Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


L, or loose, conformation. This conformation binds ADP and P
i
. It, too, is sufficiently constrained that it cannot release
bound nucleotides. The final subunit will be in the O, or open, form. This form can exist with a bound nucleotide in a
structure that is similar to those of the T and L forms, but it can also convert to form a more open conformation and
release a bound nucleotide (Figure 18.31). This structure, with one of the three β subunits in an open, nucleotide-free
state, as well as one with one of the β subunits in a nucleotide-bound O conformation, have been observed
crystallographically.
The interconversion of these three forms can be driven by rotation of the γ subunit (Figure 18.32). Suppose the γ subunit
is rotated 120 degrees in a counterclockwise direction (as viewed from the top). This rotation will change the subunit in
the T conformation into the O conformation, allowing the subunit to release the ATP that has been formed within it. The
subunit in the L conformation will be converted into the T conformation, allowing the transition of bound ADP + P
i
into
ATP. Finally, the subunit in the O conformation will be converted into the L conformation, trapping the bound ADP and
P
i
so that they cannot escape. The binding of ADP and P
i
to the subunit now in the O conformation completes the cycle.
This mechanism suggests that ATP can be synthesized by driving the rotation of the γ subunit in the appropriate
direction. Likewise, this mechanism suggests that the hydrolysis of ATP by the enzyme should drive the rotation of the γ
subunit in the opposite direction.
18.4.3. The World's Smallest Molecular Motor: Rotational Catalysis
Is it possible to observe the proposed rotation directly? Elegant experiments were performed with the use of a simple
experimental system consisting of cloned α
3
β
3
γ subunits only (Figure 18.33). The β subunits were engineered to
contain amino-terminal polyhistidine tags, which have a high affinity for nickel ions. This property of the tags allowed
the α
3
β
3
assembly to be immobilized on a glass surface that had been coated with nickel ions. The γ subunit was linked
to a fluorescently labeled actin filament to provide a long segment that could be observed under a fluorescence
microscope. Remarkably, the addition of ATP caused the actin filament to rotate unidirectionally in a counterclockwise
direction. The γ subunit was rotating, being driven by the hydrolysis of ATP. Thus, the catalytic activity of an individual
molecule could be observed. The counterclockwise rotation is consistent with the predicted mechanism for hydrolysis
because the molecule was viewed from below relative to the view shown in Figure 18.32.
More detailed analysis in the presence of lower concentrations of ATP revealed that the γ subunit rotates in 120-degree
increments, with each step corresponding to the hydrolysis of a single ATP molecule. In addition, from the results
obtained by varying the length of the actin filament and mea-suring the rate of rotation, the enzyme appears to operate
near 100% efficiency; that is, essentially all of the energy released by ATP hydrolysis is converted into rotational
motion.
18.4.4. Proton Flow Around the c Ring Powers ATP Synthesis
The direct observation of rotary motion of the γ subunit is strong evidence for the rotational mechanism for ATP
synthesis. The last remaining question is: How does proton flow through F
0
drive the rotation of the γ subunit? Howard
Berg and George Oster proposed an elegant mechanism that provides a clear answer to this question. The mechanism
depends on the structures of the a and c subunits of F
0
(Figure 18.34). The structure of the c subunit was determined both
by NMR methods and by x-ray crystallography. Each polypeptide chain forms a pair of α helices that span the
membrane. An aspartic acid residue (Asp 61) is found in the middle of the second helix. When Asp 61 is in contact with
the hydrophobic part of the membrane, the residue must be in the neutral aspartic acid form, rather than in the charged,
aspartate form. From 9 to 12 c subunits assemble into a symmetric membrane-spanning ring. Although the structure of
the a subunit has not yet been experimentally determined, a variety of evidence is consistent with a structure that
includes two proton half-channels that do not span the membrane (see Figure 18.34). Thus, protons can pass into either
of these channels, but they cannot move completely across the membrane. The a subunit directly abuts the ring
comprising the c subunits, with each half-channel directly interacting with one c subunit.

With this structure in mind, we can see how a proton gradient can drive rotation of the c ring. Suppose that the Asp 61
residues of the two c subunits that are in contact with a half-channel have given up their protons so that they are in the
charged aspartate form (Figure 18.35), which is possible because they are in relatively hydrophilic environments inside
the half-channel. The c ring cannot rotate in either direction, because such a rotation would move a charged aspartate
residue into the hydrophobic part of the membrane. A proton can move through either half-channel to protonate one of
the aspartate residues. However, it is much more likely to pass through the channel that is connected to the cytosolic side
of the membrane because the proton concentration is more than 25 times as high on this side as on the matrix side, owing
to the action of the electron-transport-chain proteins. The entry of protons into the cytosolic half-channel is further
facilitated by the membrane potential of +0.14 V (positive on the cytoplasmic side), which increases the concentration of
protons near the mouth of the cytosolic half-channel. If the aspartate residue is protonated to its neutral form, the c ring
can now rotate, but only in a clockwise direction. Such a rotation moves the newly protonated aspartic acid residue into
contact with the membrane, moves the charged aspartate residue from contact with the matrix half-channel to the
cytosolic half-channel, and moves a different protonated aspartic acid residue from contact with the membrane to the
matrix half-channel. The proton can then dissociate from aspartic acid and move through the half-channel into the proton-
poor matrix to restore the initial state. This dissociation is favored by the positive charge on a conserved arginine residue
(Arg 210) in the a subunit. Thus, the difference in proton concentration and potential on the two sides of the membrane
leads to different probabilities of protonation through the two half-channels, which yields directional rotational motion.
Each proton moves through the membrane by riding around on the rotating c ring to exit through the matrix half-channel
(Figure 18.36).
The c ring is tightly linked to the γ and ε subunits. Thus, as the c ring turns, these subunits are turned inside the α
3
β
3
hexamer unit of F
1
. The exterior column formed by the two b chains and the δ subunit prevent the α
3
β
3
hexamer from
rotating. Thus, the proton-gradient-driven rotation of the c ring drives the rotation of the γ subunit, which in turn
promotes the synthesis of ATP through the binding-change mechanism. Recall that the number of c subunits in the c ring
appears to range between 10 and 14. This number is significant because it determines the number of protons that must be
transported to generate a molecule of ATP. Each 360-degree rotation of the γ subunit leads to the synthesis and release of
three molecules of ATP. Thus, if there are 10 c subunits in the ring (as was observed in a crystal structure of yeast
mitochondrial ATP synthase), each ATP generated requires the transport of 10/3 = 3.33 protons. For simplicity, we will
assume that 3 protons must flow into the matrix for each ATP formed, but we must keep in mind that the true value may
differ.
18.4.5. ATP Synthase and G Proteins Have Several Common Features
The α and β subunits of ATP synthase are members of the P-loop NTPase family of proteins. In Chapter 15, we
learned that the signaling properties of other members of this family, the G proteins, depend on their ability to
bind nucleoside triphosphates and nucleoside diphosphates with great kinetic tenacity. They do not exchange nucleotides
unless they are stimulated to do so by interaction with other proteins. The binding-change mechanism of ATP synthase is
a variation on this theme. The three different faces of the γ subunit of ATP synthase interact with the P-loop regions of
the β subunits to favor the structures of either the NDP- or NTP-binding forms or to facilitate nucleotide release. The
conformational changes take place in an orderly way, driven by the rotation of the γ subunit.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.25. Chemiosmotic Hypothesis. Electron transfer through the respiratory chain leads to the pumping of
protons from the matrix to the cytosolic side of the inner mitochondrial membrane. The pH gradient and membrane
potential constitute a proton-motive force that is used to drive ATP synthesis.
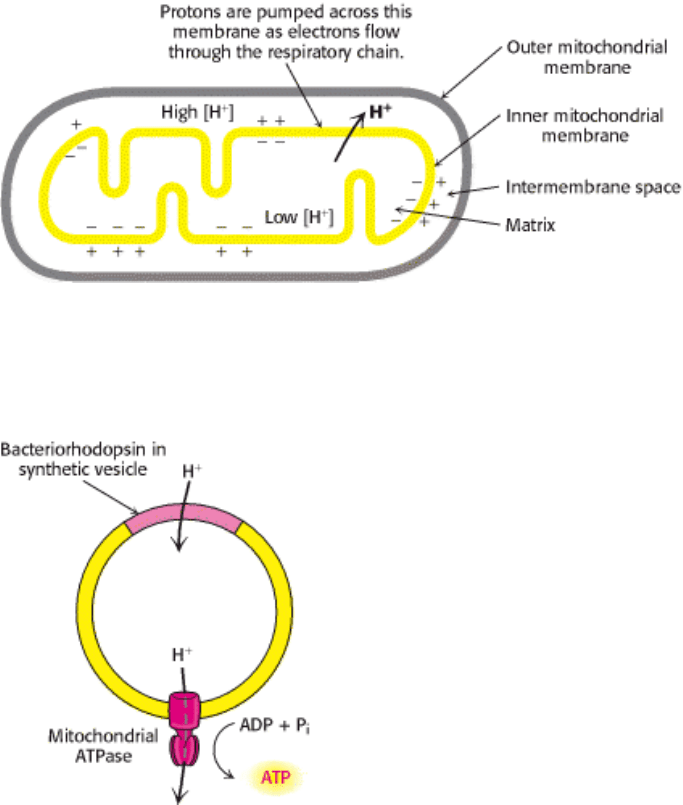
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.26. Testing the Chemiosmotic Hypothesis. ATP is synthesized when reconstituted membrane vesicles
containing bacteriorhodopsin (a light-driven proton pump) and ATP synthase are illuminated. The orientation of ATP
synthase in this reconstituted membrane is the reverse of that in the mitochondrion.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
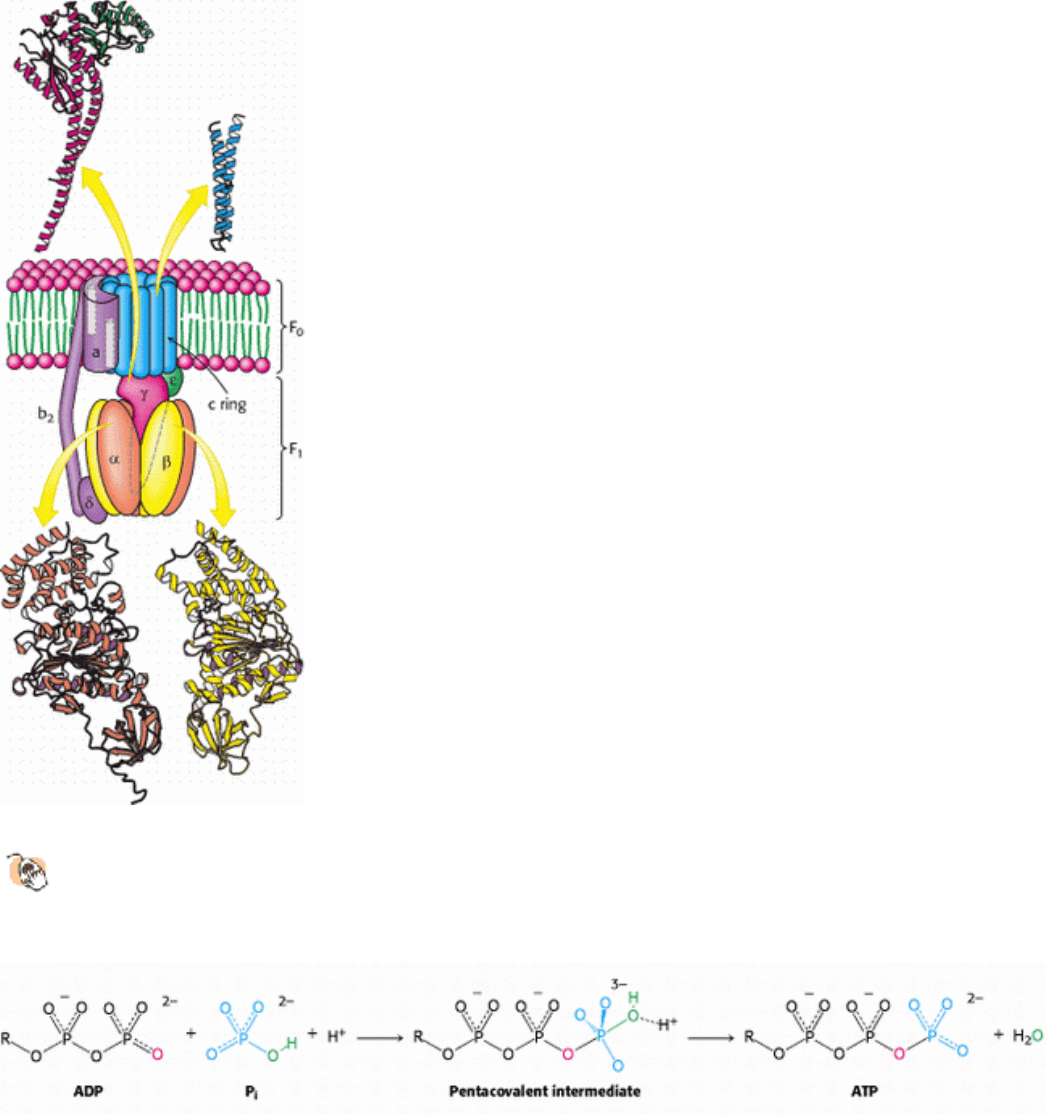
Figure 18.27. Structure of ATP Synthase.
A schematic structure is shown along with detailed structures of the
components for which structures have been determined to high resolution. The P-loop NTPase domains of the α
and β subunits are indicated by purple shading.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.28. ATP Synthesis Mechanism. One of the oxygen atoms of ADP attacks the phosphorus atom of P
i
to form
a pentacovalent intermediate, which then forms ATP and releases a molecule of H
2
O.
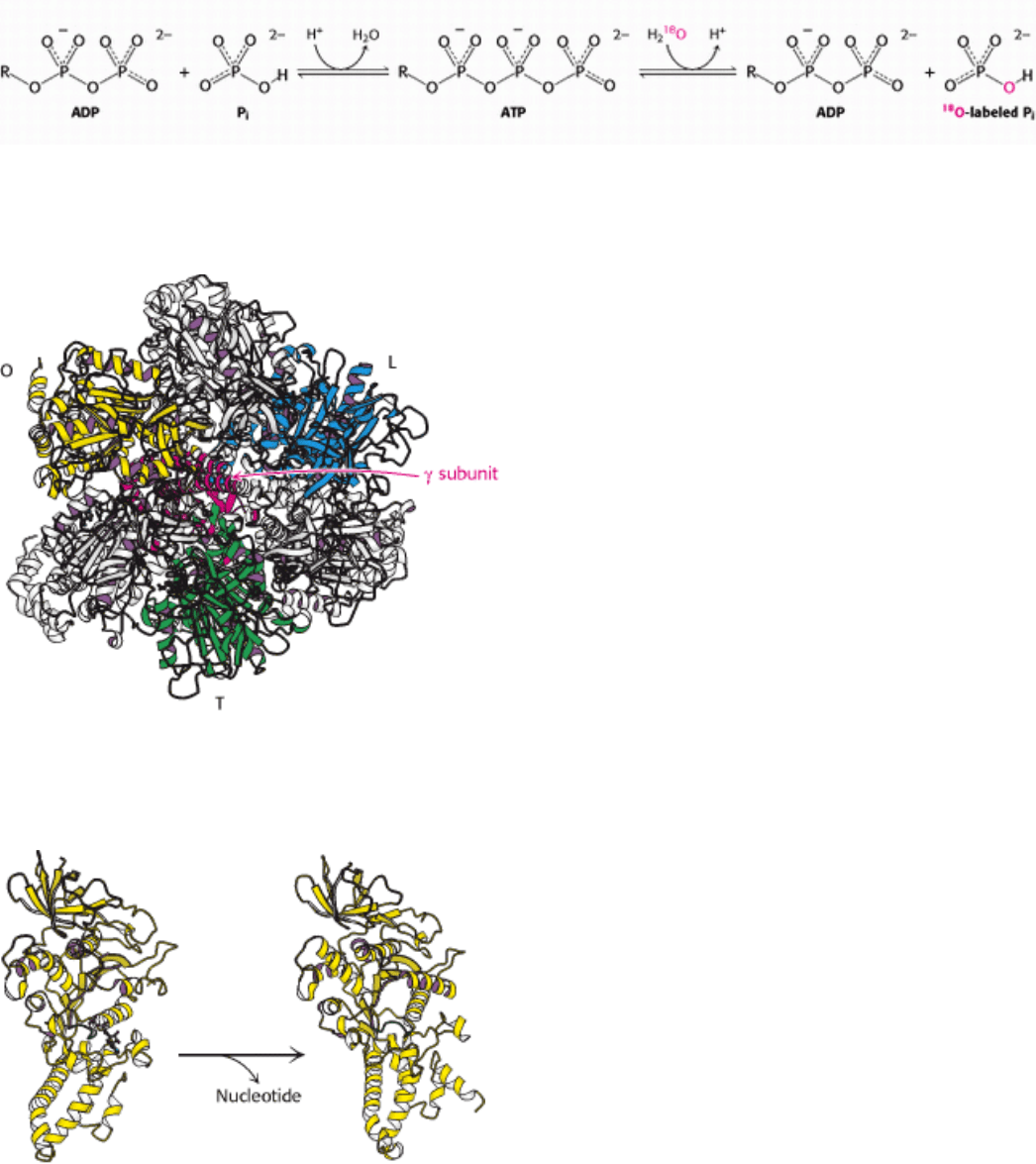
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.29. ATP Forms Without a Proton-Motive Force But Is Not Released. The results of isotope-exchange
experiments indicate that enzyme-bound ATP is formed from ADP and P
i
in the absence of a proton-motive force.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.30. ATP Synthase Nucleotide-Binding Sites Are Not Equivalent. The γ subunit passes through the center
of the α
3
β
3
hexamer and makes the nucleotide-binding sites in the β subunits distinct from one another.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.31. ATP Release From the β subunit in the open form. Unlike the tight and loose forms, the open form of
the β subunit can change conformation sufficiently to release bound nucleotides.
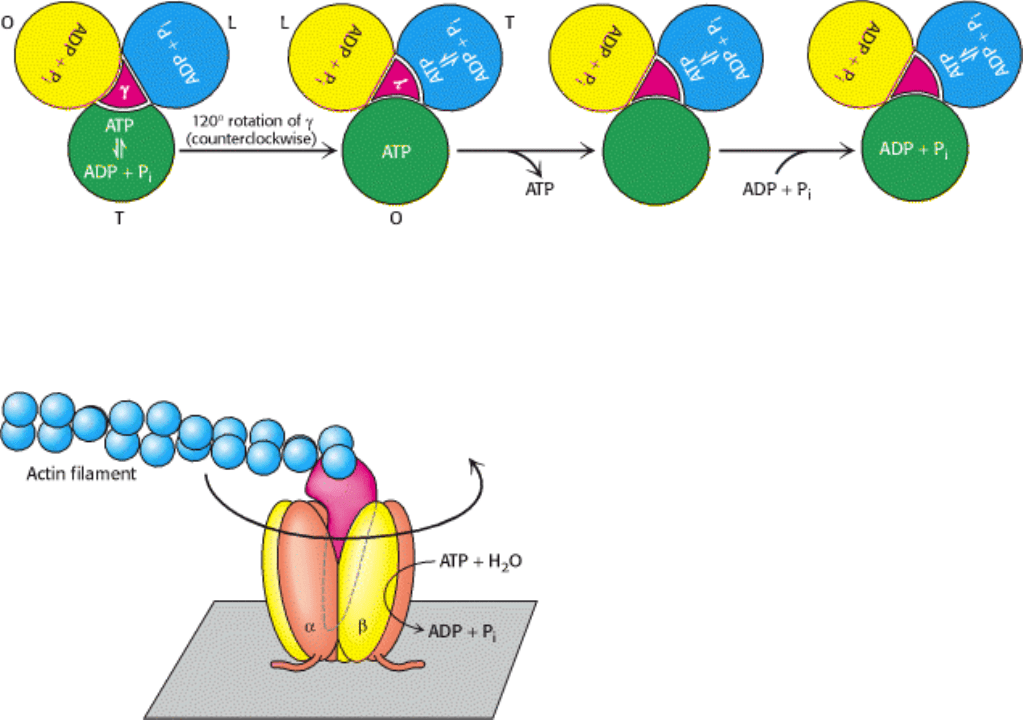
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.32. Binding-Change Mechanism for ATP Synthase. The rotation of the γ subunit interconverts the three β
subunits. The subunit in the T (tight) form, which contains newly synthesized ATP that cannot be released, is converted
into the O (open) form. In this form, it can release ATP and then bind ADP and P
i
to begin a new cycle.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.33. Direct Observation of ATP-Driven Rotation in ATP Synthase. The α
3
β
3
hexamer of ATP synthase
is fixed to a surface, with the γ subunit projecting upward and linked to a fluorescently labeled actin filament. The
addition and subsequent hydrolysis of ATP result in the counterclockwise rotation of the γ subunit, which can be directly
seen under a fluorescence microscope.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
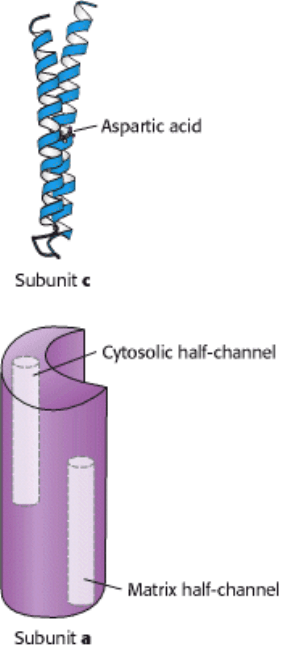
Figure 18.34. Components of the Proton-Conducting Unit of ATP Synthase. The c subunit consists of two α helices
that span the membrane. An aspartic acid residue in the second helix lies on the center of the membrane. The structure of
the a subunit has not yet been directly observed, but it appears to include two half-channels that allow protons to enter
and pass partway but not completely through the membrane.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
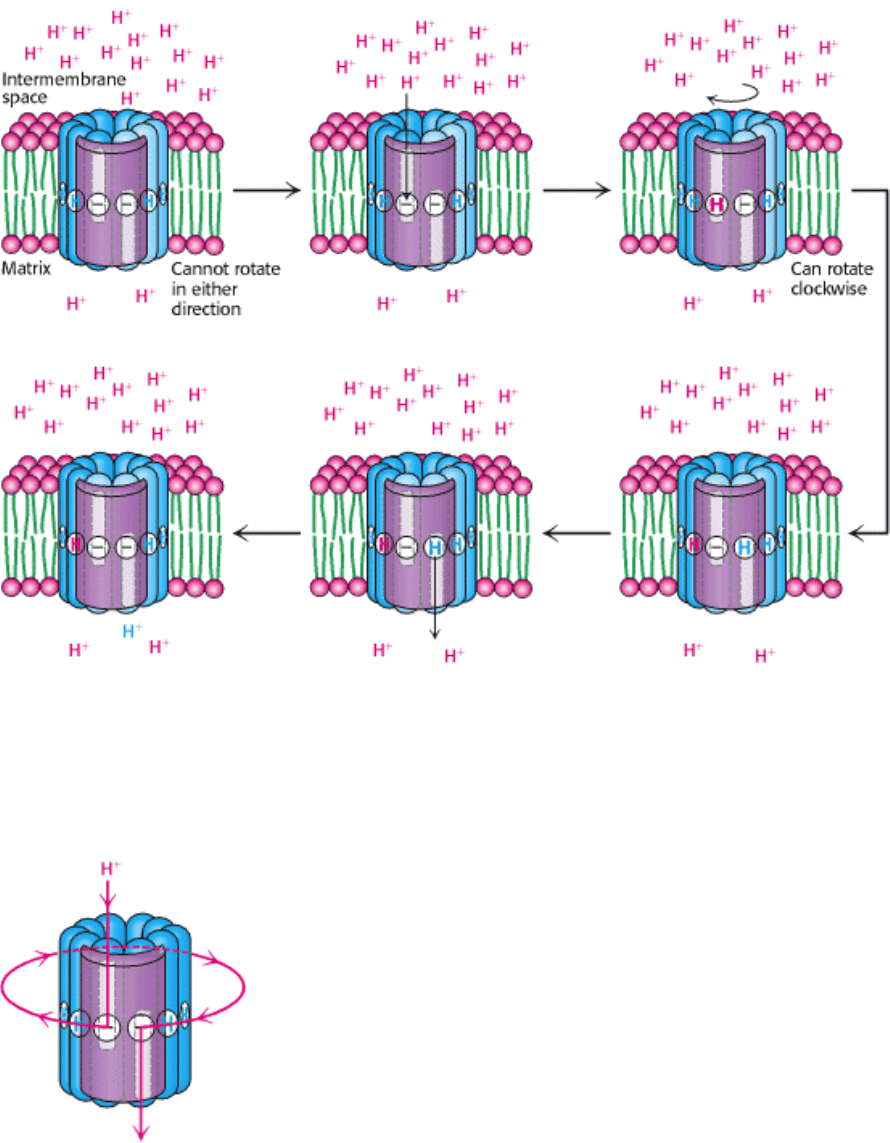
Figure 18.35. Proton Motion Across the Membrane Drives Rotation of the C Ring. A proton enters from the
intermembrane space into the cytosolic half-channel to neutralize the charge on an aspartate residue in a c subunit. With
this charge neutralized, the c ring can rotate clockwise by one c subunit, moving an aspartic acid residue out of the
membrane into the matrix half-channel. This proton can move into the matrix, resetting the system to its initial state.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation 18.4. A Proton Gradient Powers the Synthesis of ATP
Figure 18.36. Proton Path Through the Membrane. Each proton enters the cytosolic half-channel, follows a complete
rotation of the c ring, and exits through the other half-channel into the matrix.
II. Transducing and Storing Energy 18. Oxidative Phosphorylation
18.5. Many Shuttles Allow Movement Across the Mitochondrial Membranes
The inner mitochondrial membrane must be impermeable to most molecules, yet much exchange has to take place
between the cytosol and the mitochondria. This exchange is mediated by an array of membrane-spanning transporter
proteins (Section 13.4).
18.5.1. Electrons from Cytosolic NADH Enter Mitochondria by Shuttles
Recall that the glycolytic pathway generates NADH in the cytosol in the oxidation of glyceraldehyde 3-phosphate, and
NAD
+
must be regenerated for glycolysis to continue. How is cytosolic NADH reoxidized under aerobic conditions?
NADH cannot simply pass into mitochondria for oxidation by the respiratory chain, because the inner mitochondrial
membrane is impermeable to NADH and NAD
+
. The solution is that electrons from NADH, rather than NADH itself, are
carried across the mitochondrial membrane. One of several means of introducing electrons from NADH into the electron
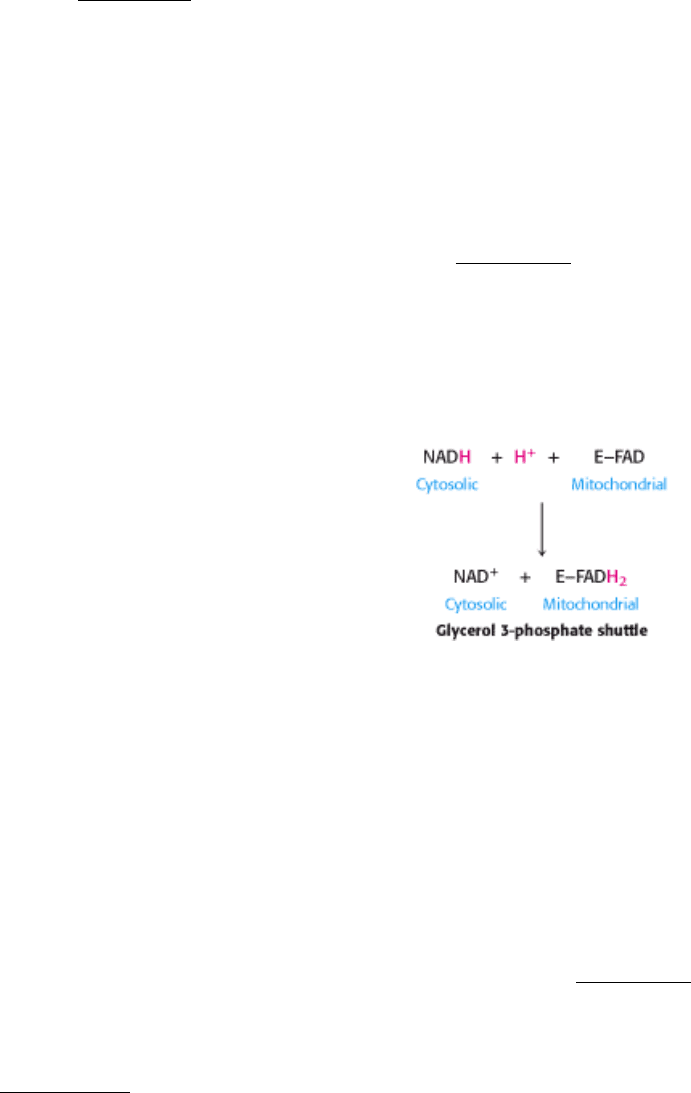
transport chain is the glycerol 3-phosphate shuttle (Figure 18.37). The first step in this shuttle is the transfer of a pair of
electrons from NADH to dihydroxyacetone phosphate, a glycolytic intermediate, to form glycerol 3-phosphate.This
reaction is catalyzed by a glycerol 3-phosphate dehydrogenase in the cytosol. Glycerol 3-phosphate is reoxidized to
dihydroxyacetone phosphate on the outer surface of the inner mitochondrial membrane by a membrane-bound isozyme
of glycerol 3-phosphate dehydrogenase. An electron pair from glycerol 3-phosphate is transferred to a FAD prosthetic
group in this enzyme to form FADH
2
. This reaction also regenerates dihydroxyacetone phosphate.
The reduced flavin transfers its electrons to the electron carrier Q, which then enters the respiratory chain as QH
2
. When
cytosolic NADH transported by the glycerol 3-phosphate shuttle is oxidized by the respiratory chain, 1.5 rather than 2.5
ATP are formed. The yield is lower because FAD rather than NAD
+
is the electron acceptor in mitochondrial glycerol 3-
phosphate dehydrogenase. The use of FAD enables electrons from cytosolic NADH to be transported into mitochondria
against an NADH concentration gradient. The price of this transport is one molecule of ATP per two electrons. This
glycerol 3-phosphate shuttle is especially prominent in muscle and enables it to sustain a very high rate of oxidative
phosphorylation. Indeed, some insects lack lactate dehydrogenase and are completely dependent on the glycerol 3-
phosphate shuttle for the regeneration of cytosolic NAD
+
.
In the heart and liver, electrons from cytosolic NADH are brought into mitochondria by the malate-aspartate shuttle,
which is mediated by two membrane carriers and four enzymes (Figure 18.38). Electrons are transferred from NADH in
the cytosol to oxaloacetate, forming malate, which traverses the inner mitochondrial membrane and is then reoxidized by
NAD
+
in the matrix to form NADH in a reaction catalyzed by the citric acid cycle enzyme malate dehydrogenase. The
resulting oxaloacetate does not readily cross the inner mitochondrial membrane, and so a transamination reaction
(Section 23.3.1) is needed to form aspartate, which can be transported to the cytosolic side. Mitochondrial glutamate
donates an amino group, forming aspartate and α -ketoglutarate. In the cytoplasm, aspartate is then deaminated to form
oxaloacetate and the cycle is restarted. This shuttle, in contrast with the glycerol 3-phosphate shuttle, is readily
reversible. Consequently, NADH can be brought into mitochondria by the malate- aspartate shuttle only if the NADH/
NAD
+
ratio is higher in the cytosol than in the mitochondrial matrix. This versatile shuttle also facilitates the exchange
of key intermediates between mitochondria and the cytosol.

18.5.2. The Entry of ADP into Mitochondria Is Coupled to the Exit of ATP by ATP-
ADP Translocase
The major function of oxidative phosphorylation is to generate ATP from ADP. However, ATP and ADP do not diffuse
freely across the inner mitochondrial membrane. How are these highly charged molecules moved across the inner
membrane into the cytosol? A specific transport protein, ATP-ADP translocase (also called adenine nucleotide
translocase or ANT), enables these molecules to traverse this permeability barrier. Most important, the flows of ATP and
ADP are coupled. ADP enters the mitochondrial matrix only if ATP exits, and vice versa. The reaction catalyzed by the
translocase, which acts as an antiporter, is
ATP-ADP translocase is highly abundant, constituting about 14% of the protein in the inner mitochondrial membrane.
The translocase, a dimer of identical 30-kd subunits, contains a single nucleotide-binding site that alternately faces the
matrix and cytosolic sides of the membrane (Figure 18.39). ATP and ADP (both devoid of Mg
2+
) are bound with nearly
the same affinity. In the presence of a positive membrane potential (as would be the case for an actively respiring
mitochondrion), the rate of binding-site eversion from the matrix to the cytosolic side is more rapid for ATP than for
ADP because ATP has one more negative charge. Hence, ATP is transported out of the matrix about 30 times as rapidly
as is ADP, which leads to a higher phosphoryl transfer potential on the cytosolic side than on the matrix side. The
translocase does not evert at an appreciable rate unless a molecule of ADP is bound at the open, cytosolic site, which
then everts to the mitochondrial matrix side. This feature ensures that the entry of ADP into the matrix is precisely
coupled to the exit of ATP. The other side of the coin is that the membrane potential and hence the proton-motive force
are decreased by the exchange of ATP for ADP, which results in a net transfer of one negative charge out of the matrix.
ATP-ADP exchange is energetically expensive; about a quarter of the energy yield from electron transfer by the
respiratory chain is consumed to regenerate the membrane potential that is tapped by this exchange process. The
inhibition of this process leads to the subsequent inhibition of cellular respiration as well (Section 18.6.3).
18.5.3. Mitochondrial Transporters for Metabolites Have a Common Tripartite Motif
ATP-ADP translocase is but one of many mitochondrial transporters for ions and charged metabolites (Figure 18.40).
For historical reasons, these transmembrane proteins are sometimes called carriers. Recall that some of them function as
symporters and others as antiporters (Section 13.4). The phosphate carrier, which works in concert with ATP-ADP
translocase, mediates the electroneutral exchange of H
2
PO
4
-
for OH
-
(or, indistinguishably, the electroneutral symport
of H
2
PO
4
-
and H
+
). The combined action of these two transporters leads to the exchange of cytosolic ADP and P
i
for
matrix ATP at the cost of an influx of one H
+
. The dicarboxylate carrier enables malate, succinate, and fumarate to be
exported from mitochondria in exchange for P
i
. The tricarboxylate carrier transports citrate and H
+
in exchange for
malate. Pyruvate in the cytosol enters the mitochondrial matrix in exchange for OH
-
(or together with H
+
) by means of
the pyruvate carrier. These mitochondrial transporters and more than five others have a common structural motif. They
are constructed from three tandem repeats of a 100-residue module, each containing two putative transmembrane
segments (Figure 18.41).
