Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

II. Transducing and Storing Energy 22. Fatty Acid Metabolism 22.5. Acetyl Coenzyme A Carboxylase Plays a Key Role in Controlling Fatty Acid Metabolism
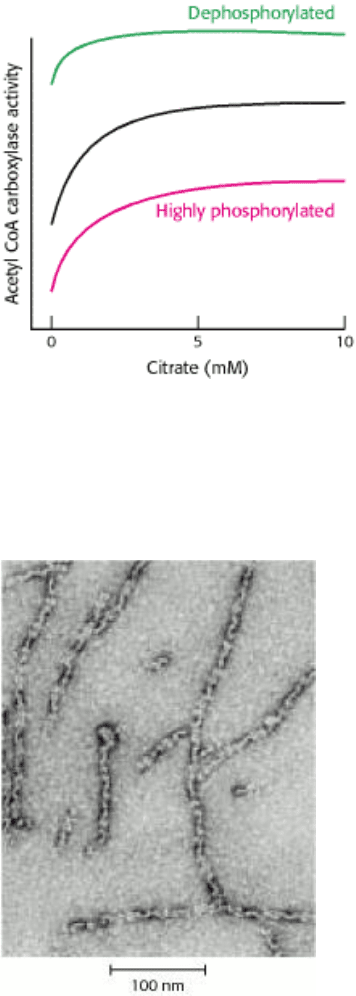
Figure 22.27. Dependence of the Catalytic Activity of Acetyl CoA Carboxylase on the Concentration of Citrate.
The dephosphorylated form of the carboxylase is highly active even when citrate is absent. Citrate partly overcomes the
inhibition produced by phosphorylation. [After G. M. Mabrouk, I. M. Helmy, K. G. Thampy, and S. J. Wakil. J. Biol.
Chem. 265(1990):6330.]
II. Transducing and Storing Energy 22. Fatty Acid Metabolism 22.5. Acetyl Coenzyme A Carboxylase Plays a Key Role in Controlling Fatty Acid Metabolism
Figure 22.28. Filaments of Acetyl CoA Carboxylase. The electron micrograph shows the enzymatically active
filamentous form of acetyl CoA carboxylase from chicken liver. The inactive form is an octamer of 265-kd subunits.
[Courtesy of Dr. M. Daniel Lane.]
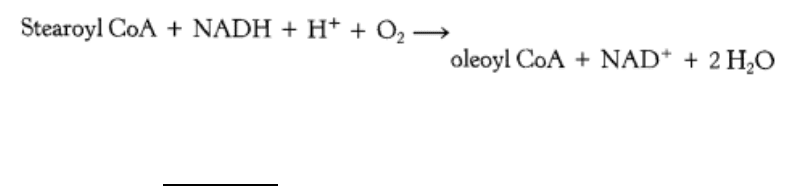
II. Transducing and Storing Energy 22. Fatty Acid Metabolism
22.6. Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory
Enzyme Systems
The major product of the fatty acid synthase is palmitate. In eukaryotes, longer fatty acids are formed by elongation
reactions catalyzed by enzymes on the cytosolic face of the endoplasmic reticulum membrane. These reactions add two-
carbon units sequentially to the carboxyl ends of both saturated and unsaturated fatty acyl CoA substrates. Malonyl CoA
is the two-carbon donor in the elongation of fatty acyl CoAs. Again, condensation is driven by the decarboxylation of
malonyl CoA.
22.6.1. Membrane-Bound Enzymes Generate Unsaturated Fatty Acids
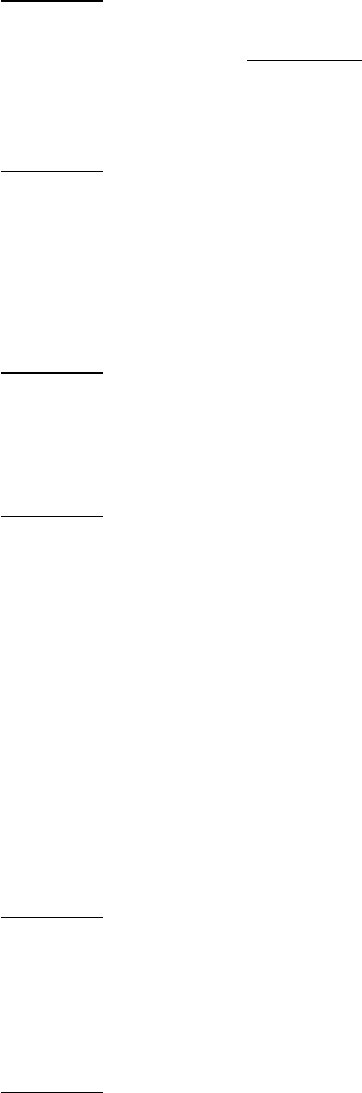
Endoplasmic reticulum systems also introduce double bonds into long-chain acyl CoAs. For example, in the conversion
of stearoyl CoA into oleoyl CoA, a cis-∆
9
double bond is inserted by an oxidase that employs molecular oxygen and
NADH (or NADPH).
This reaction is catalyzed by a complex of three membrane-bound enzymes: NADH-cytochrome b
5
reductase,
cytochrome b
5
, and a desaturase (Figure 22.29). First, electrons are transferred from NADH to the FAD moiety of
NADH-cytochrome b
5
reductase.
The heme iron atom of cytochrome b
5
is then reduced to the Fe
2+
state. The nonheme iron atom of the desaturase is
subsequently converted into the Fe
2+
state, which enables it to interact with O
2
and the saturated fatty acyl CoA
substrate. A double bond is formed and two molecules of H
2
O are released. Two electrons come from NADH and two
from the single bond of the fatty acyl substrate.
A variety of unsaturated fatty acids can be formed from oleate by a combination of elongation and desaturation reactions.
For example, oleate can be elongated to a 20:1 cis-∆
11
fatty acid. Alternatively, a second double bond can be inserted to
yield an 18:2 cis-∆
6
,∆
9
fatty acid. Similarly, palmitate (16:0) can be oxidized to palmitoleate (16:1 cis-∆
9
), which can
then be elongated to cis-vaccenate (18:1 cis-∆
11
).
Unsaturated fatty acids in mammals are derived from either palmitoleate (16:1), oleate (18:1), linoleate (18:2), or
linolenate (18:3). The number of carbon atoms from the ω end of a derived unsaturated fatty acid to the nearest double
bond identifies its precursor.

Mammals lack the enzymes to introduce double bonds at carbon atoms beyond C-9 in the fatty acid chain. Hence,
mammals cannot synthesize linoleate (18:2 cis-∆
9
, ∆
12
) and linolenate (18:3 cis-∆
9
, ∆
12
, ∆
15
). Linoleate and
linolenate are the two essential fatty acids. The term essential means that they must be supplied in the diet because they
are required by an organism and cannot be endogenously synthesized. Linoleate and linolenate furnished by the diet are
the starting points for the synthesis of a variety of other unsaturated fatty acids.
22.6.2. Eicosanoid Hormones Are Derived from Polyunsaturated Fatty Acids
Arachidonate, a 20:4 fatty acid derived from linoleate, is the major precursor of several classes of signal molecules:
prostaglandins, prostacyclins, thromboxanes, and leukotrienes (Figure 22.30).
A prostaglandin is a 20-carbon fatty acid containing a 5-carbon ring (Figure 22.31). A series of prostaglandins is
fashioned by reductases and isomerases. The major classes are designated PGA through PGI; a subscript denotes the
number of carbon-carbon double bonds outside the ring. Prostaglandins with two double bonds, such as PGE
2
, are
derived from arachidonate; the other two double bonds of this precursor are lost in forming a five-membered ring.
Prostacyclin and thromboxanes are related compounds that arise from a nascent prostaglandin. They are generated by
prostacylin synthase and thromboxane synthase respectively. Alternatively, arachidonate can be converted into
leukotrienes by the action of lipoxygenase. These compounds, first found in leukocytes, contain three conjugated double
bonds
hence, the name. Prostaglandins, prostacyclin, thromboxanes, and leukotrienes are called eicosanoids (from the
Greek eikosi, "twenty") because they contain 20 carbon atoms.
Prostaglandins and other eicosanoids are local hormones because they are short-lived. They alter the activities both of
the cells in which they are synthesized and of adjoining cells by binding to 7TM receptors. The nature of these effects
may vary from one type of cell to another, in contrast with the more uniform actions of global hormones such as insulin
and glucagon. Prostaglandins stimulate inflammation, regulate blood flow to particular organs, control ion transport
across membranes, modulate synaptic transmission, and induce sleep.
Recall that aspirin blocks access to the active site of the enzyme that converts arachidonate into prostaglandin H
2
(Section 12.5.2). Because arachidonate is the precursor of other prostaglandins, prostacyclin, and thromboxanes,
blocking this step affects many signaling pathways. It accounts for the wide-ranging effects that aspirin and related
compounds have on inflammation, fever, pain, and blood clotting.

II. Transducing and Storing Energy 22. Fatty Acid Metabolism 22.6. Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory Enzyme Systems
Figure 22.29. Electron-Transport Chain in the Desaturation of Fatty Acids.
II. Transducing and Storing Energy 22. Fatty Acid Metabolism 22.6. Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory Enzyme Systems
Figure 22.30. Arachidonate Is the Major Precursor of Eicosanoid Hormones. Prostaglandin synthase catalyzes the
first step in a pathway leading to prostaglandins, prostacyclins, and thromboxanes. Lipoxygenase catalyzes the initial
step in a pathway leading to leukotrienes.
II. Transducing and Storing Energy 22. Fatty Acid Metabolism 22.6. Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory Enzyme Systems
Figure 22.31. Structures of Several Eicosanoids.
II. Transducing and Storing Energy 22. Fatty Acid Metabolism
Summary
Triacylglycerols Are Highly Concentrated Energy Stores
Fatty acids are physiologically important as (1) components of phospholipids and glycolipids, (2) hydrophilic modifiers
of proteins, (3) fuel molecules, and (4) hormones and intracellular messengers. They are stored in adipose tissue as
triacylglycerols (neutral fat).
The Utilization of Fatty Acids as Fuel Requires Three Stages of Processing
Triacylglycerols can be mobilized by the hydrolytic action of lipases that are under hormonal control. Fatty acids are
activated to acyl CoAs, transported across the inner mitochondrial membrane by carnitine, and degraded in the
mitochondrial matrix by a recurring sequence of four reactions: oxidation by FAD, hydration, oxidation by NAD
+
, and
thiolysis by CoA. The FADH
2
and NADH formed in the oxidation steps transfer their electrons to O
2
by means of the
respiratory chain, whereas the acetyl CoA formed in the thiolysis step normally enters the citric acid cycle by condensing
with oxaloacetate. Mammals are unable to convert fatty acids into glucose, because they lack a pathway for the net
production of oxaloacetate, pyruvate, or other gluconeogenic intermediates from acetyl CoA.
Certain Fatty Acids Require Additional Steps for Degradation
Fatty acids that contain double bonds or odd numbers of carbon atoms require ancillary steps to be degraded. An
isomerase and a reductase are required for the oxidation of unsaturated fatty acids, whereas propionyl CoA derived from
chains with odd numbers of carbons requires a vitamin B
12
-dependent enzyme to be converted into succinyl CoA.
Fatty Acids Are Synthesized and Degraded by Different Pathways
Fatty acids are synthesized in the cytosol by a different pathway from that of β oxidation. Synthesis starts with the
carboxylation of acetyl CoA to malonyl CoA, the committed step. This ATP-driven reaction is catalyzed by acetyl CoA
carboxylase, a biotin enzyme. The intermediates in fatty acid synthesis are linked to an acyl carrier protein. Acetyl ACP
is formed from acetyl CoA, and malonyl ACP is formed from malonyl CoA. Acetyl ACP and malonyl ACP condense to
form acetoacetyl ACP, a reaction driven by the release of CO
2
from the activated malonyl unit. A reduction, a
dehydration, and a second reduction follow. NADPH is the reductant in these steps. The butyryl ACP formed in this way
is ready for a second round of elongation, starting with the addition of a two-carbon unit from malonyl ACP. Seven
rounds of elongation yield palmitoyl ACP, which is hydrolyzed to palmitate. In higher organisms, the enzymes carrying
out fatty acid synthesis are covalently linked in a multifunctional enzyme complex. A reaction cycle based on the
formation and cleavage of citrate carries acetyl groups from mitochondria to the cytosol. NADPH needed for synthesis is
generated in the transfer of reducing equivalents from mitochondria by the malate-pyruvate shuttle and by the pentose
phosphate pathway.
Acetyl Coenzyme A Carboxylase Plays a Key Role in Controlling Fatty Acid
Metabolism
Fatty acid synthesis and degradation are reciprocally regulated so that both are not simultaneously active. Acetyl CoA
carboxylase, the essential control site, is stimulated by insulin and inhibited by glucagon and epinephrine. These
hormonal effects are mediated by changes in the amounts of the active dephosphorylated and inactive phosphorylated
forms of the carboxylase. Citrate, which signals an abundance of building blocks and energy, allosterically stimulates the
carboxylase. Glucagon and epinephrine stimulate triacylglycerol breakdown by activating the lipase. Insulin, in contrast,
inhibits lipolysis. In times of plenty, fatty acyl CoAs do not enter the mitochondrial matrix because malonyl CoA inhibits

carnitine acyltransferase I.
Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory Enzyme
Systems
Fatty acids are elongated and desaturated by enzyme systems in the endoplasmic reticulum membrane. Desaturation
requires NADH and O
2
and is carried out by a complex consisting of a flavoprotein, a cytochrome, and a nonheme iron
protein. Mammals lack the enzymes to introduce double bonds distal to C-9, and so they require linoleate and linolenate
in their diets.
Arachidonate, an essential precursor of prostaglandins and other signal molecules, is derived from linoleate. This 20:4
polyunsaturated fatty acid is the precursor of several classes of signal molecules
prostaglandins, prostacyclins,
thromboxanes, and leukotrienes that act as messengers and local hormones because of their transience. They are called
eicosanoids because they contain 20 carbon atoms. Prostaglandin synthase, the enzyme catalyzing the first step in the
synthesis of all eicosanoids except the leukotrienes, consists of a cyclooxygenase and a hydroperoxidase. Aspirin
(acetylsalicylate), an anti-inflammatory and antithrombotic drug, irreversibly blocks the synthesis of these eicosanoids.
Key Terms
triacylglycerol (neutral fat, triacylglyceride)
bile salt
chylomicron
acyl adenylate
carnitine
β-oxidation pathway
vitamin B
12
(cobalamin)
peroxisome
ketone body
acyl carrier protein (ACP)
fatty acid synthase
malonyl CoA
acetyl CoA carboxylase
megasynthase
polyketide
nonribosomal peptide

AMP-dependent protein kinase (AMPK)
protein phosphatase 2A
arachidonate
prostaglandin
eicosanoid
II. Transducing and Storing Energy 22. Fatty Acid Metabolism
Problems
1.
After lipolysis. Write a balanced equation for the conversion of glycerol into pyruvate. Which enzymes are required
in addition to those of the glycolytic pathway?
See answer
2.
From fatty acid to ketone body. Write a balanced equation for the conversion of stearate into acetoacetate.
See answer
3.
Counterpoint. Compare and contrast fatty acid oxidation and synthesis with respect to
(a) site of the process.
(b) acyl carrier.
(c) reductants and oxidants.
(d) stereochemistry of the intemediates.
(e) direction of synthesis or degradation.
(f) organization of the enzyme system.
See answer
4.
Sources. For each of the following unsaturated fatty acids, indicate whether the biosynthetic precursor in animals is
palmitoleate, oleate, linoleate, or linolenate.
(a) 18:1 cis-∆
11
(b) 18:3 cis-∆
6
,∆
9
,∆
12
(c) 20:2 cis-∆
11
,∆
14
(d) 20:3 cis-∆
5
,∆
8
,∆
11

(e) 22:1 cis-∆
13
(f) 22:6 cis-∆
4
,∆
7
,∆
10
,∆
13
,∆
16
,∆
19
See answer
5.
Tracing carbons. Consider a cell extract that actively synthesizes palmitate. Suppose that a fatty acid synthase in this
preparation forms one molecule of palmitate in about 5 minutes. A large amount of malonyl CoA labeled with
14
C in
each carbon of its malonyl unit is suddenly added to this system, and fatty acid synthesis is stopped a minute later by
altering the pH. The fatty acids in the supernatant are analyzed for radioactivity. Which carbon atom of the palmitate
formed by this system is more radioactive
C-1 or C-14?
See answer
6.
Driven by decarboxylation. What is the role of decarboxylation in fatty acid synthesis? Name another key reaction in
a metabolic pathway that employs this mechanistic motif.
See answer
7.
Kinase surfeit. Suppose that a promoter mutation leads to the overproduction of protein kinase A in adipose cells.
How might fatty acid metabolism be altered by this mutation?
See answer
8.
An unaccepting mutant. The serine residue in acetyl CoA carboxylase that is the target of the AMP-dependent
protein kinase is mutated to alanine. What is a likely consequence of this mutation?
See answer
9.
Blocked assets. The presence of a fuel molecule in the cytosol does not ensure that it can be effectively used. Give
two examples of how impaired transport of metabolites between compartments leads to disease.
See answer
10.
Elegant inversion. Peroxisomes have an alternative pathway for oxidizing polyunsaturated fatty acids. They contain
a hydratase that converts
d-3-hydroxyacyl CoA into trans-∆
2
-enoyl CoA. How can this enzyme be used to oxidize
CoAs containing a cis double bond at an even-numbered carbon atom (e.g., the cis-∆
12
double bond of linoleate)?
See answer
11.
Covalent catastrophe. What is a potential disadvantage of having many catalytic sites together on one very long
polypeptide chain?
See answer
12.
Missing acyl CoA dehydrogenases. A number of genetic deficiencies in acyl CoA dehydrogenases have been
described. This deficiency presents early in life after a period of fasting. Symptoms include vomiting, lethargy, and
sometimes coma. Not only are blood levels of glucose low (hypoglycemia), but starvation-induced ketosis is
absent. Provide a biochemical explanation for these last two observations.
See answer
13.
Effects of clofibrate. High blood levels of triacylglycerides are associated with heart attacks and strokes. Clofibrate,
a drug that increases the activity of peroxisomes, is sometimes used to treat patients with such a condition. What is
the biochemical basis for this treatment?
See answer
14.
A different kind of enzyme. Figure 22.27 shows the response of acetyl CoA carboxylase to varying amounts of
citrate. Explain this effect in light of the allosteric effects that citrate has on the enzyme. Predict the effects of
increasing concentrations of palmitoyl CoA.
See answer
Mechanism Problems
15.
Variation on a theme. Thiolase is homologous in structure to the condensing enzyme. On the basis of this
observation, propose a mechanism for the cleavage of 3-ketoacyl CoA by CoA.
See answer
16.
Two plus three to make four. Propose a reaction mechanism for the condensation of an acetyl unit with a malonyl
unit to form an acetoacetyl unit in fatty acid synthesis.
See answer
Chapter Integration Problems
17.
Ill-advised diet. Suppose that, for some bizarre reason, you decided to exist on a diet of whale and seal blubber,
exclusively.
(a) How would lack of carbohydrates affect your ability to utilize fats?
(b) What would your breath smell like?
(c) One of your best friends, after trying unsuccessfully to convince you to abandon this diet, makes you promise to
consume a healthy dose of odd-chain fatty acids. Does your friend have your best interests at heart? Explain.
See answer
18.
Fats to glycogen. An animal is fed stearic acid that is radioactively labeled with [
14
C]carbon. A liver biopsy reveals
the presence of
14
C-labeled glycogen. How is this possible in light of the fact that animals cannot convert fats into
carbohydrates?
See answer
Data Interpretation Problem
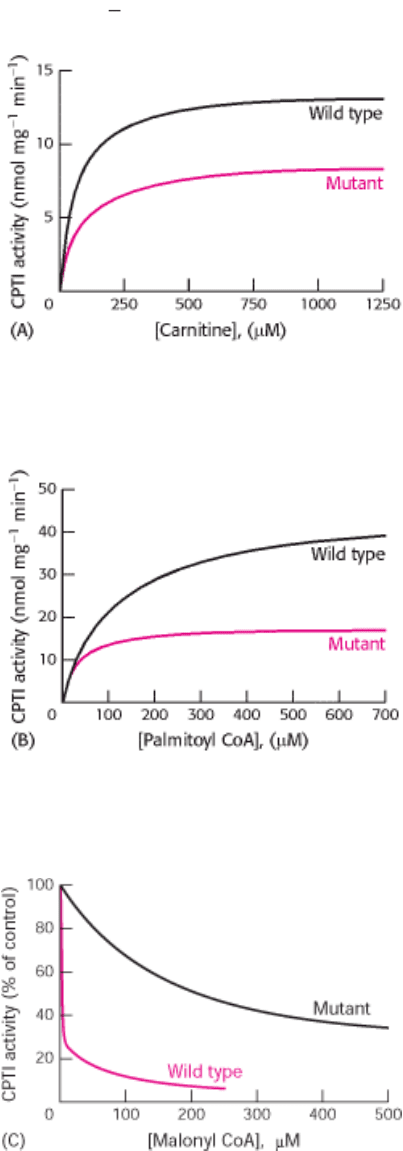
19.
Mutant enzyme. Carnitine palmitoyl transferase I (CPTI) catalyzes the conversion of long-chain acyl CoA into acyl
carnitine, a prerequisite for transport into mitochondria and subsequent degradation. A mutant enzyme was
constructed with a single amino acid change at position 3 of glutamic acid for alanine. Figures A through C show
data from studies performed to identify the effect of the mutation [data from J. Shi, H. Zhu, D. N. Arvidson, and G.
J. Wodegiorgis. J. Biol. Chem. 274(1999): 9421 9426].
(a) What is the effect of the mutation on enzyme activity when the concentration of carnitine is varied? What are
the K
M
and V
max
values for the wild-type and mutant enzymes?
(b) What is the effect when the experiment is repeated with varying concentrations of palmitoyl CoA? What are the
K
M
and V
max
values for the wild-type and mutant enzymes?
(c) Figure C shows the inhibitory effect of malonyl CoA on the wild-type and mutant enzymes. Which enzyme is
more sensitive to malonyl CoA inhibition?
(d) Suppose that palmitoyl CoA = 100 µM, carnitine = 100 µM, and malonyl CoA = 10 µM. Under these
conditions, what is the most prominent effect of the mutation on the properties of the enzyme?
