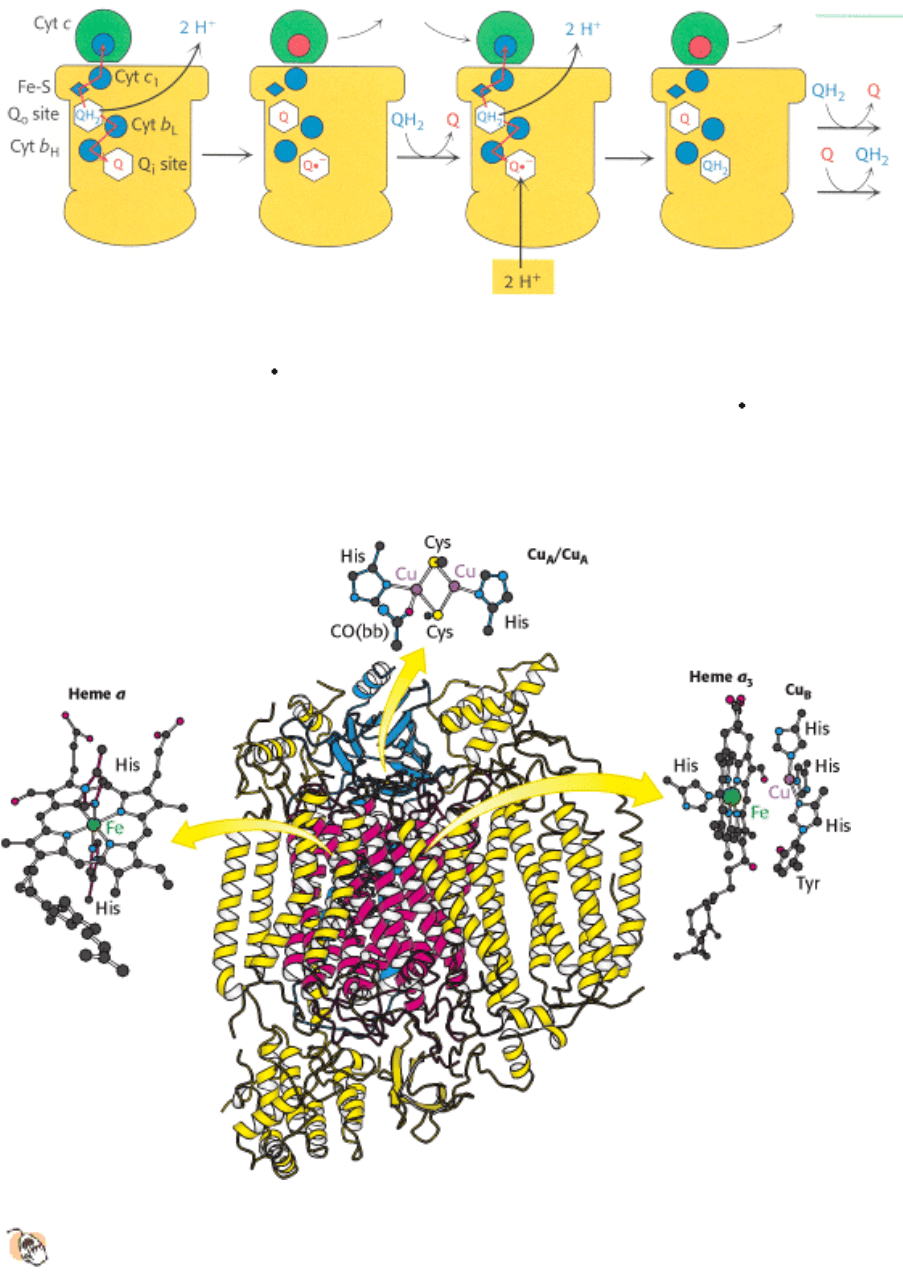
Figure 18.18. Structure of Cytochrome C Oxidase.
This enzyme consists of 13 polypeptide chains. The major
prosthetic groups include Cu
A
/Cu
A
, heme a, and heme a
3
-Cu
B
. Heme a
3
-Cu
B
is the site of the reduction of
oxygen to water. CO(bb) is a carbonyl group of the peptide backbone.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
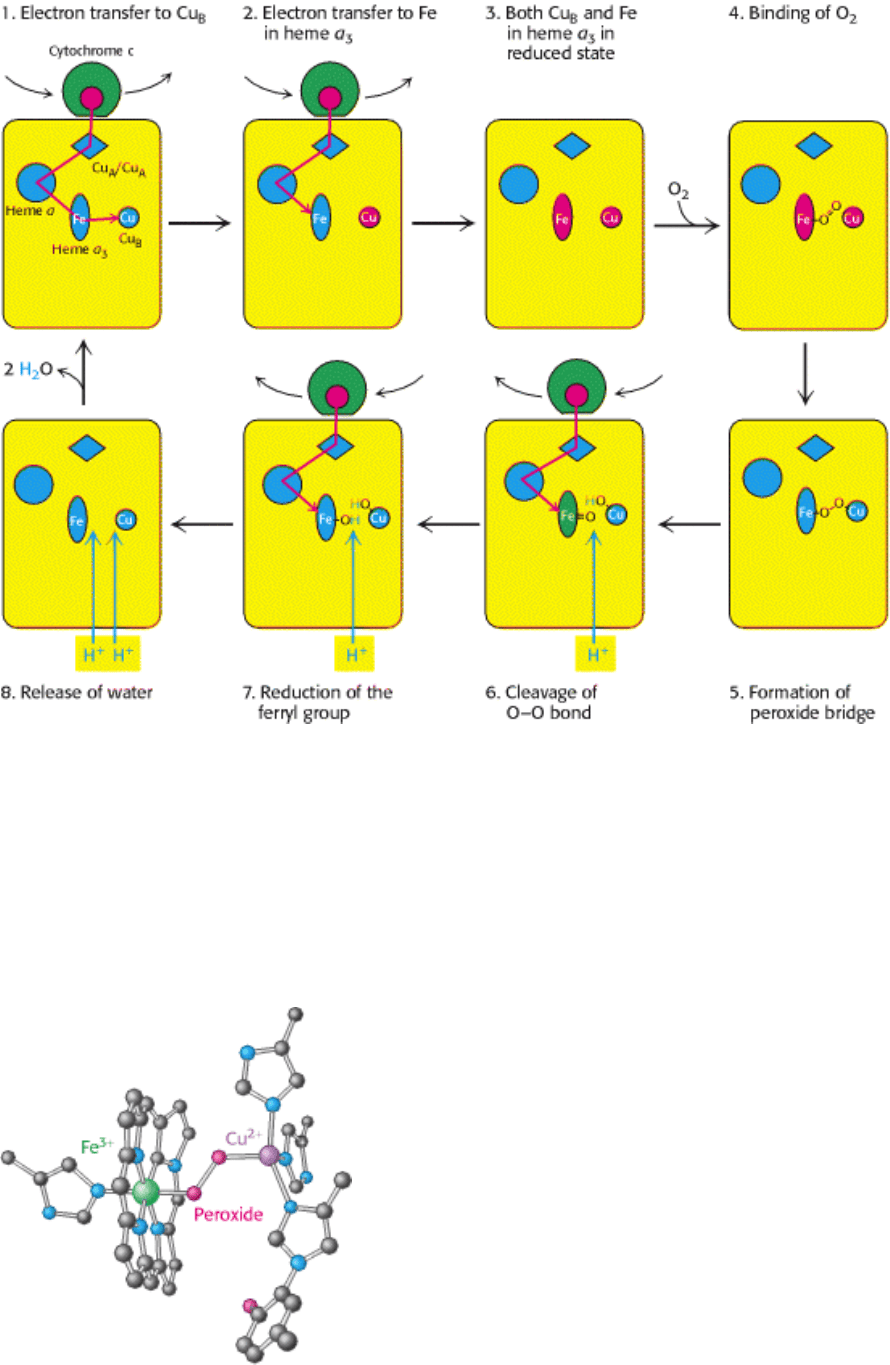
Figure 18.19. Cytochrome Oxidase Mechanism. The cycle begins with all prosthetic groups in their oxidized forms
(shown in blue). Reduced cytochrome c introduces an electron that reduces Cu
B
. A second reduced cytochrome c then
reduces the iron in heme a
3
. This Fe
2+
center then binds oxygen. Two electrons are transferred to the bound oxygen to
form peroxide, which bridges between the iron and Cu
B
. The introduction of an additional electron by a third molecule
of reduced cytochrome c cleaves the O-O bond and results in the uptake of a proton from the matrix. The introduction of
a final electron and three more protons generates two molecules of H
2
O, which are released from the enzyme to
regenerate the initial state. The four protons found in the water molecules come from the matrix.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.20. Peroxide Bridge. The oxygen bound to heme a
3
is reduced to peroxide by the presence of Cu
B
.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
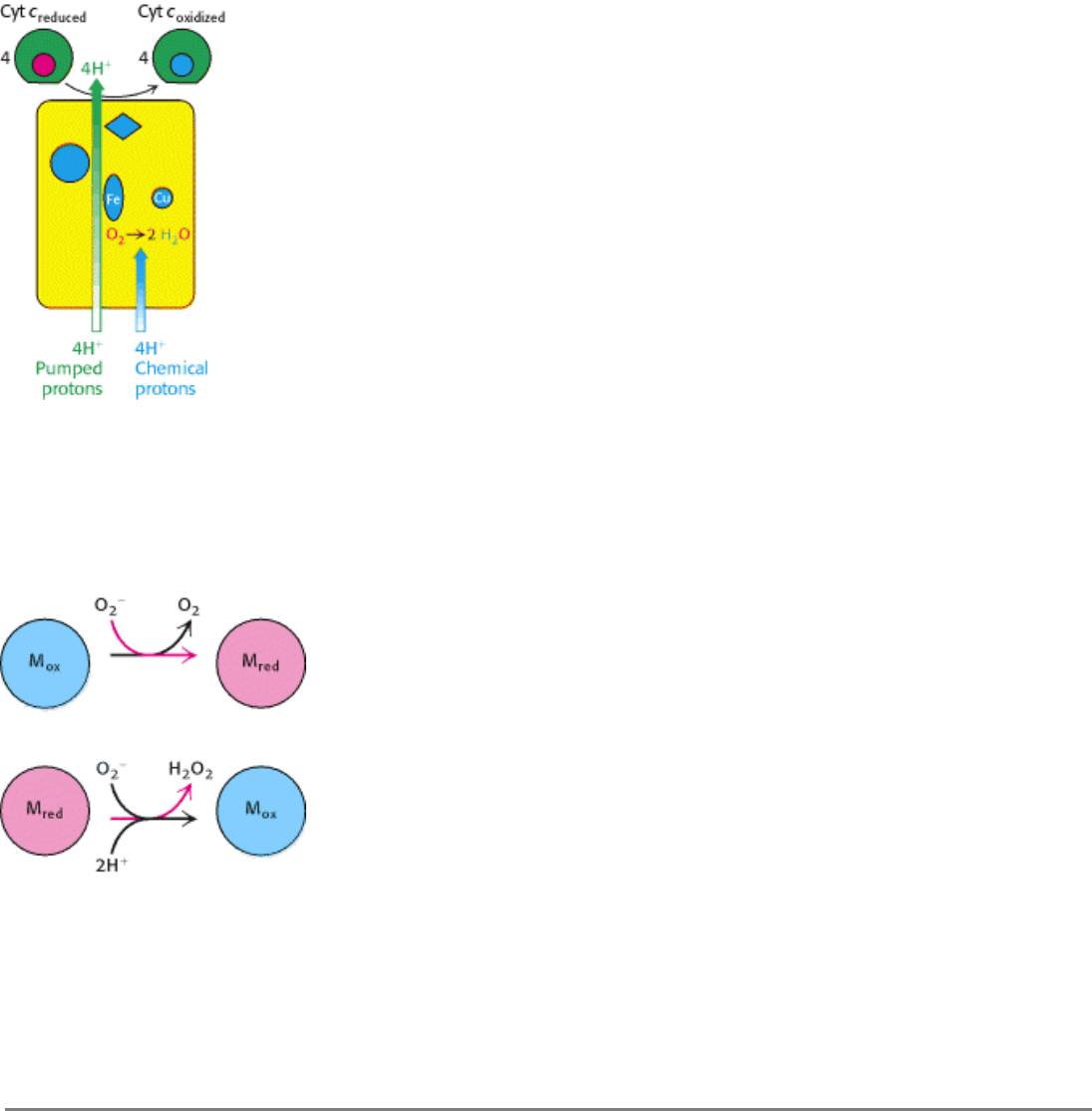
Figure 18.21. Proton Transport by Cytochrome C Oxidase. Four "chemical" protons are taken up from the matrix
side to reduce one molecule of O
2
to two molecules of H
2
O. Four additional "pumped" protons are transported out of the
matrix and released on the cytosolic side in the course of the reaction. The pumped protons double the efficiency of free-
energy storage in the form of a proton gradient for this final step in the electron-transport chain.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.22. Superoxide Dismutase Mechanism. The oxidized form of superoxide dismutase (M
ox
) reacts with one
superoxide ion to form O
2
and generate the reduced form of the enzyme (M
red
). The reduced form then reacts with a
second superoxide and two protons to form hydrogen peroxide and regenerate the oxidized form of the enzyme.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Table 18.3. Pathological and other conditions that may entail free-radical injury
Atherogenesis
Emphysema; bronchitis
Parkinson disease
Duchenne muscular dystrophy
Cervical cancer
Alcoholic liver disease
Diabetes
Acute renal failure
Down syndrome
Retrolental fibroplasia
Cerebrovascular disorders
Ischemia; reperfusion injury
Source: After D.B. Marks, A.D. Marks, and C.M. Smith, Basic Medical Biochemistry: A Clinical Approach (Williams & Wilkins,
1996, p. 331).
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
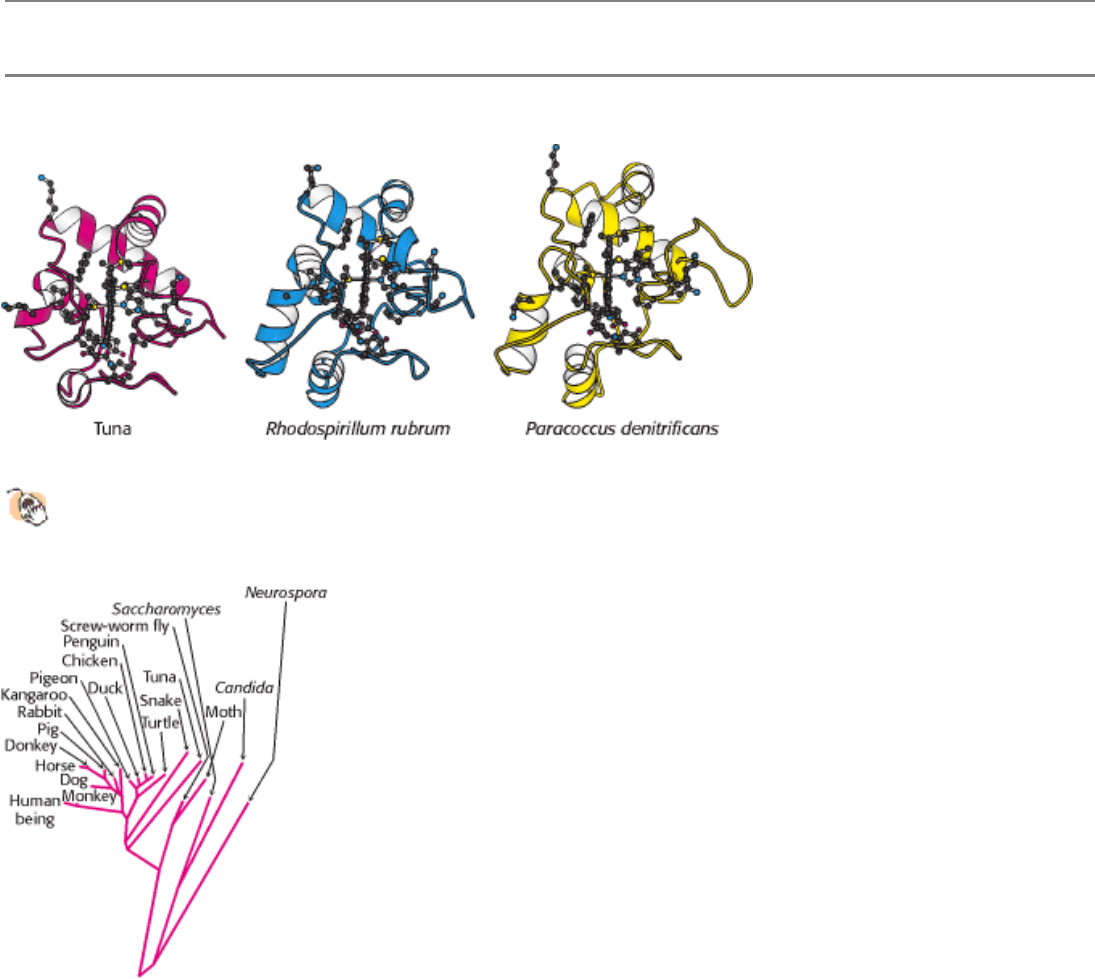
Figure 18.23. Conservation of the Three-Dimensional Structure of Cytochrome C.
The side chains are shown for the
21 conserved amino acids and the heme.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.3. The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Figure 18.24. Evolutionary Tree Constructed From Sequences of Cytochrome C. Branch lengths are proportional to
the number of amino acid changes that are believed to have occurred. This drawing is an adaptation of the work of
Walter M. Fitch and Emanuel Margoliash.

II. Transducing and Storing Energy 18. Oxidative Phosphorylation
18.4. A Proton Gradient Powers the Synthesis of ATP
Conceptual Insights, Energy Transformations in Oxidative
Phosphorylation. View this media module for an animated, interactive
summary of how electron transfer potential is converted into proton-motive
force and, finally, phosphoryl transfer potential in oxidative phosphorylation.
Thus far, we have considered the flow of electrons from NADH to O
2
, an exergonic process.
Next, we consider how this process is coupled to the synthesis of ATP, an endergonic process.
A molecular assembly in the inner mitochondrial membrane carries out the synthesis of ATP. This enzyme complex was
originally called the mitochon-drial ATPase or F
1
F
0
ATPase because it was discovered through its catalysis of the
reverse reaction, the hydrolysis of ATP. ATP synthase, its preferred name, emphasizes its actual role in the
mitochondrion. It is also called Complex V.
How is the oxidation of NADH coupled to the phosphorylation of ADP? It was first suggested that electron transfer leads
to the formation of a covalent high-energy intermediate that serves as a high phosphoryl transfer potential compound or
to the formation of an activated protein conformation, which then drives ATP synthesis. The search for such
intermediates for several decades proved fruitless.
In 1961, Peter Mitchell proposed that electron transport and ATP synthesis are coupled by a proton gradient across the
inner mitochondrial membrane rather than by a covalent high-energy intermediate or an activated protein conformation.
In his model, the transfer of electrons through the respiratory chain leads to the pumping of protons from the matrix to
the cytosolic side of the inner mitochondrial membrane. The H
+
concentration becomes lower in the matrix, and an
electrical field with the matrix side negative is generated (Figure 18.25). Mitchell's idea, called the chemiosmotic
hypothesis, was that this proton-motive force drives the synthesis of ATP by ATP synthase. Mitchell's highly innovative
hypothesis that oxidation and phosphorylation are coupled by a proton gradient is now supported by a wealth of
evidence. Indeed, electron transport does generate a proton gradient across the inner mitochondrial membrane. The pH
outside is 1.4 units lower than inside, and the membrane potential is 0.14 V, the outside being positive. As we calculated
in Section 18.2.2, this membrane potential corresponds to a free energy of 5.2 kcal (21.8 kJ) per mole of protons.
An artificial system was created to elegantly demonstrate the basic principle of the chemiosmotic hypothesis. Synthetic
vesicles containing bacteriorhodopsin, a purple-membrane protein from halobacteria that pumps protons when
illuminated, and mitochondrial ATP synthase purified from beef heart were created (Figure 18.26). When the vesicles
were exposed to light, ATP was formed. This key experiment clearly showed that the respiratory chain and ATP
synthase are biochemically separate systems, linked only by a proton-motive force.
18.4.1. ATP Synthase Is Composed of a Proton-Conducting Unit and a Catalytic Unit
Biochemical, electron microscopic, and crystallographic studies of ATP synthase have revealed many details of its
structure (Figure 18.27). It is a large, complex membrane-embedded enzyme that looks like a ball on a stick. The 85-Å-

diameter ball, called the F
1
subunit, protrudes into the mitochondrial matrix and contains the catalytic activity of the
synthase. In fact, isolated F
1
subunits display ATPase activity. The F
1
subunit consists of five types of polypeptide
chains (α
3
, β
3
, γ, δ, and ε) with the indicated stoichiometry. The α and β subunits, which make up the bulk of the F
1
,
are arranged alternately in a hexameric ring; they are homologous to one another and are members of the P-loop NTPase
family (Section 9.4.1). Both bind nucleotides but only the β subunits participate directly in catalysis. The central stalk
consists of two proteins: γ and ε. The γ subunit includes a long α-helical coiled coil that extends into the center of the α
3
β
3
hexamer. The γ subunit breaks the symmetry of the α
3
β
3
hexamer: each of the β subunits is distinct by virtue of its
interaction with a different face of γ. Distinguishing the three β subunits is crucial for the mechanism of ATP synthesis.
The F
0
subunit is a hydrophobic segment that spans the inner mitochondrial membrane. F
0
contains the proton channel
of the complex. This channel consists of a ring comprising from 10 to 14 c subunits that are embedded in the membrane.
A single a subunit binds to the outside of this ring. The proton channel depends on both the a subunit and the c ring. The
F
0
and F
1
subunits are connected in two ways, by the central γ ε stalk and by an exterior column. The exterior column
consists of one a subunit, two b subunits, and the δ subunit. As will be discussed shortly, we can think of the enzyme as
consisting of two functional components: (1) a moving unit, or rotor, consisting of the c ring and the γ ε stalk, and (2) a
stationary unit, or stator, composed of the remainder of the molecule.
18.4.2. Proton Flow Through ATP Synthase Leads to the Release of Tightly Bound
ATP: The Binding-Change Mechanism
Conceptual Insights, ATP Synthase as Motor Protein, looks further into the
chemistry and mechanics of ATP synthase rotation.
ATP synthase catalyzes the formation of ATP from ADP and orthophosphate.
The actual substrates are Mg
2+
complexes of ADP and ATP, as in all known phosphoryl transfer reactions with these
nucleotides. A terminal oxygen atom of ADP attacks the phosphorus atom of P
i
to form a pentacovalent intermediate,
which then dissociates into ATP and H
2
O (Figure 18.28). The attacking oxygen atom of ADP and the departing oxygen
atom of P
i
occupy the apices of a trigonal bipyramid.
How does the flow of protons drive the synthesis of ATP? The results of isotopic-exchange experiments unexpectedly
revealed that enzyme-bound ATP forms readily in the absence of a proton-motive force. When ADP and P
i
were added to
ATP synthase in H
2
18
O,
18
O became incorporated into P
i
through the synthesis of ATP and its subsequent hydrolysis
(Figure 18.29). The rate of incorporation of
18
O into P
i
showed that about equal amounts of bound ATP and ADP are in
equilibrium at the catalytic site, even in the absence of a proton gradient. However, ATP does not leave the catalytic site
unless protons flow through the enzyme. Thus, the role of the proton gradient is not to form ATP but to release it from
the synthase.
On the basis of these and other observations, Paul Boyer proposed a binding-change mechanism for proton-driven ATP
synthesis. This proposal states that changes in the properties of the three β subunits allow sequential ADP and P
i
binding,
ATP synthesis, and ATP release. The concepts of this initial proposal refined by more recent crystallographic and other
data yield a satisfying mechanism for ATP synthesis. As already noted, interactions with the γ subunit make the three β
subunits inequivalent (Figure 18.30). One β subunit can be in the T, or tight, conformation. This conformation binds
ATP with great avidity. Indeed, its affinity for ATP is so high that it will convert bound ADP and P
i
into ATP with an
equilibrium constant near 1, as indicated by the aforediscussed isotopic-exchange experiments. However, the
conformation of this subunit is sufficiently constrained that it cannot release ATP. A second subunit will then be in the
