Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

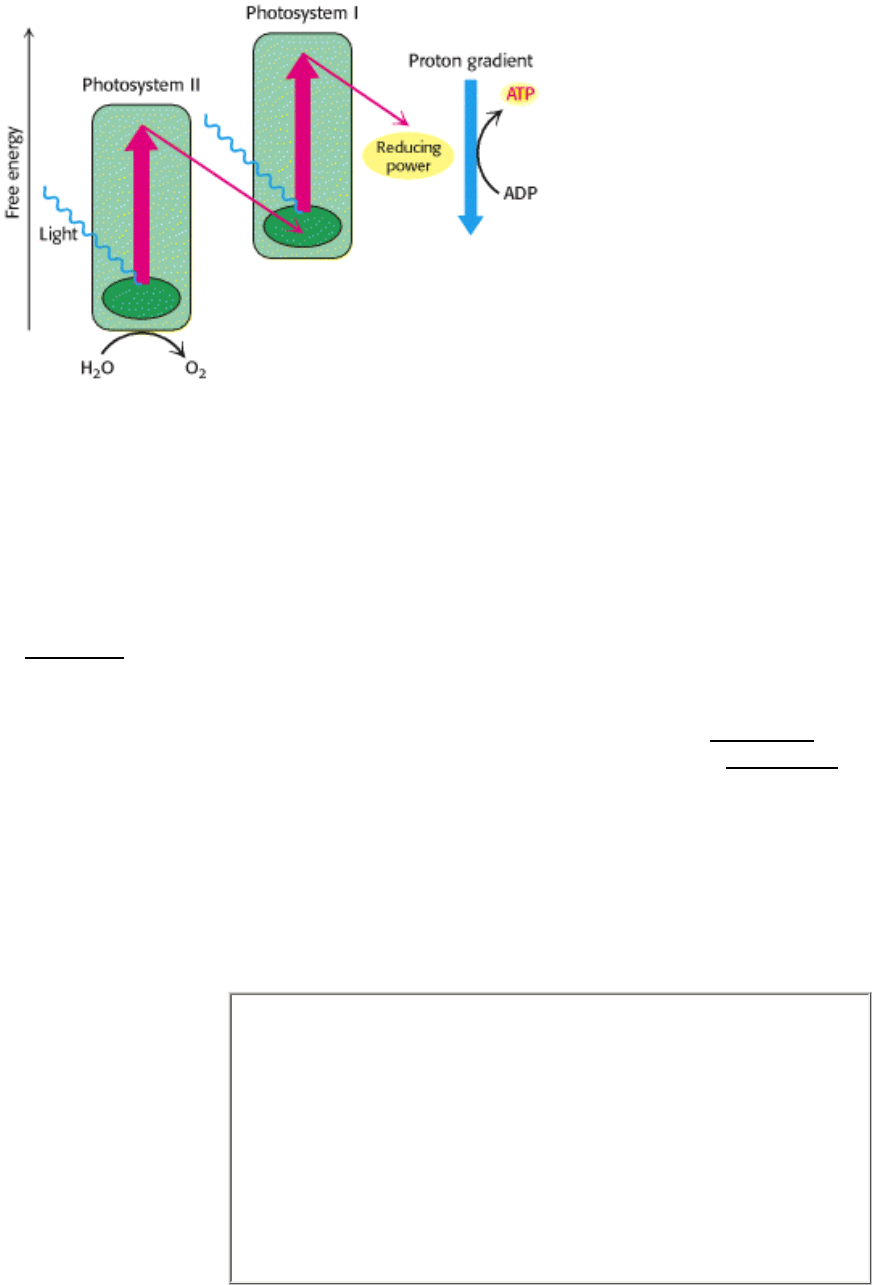
Chloroplasts (left) convert light energy into chemical energy. High-energy electrons in chloroplasts are transported
through two photosystems (right). During this transit, which culminates in the generation of reducing power, ATP is
synthesized in a manner analogous to mitochondrial ATP synthesis. Unlike as in mitochondrial electron transport,
however, electrons in chloroplasts are energized by light. [(Left) Herb Charles Ohlmeyer/Fran Heyl Associates.]
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis
19.1. Photosynthesis Takes Place in Chloroplasts
In Chapter 18, we saw that oxidative phosphorylation, the predominant means of generating ATP from fuel molecules,
was compartmentalized into mitochondria. Likewise, photosynthesis, the means of converting light into chemical energy,
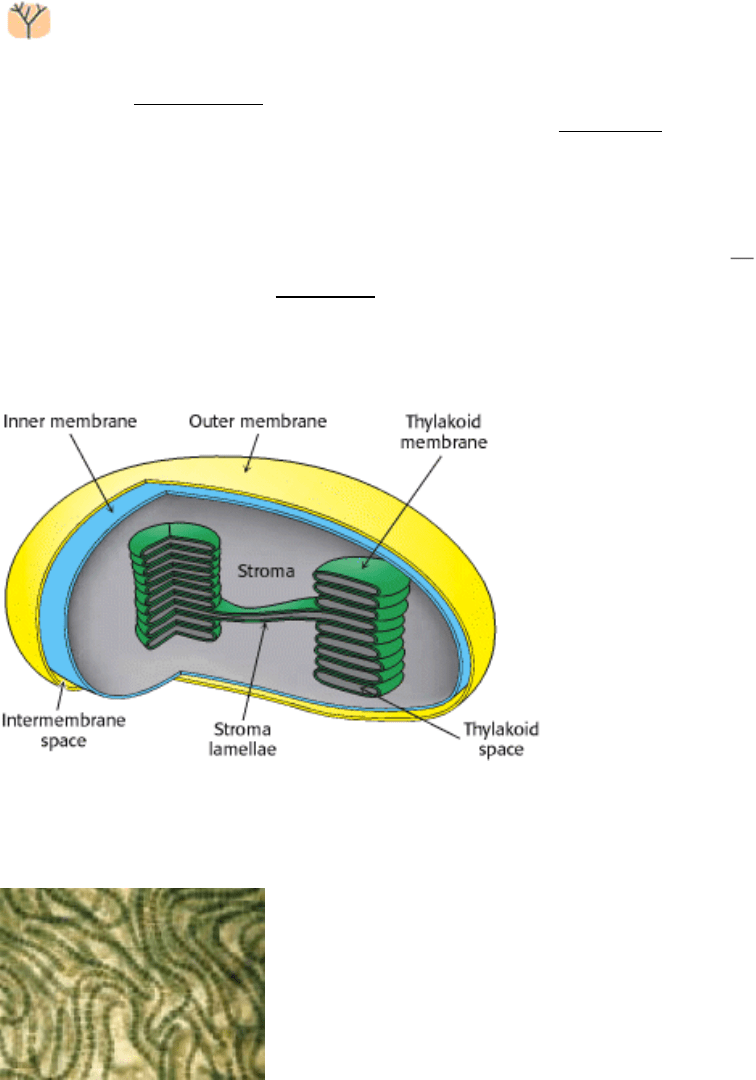
is sequestered into organelles called chloroplasts, typically 5 µm long. Like a mitochondrion, a chloroplast has an outer
membrane and an inner membrane, with an intervening intermembrane space (Figure 19.3). The inner membrane
surrounds a stroma, which is the site of the carbon chemistry of photosynthesis (Section 20.1). In the stroma are
membranous structures called thylakoids, which are flattened sacs, or discs. The thylakoid sacs are stacked to form a
granum. Different grana are linked by regions of thylakoid membrane called stroma lamellae. The thylakoid membranes
separate the thylakoid space from the stroma space. Thus, chloroplasts have three different membranes (outer, inner, and
thy-lakoid membranes) and three separate spaces (intermembrane, stroma, and thylakoid spaces). In developing
chloroplasts, thylakoids are believed to arise from invaginations of the inner membrane, and so they are analogous to the
mitochondrial cristae. Like the mitochondrial cristae, they are the site of coupled oxidation-reduction reactions that
generate the proton-motive force.
Photosynthetic catastrophe-
If photosynthesis were to cease, all higher forms of life would be
extinct in about 25 years. A milder version of such a catastrophe
ended the Cretaceous period 65.1 million years ago when a large
asteroid struck the Yucatan Peninsula of Mexico. Enough dust was
sent into the atmosphere that photosynthetic capacity was greatly
diminished, which apparently led to the disappearance of the
dinosaurs and allowed the mammals to rise to prominence.
19.1.1. The Primary Events of Photosynthesis Take Place in Thylakoid Membranes
The thylakoid membranes contain the energy-transducing machinery: light-harvesting proteins, reaction centers, electron-
transport chains, and ATP synthase. They have nearly equal amounts of lipids and proteins. The lipid composition is
highly distinctive: about 40% of the total lipids are galactolipids and 4% are sulfolipids, whereas only 10% are
phospholipids. The thylakoid membrane and the inner membrane, like the inner mitochondrial membrane, are
impermeable to most molecules and ions. The outer membrane of a chloroplast, like that of a mitochondrion, is highly
permeable to small molecules and ions. The stroma contains the soluble enzymes that utilize the NADPH and ATP
synthesized by the thylakoids to convert CO
2
into sugar. Plant leaf cells contain between 1 and 100 chloroplasts,
depending on the species, cell type, and growth conditions.
19.1.2. The Evolution of Chloroplasts
Chloroplasts contain their own DNA and the machinery for replicating and expressing it. However, chloroplasts
are not autonomous: they also contain many proteins encoded by nuclear DNA. How did the intriguing relation
between the cell and its chloroplasts develop? We now believe that, in a manner analogous to the evolution of
mitochondria (Section 18.1.2), chloroplasts are the result of endosymbiotic events in which a photosynthetic
microorganism, most likely an ancestor of a cyanobacterium (Figure 19.4), was engulfed by a eukaryotic host. Evidence
suggests that chloroplasts in higher plants and green algae are derived from a single endosymbiotic event, whereas those
in red and brown algae arose from at least one additional event.
The chloroplast genome is smaller than that of a cyanobacterium; however, like that of a cyanobacterium, it is circular
with a single start site for DNA replication, and its genes are arranged in operons
sequences of functionally related
genes under common control (Chapter 31). In the course of evolution, many of the genes of the chloroplast ancestor were
transferred to the plant cell's nucleus or, in some cases, lost entirely, thus establishing a fully dependent relation.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.1. Photosynthesis Takes Place in Chloroplasts
Figure 19.3. Diagram of a Chloroplast. [After S. L. Wolfe, Biology of the Cell, p. 130. © 1972 by Wadsworth
Publishing Company, Inc. Adapted by permission of the publisher.]
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.1. Photosynthesis Takes Place in Chloroplasts
Figure 19.4. Cyanobacteria. A colony of the photosynthetic filamentous cyanobacteria Anabaena shown at 450×
magnification. Ancestors of these bacteria are thought to have evolved into present-day chloroplasts. [James W.
Richardson/Visuals Unlimited.]

II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis
19.2. Light Absorption by Chlorophyll Induces Electron Transfer
The trapping of light energy is the key to photosynthesis. The first event is the absorption of light by a photoreceptor
molecule. The principal photoreceptor in the chloroplasts of most green plants is chlorophyll a, a substituted tetrapyrrole
(Figure 19.5). The four nitrogen atoms of the pyrroles are coordinated to a magnesium ion. Unlike a porphyrin such as
heme, chlorophyll has a reduced pyrrole ring. Another distinctive feature of chlorophyll is the presence of phytol, a
highly hydrophobic 20-carbon alcohol, esterified to an acid side chain.
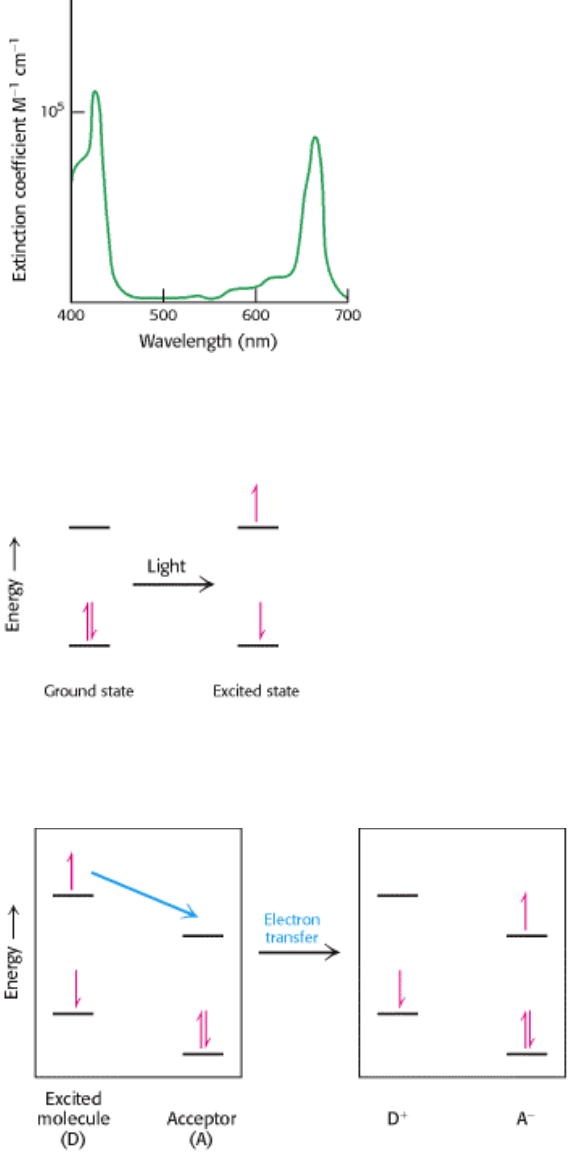
Chlorophylls are very effective photoreceptors because they contain networks of alternating single and double bonds.
Such compounds are called polyenes. They have very strong absorption bands in the visible region of the spectrum,
where the solar output reaching Earth also is maximal (Figure 19.6). The peak molar absorption coefficient (ε, Section
3.1) of chlorophyll a is higher than 10
5
M
-1
cm
-1
, among the highest observed for organic compounds.
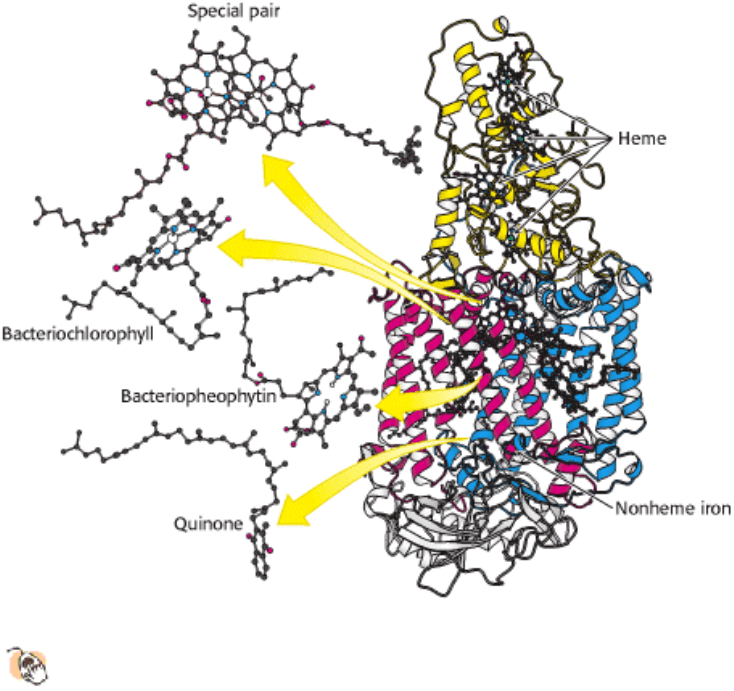
What happens when light is absorbed by a molecule such as chlorophyll? The energy from the light excites an electron
from its ground energy level to an excited energy level (Figure 19.7). This high-energy electron can have several fates.
For most compounds that absorb light, the electron simply returns to the ground state and the absorbed energy is
converted into heat. However, if a suitable electron acceptor is nearby, the excited electron can move from the initial
molecule to the acceptor (Figure 19.8). This process results in the formation of a positive charge on the initial molecule
(due to the loss of an electron) and a negative charge on the acceptor and is, hence, referred to as photoinduced charge
separation. The site where the separational change occurs is called the reaction center. We shall see how the
photosynthetic apparatus is arranged to make photoinduced charge separation extremely efficient. The electron, extracted
from its initial site by absorption of light, can reduce other species to store the light energy in chemical forms.
19.2.1. Photosynthetic Bacteria and the Photosynthetic Reaction Centers of Green
Plants Have a Common Core
Photosynthesis in green plants is mediated by two kinds of membrane-bound, light-sensitive complexes
photosystem I
(PS I) and photosystem II (PS II). Photosystem I typically includes 13 polypeptide chains, more than 60 chlorophyll
molecules, a quinone (vitamin K
1
), and three 4Fe-4S clusters. The total molecular mass is more than 800 kd.
Photosystem II is only slightly less complex with at least 10 polypeptide chains, more than 30 chlorophyll molecules, a
nonheme iron ion, and four manganese ions. Photosynthetic bacteria such as Rhodopseudomonas viridis contain a
simpler, single type of photosynthetic reaction center, the structure of which was revealed at atomic resolution. The
bacterial reaction center consists of four polypeptides: L (31 kd), M (36 kd), and H (28 kd) subunits and C, a c-type
cytochrome (Figure 19.9). The results of sequence comparisons and low-resolution structural studies of photosystems I
and II revealed that the bacterial reaction center is homologous to the more complex plant systems. Thus, we begin our
consideration of the mechanisms of the light reactions within the bacterial photosynthetic reaction center, with the
understanding that many of our observations will apply to the plant systems as well.
19.2.2. A Special Pair of Chlorophylls Initiates Charge Separation
The L and M subunits form the structural and functional core of the bacterial photosynthetic reaction center (see Figure
19.9). Each of these homologous subunits contains five transmembrane helices. The H subunit, which has only one
transmembrane helix, lies on the cytoplasmic side of the membrane. The cytochrome subunit, which contains four c-type
hemes, lies on the opposite periplasmic side. Four bacteriochlorophyll b (BChl-b) molecules, two bacteriopheophytin b
(BPh) molecules, two quinones (Q
A
and Q
B
), and a ferrous ion are associated with the L and M subunits.
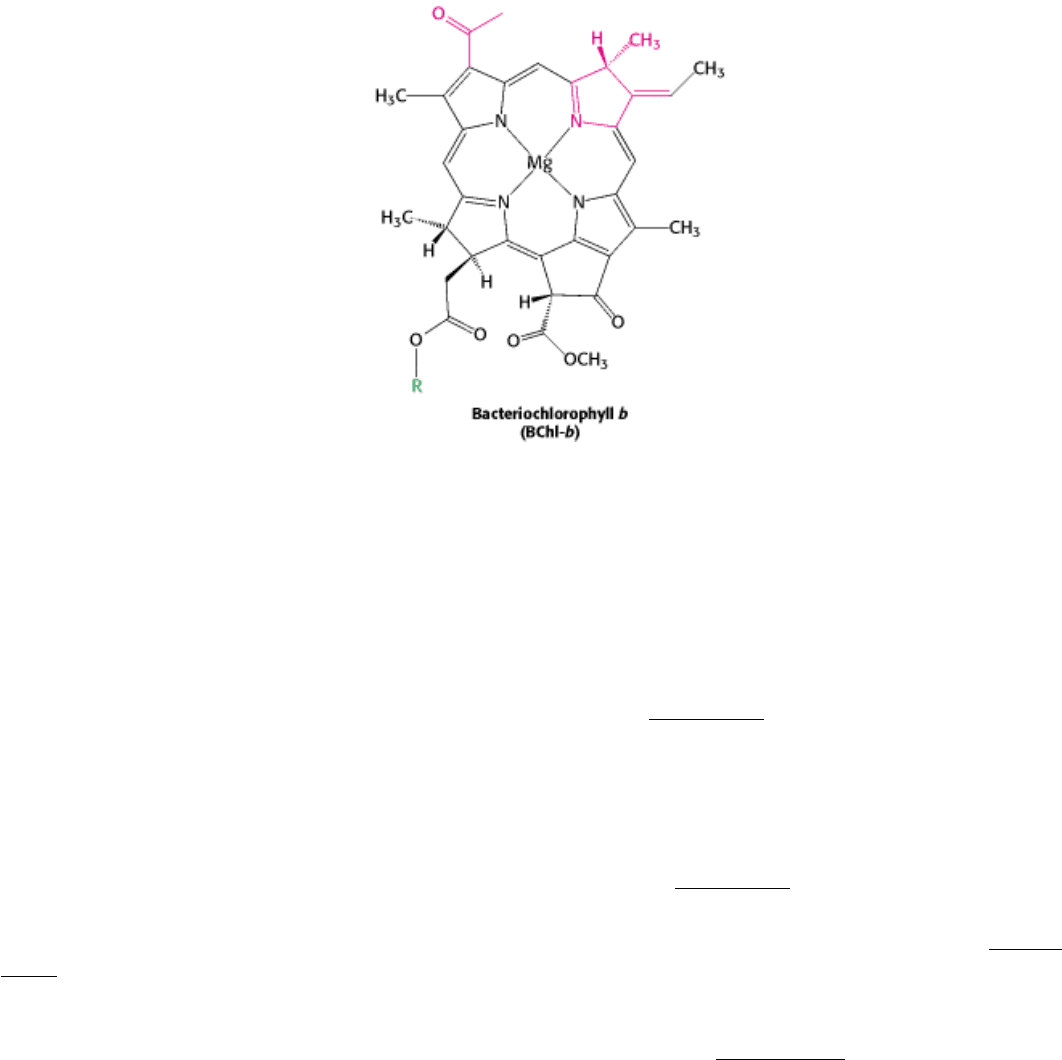
Bacteriochlorophylls are similar to chlorophylls, except for the reduction of an additional pyrrole ring and some other
minor differences that shift their absorption maxima to the near infrared, to wavelengths as long as 1000 nm.
Bacteriopheophytin is the term for a bacteriochlorophyll that has two protons instead of a magnesium ion at its center.
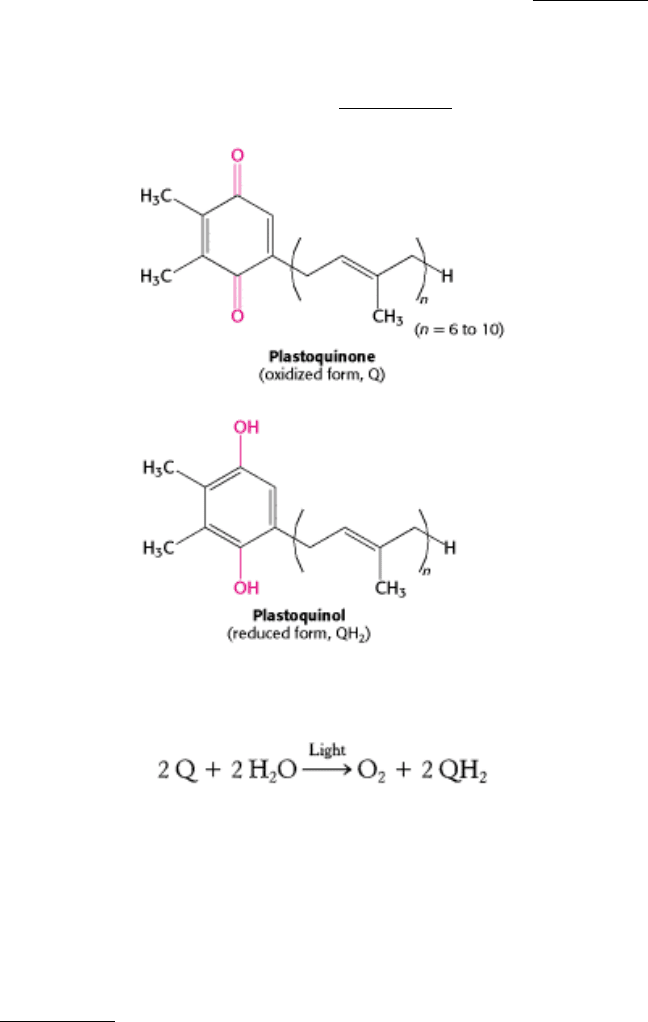
The reaction begins with light absorption by a dimer of BChl-b molecules that lie near the periplasmic side of the
membrane. This dimer, called a special pair because of its fundamental role in photosynthesis, absorbs light maximally
at 960 nm, in the infrared near the edge of the visible region. For this reason, the special pair is often referred to as P960
(P stands for pigment). Excitation of the special pair leads to the ejection of an electron, which is transferred through
another molecule of BChl-b to the bacteriopheophytin in the L subunit (Figure 19.10, steps 1 and 2). This initial charge
separation, which yields a positive charge on the special pair (P960
+
) and a negative charge on BPh, occurs in less than
10 picoseconds (10
-11
seconds). Interestingly, only one of the two possible paths within the nearly symmetric L-M dimer
is utilized. In their high-energy states, P960
+
and BPh
-
could undergo charge recombination; that is, the electron on BPh
-
could move back to neutralize the positive charge on the special pair. Its return to the special pair would waste a valuable
high-energy electron and simply convert the absorbed light energy into heat. Three factors in the structure of the reaction
center work together to suppress charge recombination nearly completely (Figure 19.10, steps 3 and 4). First, another
electron acceptor, a tightly bound quinone (Q
A
), is less than 10 Å away from BPh
-
, and so the electron is rapidly
transferred farther away from the special pair. Recall that electron-transfer rates depend strongly on distance (Section
18.2.3). Second, one of the hemes of the cytochrome subunit is less than 10 Å away from the special pair, and so the
positive charge is neutralized by the transfer of an electron from the reduced cytochrome. Finally, the electron transfer
from BPh
-
to the positively charged special pair is especially slow: the transfer is so thermodynamically favorable that it
takes place in the inverted region where electron-transfer rates become slower (Section 18.2.3). Thus, electron transfer
proceeds efficiently from BPh
-
to Q
A
.
From Q
A
, the electron moves to a more loosely associated quinone, Q
B
. The absorption of a second photon and the
movement of a second electron down the path from the special pair completes the two-electron reduction of Q
B
from Q
to QH
2
. Because the Q
B
-binding site lies near the cytoplasmic side of the membrane, two protons are taken up from the
cytoplasm, contributing to the development of a proton gradient across the cell membrane (Figure 19.10, steps 5, 6, and
7).
How does the cytochrome subunit of the reaction center regain an electron to complete the cycle? The reduced quinone
(QH
2
) is reoxidized to Q by complex III of the respiratory electron-transport chain (Section 18.3.3). The electrons from
the reduced quinone are transferred through a soluble cytochrome c intermediate, called cytochrome c
2
, in the periplasm
to the cytochrome subunit of the reaction center. The flow of electrons is thus cyclic. The proton gradient generated in
the course of this cycle drives the generation of ATP through the action of ATP synthase.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.2. Light Absorption by Chlorophyll Induces Electron Transfer
Figure 19.5. Chlorophyll. Like heme, chlorophyll a is a cyclic tetrapyrrole. One of the pyrrole rings (shown in red) is
reduced. A phytol chain (shown in green) is connected by an ester linkage. Magnesium ion binds at the center of the
structure.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.2. Light Absorption by Chlorophyll Induces Electron Transfer
Figure 19.6. Light Absorption By Chlorophyll A. Chlorophyll a absorbs visible light efficiently as judged by the
extinction coefficients near 10
5
M
-1
cm
-1
.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.2. Light Absorption by Chlorophyll Induces Electron Transfer
Figure 19.7. Light Absorption. The absorption of light leads to the excitation of an electron from its ground state to a
higher energy level.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.2. Light Absorption by Chlorophyll Induces Electron Transfer
Figure 19.8. Photoinduced Charge Separation. If a suitable electron acceptor is nearby, an electron that has been
moved to a high energy level by light absorption can move from the excited molecule to the acceptor.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.2. Light Absorption by Chlorophyll Induces Electron Transfer
Figure 19.9. Bacterial Photosynthetic Reaction Center.
The core of the reaction center from Rhodopseudomonas
viridis consists of two similar chains: L (red) and M (blue). An H chain (white) and a cytochrome subunit (yellow)
complete the structure. A chain of prosthetic groups, beginning with a special pair of bacteriochlorophylls and
ending at a bound quinone, runs through the structure from top to bottom in this view.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.2. Light Absorption by Chlorophyll Induces Electron Transfer
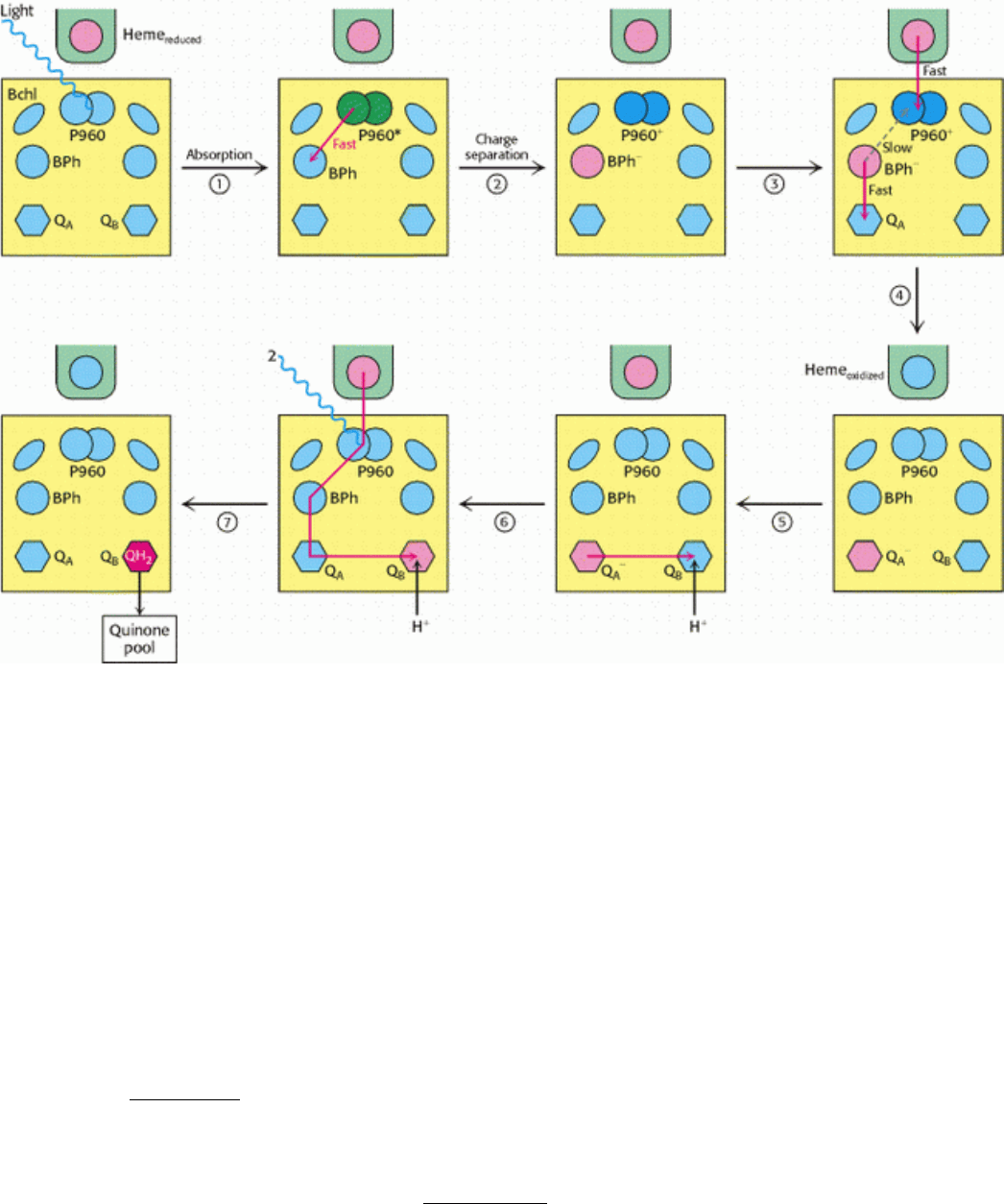
Figure 19.10. Electron Chain in the Photosynthetic Bacterial Reaction Center. The absorption of light by the special
pair (P960) results in the rapid transfer of an electron from this site to a bacteriopheophytin (BPh), creating a
photoinduced charge separation (steps 1 and 2). (The asterisk on P960 stands for excited state.) The possible return of the
electron from the pheophytin to the oxidized special pair is suppressed by the "hole" in the special pair being refilled
with an electron from the cytochrome subunit and the electron from the pheophytin being transferred to a quinone (Q
A
)
that is farther away from the special pair (steps 3 and 4). The reduction of a quinone (Q
B
) on the periplasmic side of the
membrane results in the uptake of two protons from the periplasmic space (steps 5 and 6). The reduced quinone can
move into the quinone pool in the membrane (step 7).
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis
19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic
Photosynthesis
Photosynthesis by oxygen-evolving organisms depends on the interplay of two photosystems, linked by common
intermediates (Figure 19.11). These two systems were discovered because of slight differences in the wavelengths of
light to which they respond. Photosystem I responds to light with wavelengths shorter than 700 nm, whereas
photosystem II responds to wavelengths shorter than 680 nm. Under normal conditions, electrons flow first through
photosystem II, then through cytochrome bf, a membrane-bound complex homologous to Q-cytochrome c
oxidoreductase from oxidative phosphorylation (Section 18.3.3), and then through photosystem I. The electrons are
derived from water: two molecules of H
2
O are oxidized to generate a molecule of O
2
for every four electrons sent
through this electron-transport chain. The electrons end up reducing NADP
+
to NADPH, a versatile reagent for driving
biosynthetic processes. These processes generate a proton gradient across the thylakoid membrane that drives the
formation of ATP.
19.3.1. Photosystem II Transfers Electrons from Water to Plastoquinone and Generates
a Proton Gradient
Photosystem II of green plants is reasonably similar to the bacterial reaction center (Figure 19.12). The core of
photosystem II is formed by D1 and D2, a pair of similar 32-kd subunits that span the thylakoid membrane. These
subunits are homologous to the L and M chains of the bacterial reaction center. Unlike the bacterial system, photosystem
II contains a large number of additional subunits that bind additional chlorophylls and increase the efficiency with which
light energy is absorbed and transferred to the reaction center (Section 19.5).
The overall reaction catalyzed by photosystem II is:
in which Q represents plastoquinone and QH
2
represents plastoquinol. This reaction is similar to one catalyzed by the
bacterial system in that a quinone is converted from its oxidized into its reduced form. However, instead of obtaining the
electrons for this reduction from a reduced cytochrome c molecule, photosystem II extracts the electrons from water,
generating molecular oxygen. This remarkable reaction takes place at a special center containing four manganese ions.
The photochemistry of photosystem II begins with excitation of a special pair of chlorophyll molecules that are bound by
the D1 and D2 subunits (Figure 19.13). This pair of molecules is analogous to the special pair in the bacterial reaction
center, but it absorbs light at shorter wavelengths (maximum absorbance at 680 nm) because it consists of chlorophyll a
molecules rather than bacteriochlorophyll. The special pair is often called P680. On excitation, P680 rapidly transfers an
electron to a nearby pheophytin (chlorophyll with two H
+
ions in place of the central Mg
2+
ion). From there, the electron
is transferred first to a tightly bound plastoquinone at site Q
A
and then to an exchangeable plastoquinone at site Q
B
. This
electron flow is entirely analogous to that in the bacterial system. With the arrival of a second electron and the uptake of
two protons, the exchangeable plastoquinone is reduced to QH
2
.
The major difference between the bacterial system and photosystem II is the source of the electrons that are used to
neutralize the positive charge formed on the special pair. P680
+
, a very strong oxidant, extracts electrons from water
molecules bound at the manganese center. The structure of this center, which includes four manganese ions, a calcium
ion, a chloride ion, and a tyrosine residue that forms a radical, has not been fully established, although the results of
extensive spectroscopic studies and a recent X-ray crystallographic study at moderate resolution have provided many
constraints. Manganese was apparently evolutionarily selected for this role because of its ability to exist in multiple
oxidation states (Mn
2+
, Mn
3+
, Mn
4+
, Mn
5+
) and to form strong bonds with oxygen-containing species. The manganese
center, in its reduced form, oxidizes two molecules of water to form a single molecule of oxygen. Each time the
absorbance of a photon kicks an electron out of P680, the positively charged special pair extracts an electron from the
manganese center (Figure 19.14). Thus four photochemical steps are required to extract the electrons and reduce the
manganese center (Figure 19.15). The four electrons harvested from water are used to reduce two molecules of Q to QH
2
.
Photosystem II spans the thylakoid membrane such that the site of quinone reduction is on the side of the stroma,
whereas the manganese center and, hence, the site of water oxidation lies in the thylakoid lumen. Thus, the two protons
that are taken up with the reduction of each molecule of plastoquinone come from the stroma, and the four protons that
are liberated in the course of water oxidation are released into the lumen. This distribution of protons generates a proton
gradient across the thylakoid membrane characterized by an excess of protons in the thylakoid lumen compared with the
stroma (Figure 19.16). Thus, the direction of the proton gradient is the reverse of that generated during oxidative
phosphorylation, which depletes the mitochondrial matrix of protons. As we shall see, this difference is consistent with
the reversed orientations of other membrane proteins, including ATP synthase.
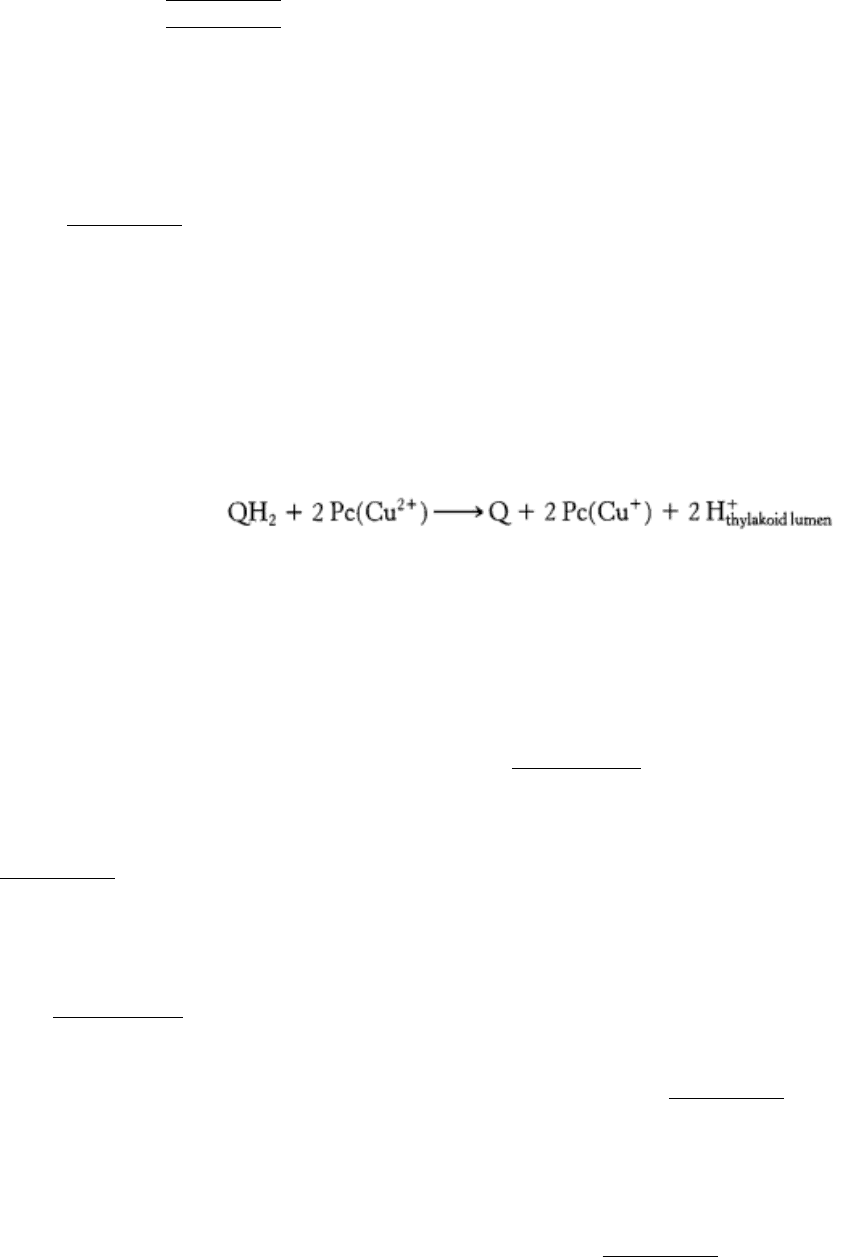
19.3.2. Cytochrome bf Links Photosystem II to Photosystem I
The plastoquinol (QH
2
) produced by photosystem II contributes its electrons to continue the electron chain that
terminates at photosystem I. These electrons are transferred, one at a time, to plastocyanin (Pc), a copper protein in the
thylakoid lumen.
The two protons from plastoquinol are released into the thylakoid lumen. This reaction is reminiscent of that catalyzed
by ubiquinol cytochrome c oxidoreductase in oxidative phosphorylation. Indeed, most components of the enzyme
complex that catalyzes the reaction, the cytochrome bf complex, are homologous to those of ubiquinol cytochrome c
oxidoreductase. The cytochrome bf complex includes four subunits: a 23-kd cytochrome with two b-type hemes, a 20-kd
Rieske-type Fe-S protein, a 33-kd cytochrome f with a c-type cytochrome, and a 17-kd chain.
This complex catalyzes the reaction through the Q cycle (Section 18.3.4). In the first half of the Q cycle, plastoquinol is
oxidized to plastoquinone, one electron at a time. The electrons from plastoquinol flow through the Fe-S protein to
convert oxidized plastocyanin into its reduced form. Plastocyanin is a small, soluble protein with a single copper ion
bound by a cysteine residue, two histidine residues, and a methionine residue in a distorted tetrahedral arrangement
(Figure 19.17). This geometry facilitates the interconversion between the Cu
2+
and the Cu
+
states and sets the reduction
potential at an appropriate value relative to that of plastoquinol. Plastocyanin is intensely blue in color in its oxidized
form, marking it as a member of the "blue copper protein," or type I copper protein family.
The oxidation of plastoquinol results in the release of two protons into the thylakoid lumen. In the second half of the Q
cycle (Section 18.3.4), cytochrome bf reduces a second molecule of plastoquinone from the Q pool to plastoquinol,
taking up two protons from one side of the membrane, and then reoxidizes plastoquinol to release these protons on the
other side. The enzyme is oriented so that protons are released into the thylakoid lumen and taken up from the stroma,
contributing further to the proton gradient across the thylakoid membrane (Figure 19.18).
19.3.3. Photosystem I Uses Light Energy to Generate Reduced Ferredoxin, a Powerful
Reductant
The final stage of the light reactions is catalyzed by photosystem I (Figure 19.19). The core of this system is a pair of
similar subunits psaA (83 kd) and psaB (82 kd). These subunits are quite a bit larger than the core subunits of
photosystem II and the bacterial reaction center. Nonetheless, they appear to be homologous; the terminal 40% of each
subunit is similar to a corresponding subunit of photosytem II. A special pair of chlorophyll a molecules lies at the center
