Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.


© 1999 by CRC Press LLC
one atomic site. As a result, no Ir–Ir adhesion was observed. Because Ir–Ir adhesion is stronger than
Ir–Pb adhesion, the separation force was less in this case than it was in the absence of the Pb monolayer
(Raffi-Tabar et al., 1992). In addition, the Ir substrate was not deformed as a result of the indentation
because the Ir tip did not wet the substrate.
When a Pb tip was used to indent an Ir-covered Pb substrate, the Pb tip atoms wetted the Ir monolayer
during the JC. As a result of the JC, the contact area between the tip and the Ir monolayer was larger
and there was no discernible crystal structure present in the Pb tip. Instead, the tip appeared to have the
structure and properties of a liquid drop wetting a surface. Because of the presence of the Ir monolayer,
continued indentation of the tip did not result in the formation of any adhesive Pb–Pb bonds. During
pull off, the Pb tip formed a connective neck, which decreased in width, as it separated from the
monolayer–substrate system. This was largely due to the Pb–Pb interaction that is small compared with
the Ir–Pb interaction. The radius of this connective neck of atoms was smaller than it was in the absence
of the Ir layer. As a result, the pull-off force (i.e., the force of adhesion) was less when the Ir layer was
present.
In summary, a reduction in the force of adhesion was observed when a monolayer film was placed
between the tip and the substrate. In the Ir/Pb/Ir case, formation of strong Ir–Ir adhesion was prevented
by the presence of the Pb film; therefore, the pull-off force was reduced. In the Pb/Ir/Pb case, the smaller
radius of the connective neck between the tip and substrate was responsible for the reduction in the force
of adhesion.
Molecular dynamics has also been used to simulate indentation of an n-hexadecane-covered Au(001)
substrate with an Ni tip (Landman et al., 1992). The forces governing the metal–metal interactions were
derived from EAM potentials. A so-called united-atom model (Ryckaert and Bellmans, 1978) was used
to model the n-hexadecane film. In this model, the hydrogen and carbon atoms were treated as one
united atom and the bonds between united atoms were held rigid. The interchain forces and the inter-
action of the chain molecules with the metallic tip and substrate were both modeled using an LJ potential
energy function. The size of the metallic tip and substrate were the same as in a previous study (Landman
et al., 1990). The hexadecane film consisted of 73 alkane molecules. The film was equilibrated on a 300
K Au surface and indentation was performed as described earlier (Landman et al., 1990).
Equilibration of the film with the Au surface resulted in a partially ordered film where molecules in
the layer closest to the Au substrate were oriented parallel to the surface plane. When the Ni tip was
lowered, the film “swelled up” to meet and partially wet the tip. Continued approach of the tip toward
the film caused the film to flatten and some of the alkane molecules to wet the sides of the tip. Lowering
the tip farther caused drainage of the top layer of alkane molecules from underneath the tip, increased
wetting of the sides of the tip, “pinning” of hexadecane molecules under the tip, and deformation of the
gold substrate beneath the tip. At this stage of the simulation, the force between the Ni tip and the film/Au
substrate was repulsive. In contrast, the force between the tip and the substrate had been attractive when
the alkane film was not present (Landman et al., 1990). Further lowering of the tip resulted in the drainage
of the pinned alkane molecules, inward deformation of the substrate, and eventual formation of an
intermetallic contact by surface Au atoms moving toward the Ni tip, which was concomitant with the
force between the tip and the substrate becoming attractive.
The effect of indenter shape on the compression of self-assembled monolayers was examined using
MD simulations by Tupper et al. (1994); 64 chains of hexadecanethiol were chemisorbed on an Au(111)
substrate composed of 192 atoms. A flat compressing surface, also composed of 192 atoms, and an
asperity, which was ¼ the size of the flat surface at the point of contact, were used to compress the
hexadecanethiol film in separate studies. Equilibration of the hexadecanethiol films, prior to compression,
resulted in highly ordered films in which the sulfur head group was bound to the threefold hollow sites
in a hexagonal array with a nearest-neighbor distance of 4.99 Å. The temperature of the system was
maintained at 300 K while the films were compressed by moving the flat surface (or the asperity) closer
to the films at a constant velocity of 100 m/s. The potential energy, load, and average tilt angle of the
film molecules were monitored during the simulation.

© 1999 by CRC Press LLC
For the compression of the monolayer film by the flat surface, there was a reversible change in the tilt
angle of the chain-tail group normal to the surface. Prior to compression, the tail groups were tilted
uniformly at an angle of 28° with respect to the surface normal. As the surface and the film were brought
into close proximity, the tilt angle decreased to approximately 20° during the JC. Compression of the
film to a load of 1.0 nN/molecule caused the tilt angle to increase steadily to 48°. This uniform change
in tilt angle of the tail groups suggested that a structural change had occurred. This was confirmed by
examination of the structure factors for the sulfur head groups. The structure factors indicated that the
head groups had converted from their original hexagonal packing to oblique packing. Because this change
was also observed in the tail groups, this suggested that the film had undergone a uniform structural
change to form an ordered structure (Tupper and Brenner, 1994). This structural rearrangement was
largely due to the uniform compression of the bonds in the chains accompanied by the formation of few
gauche defects in the hexadecanethiol film. When the compressing surface was retracted, the average tilt
angle of the tail groups returned to its equilibrium value. Thus, this structural rearrangement was shown
to be reversible. The signature of this transition appeared as a subtle slope change in the force vs. distance
curve. A similar effect was observed in experimental data generated by Joyce et al. (1992).
Conversely, a large number of random structural changes of the film resulted when an asperity was
used to compress the film. During compression, the average tilt angle of the tail groups varied nonuni-
formly between 20° and 30°. In addition, the distribution of bond lengths was much broader than for
the flat surface compression. The average bond angle also decreased and a large number of gauche defects
were formed in the film. Finally, the structure factors calculated for the sulfur head groups suggested
that the sulfur head groups were disordered. By comparing the force profiles from these two indentations
Tupper et al. (1994) concluded that an asperity can approach the substrate much closer than the flat
surface before disrupting the film. This conclusion agrees with the hypothesis that surface asperities that
penetrate these thick insulating films may play a crucial role in the STM imaging of these films (Liu and
Salmeron, 1994).
11.3.3 Indentation of Nonmetals
Large-scale MD simulations have also been used to investigate nanometer-sized indentation processes in
nonmetallic systems. For example, Kallman et al. (1993) examined the microstructure of amorphous and
crystalline silicon before, during, and after indentation. This was done in an effort to examine the assertion
that Si directly beneath the indenter undergoes two different pressure-induced, solid–solid phase trans-
formations during indentation involving the high-pressure β-Sn structure (above 100 kbars) and a
thermodynamically unstable amorphous phase. The constant-temperature MD simulations contained
over 350,000 substrate silicon atoms. By using both a “smooth” continuum tip and rigid, but atomically
“rough” tetrahedral tip, both amorphous and crystalline silicon were indented using two different inden-
tation speeds (2.7 km/s, or ⅓ the longitudinal speed of sound in Si, and approximately 0.3 km/s).
Interatomic forces governing the motion of the silicon atoms were derived from the many-body silicon
potential of Stillinger and Weber (1985). Both substrates were also indented at a number of temperatures,
the highest temperature being close to the melting point of silicon.
Phase transitions during the simulated indentation were monitored by calculating both a diffraction
pattern and the angle-averaged pair distribution function G(r). Because bar-code plots of G(r) differ
qualitatively for different phase of silicon, they reveal the structural state of the silicon during indentation,
when examined in conjunction with the calculated diffraction patterns.
At the highest indentation rate and the lowest temperature, Kallman et al. (1993) determined that
amorphous and crystalline Si have similar yield strengths (138 and 179 kbar). Near the melting temper-
ature and at the slowest indentation rate, lower yield strengths (30 kbar) were observed for both amor-
phous and crystalline Si. Thus, the simulated nanoyield strength of Si depended on structure, rate of
deformation, and temperature of the sample. Amorphous silicon did not show any sign of crystallization
upon indentation. Conversely, indentation of the crystalline silicon close to its melting point did show

© 1999 by CRC Press LLC
a tendency to transform to the amorphous phase near the indenter surface. No evidence of the transfor-
mation to the β-Sn structure under warm indentation was found.
Ionic solids also show interesting behavior upon indentation. The interaction of a CaF
2
tip with a CaF
2
substrate was examined using constant-temperature MD simulations by Landman et al. (1992). The
substrate was composed of 242 static and 2904 dynamic ions. The stacking sequence was ABAABA …,
where A and B correspond to F
–
and Ca
+2
layers, respectively. The tip was a (111)-faceted microcrystal
that contained nine (111) layers. Indentation was performed by repeatedly moving the tip 0.5 Å closer
to the substrate and allowing the system to equilibrate. The energy was described as a sum of pairwise
interactions between ions, where the potential between ions was composed of both Coulomb and
repulsive contributions, as well as a van der Waals dispersion interaction that was parameterized by fitting
to experimental data. The long-range Coulomb interactions were treated via the Ewald summation
method and the temperature was maintained at 300 K.
The attractive force between the tip and the substrate increased gradually as the tip approached the
substrate. At the critical distance of 2.3 Å, the attractive force increased dramatically; this was accompanied
by increased interlayer spacing (i.e., elongation) in the tip. This process was similar to the JC phenomenon
observed in metals; however, the amount of elongation (0.35 Å) is much smaller in this case. Decrement-
ing the distance between the tip holder and the substrate further caused an increase in the attractive
energy until an extremum value was reached. Continued indentation resulted in a repulsive tip–substrate
interaction, compression of the tip, and ionic bonding between the tip and substrate. These bonds were
responsible for the observed hysteresis in the force curve and upon retraction from the substrate ultimately
led to plastic deformation of the tip and its fracture.
As noted earlier, the AFM can be used to measure nanomechanical properties (such as elastic modulus
and hardness) with depth and force resolution superior to other methods (Burnham et al., 1990; Burnham
and Colton, 1993). One way to do this is to relate the shape and the slope of the repulsive wall region
of the force curve for an elastic indentation to the Young’s modulus of the material that is indented. With
this in mind, MD simulations have been used to simulate indentation at the atomic scale to elucidate
the strengths, limitations, and interpretation of nanoindentation for characterizing materials and thin-
film properties.
The simulated indentation of various hydrocarbon substrates using an sp
3
-hybridized indenter was
first performed by Harrison et al. (1992a). In a series of 300 K simulations, a hydrogen-terminated, sp
3
-
hybridized tip was used to indent the (111) surface of a (1 × 1) hydrogen-terminated diamond substrate,
the (100) surface of a (2 × 1) hydrogen-terminated diamond substrate, and the basal plane of a graphite
substrate. The indentation rate was approximately 0.3 km/s. This rate is orders of magnitude faster than
experimental indentation rates, but much slower than the propagation of sound in diamond (12 to
18 km/s). The particle forces were derived from a reactive empirical bond-order potential (REBO) that
is unique among hydrocarbon potentials in its ability to model chemical reactions (Brenner, 1990; Brenner
et al., 1991).
Elastic indentations were performed on all three substrates and force curves were generated. The
loading portion of the force curves for the elastic indentation of each of these substrates were all
approximately linear, with slopes S
111
> S
100
S
graphite
. Experimental indentation of diamond (100) and
graphite using an AFM also resulted in linear loading curves and the curve for diamond (100) had a
larger slope than the curve for graphite. Because the indentation of diamond (111) involved both the
compression of, and the changing of angles between, carbon–carbon bonds, while indentation of the
(100) surface only involved the latter (Harrison et al., 1991), the (100) surface was softer. While these
simulations provided the correct qualitative information regarding the relative hardness of the diamond
and graphite substrates, the quantitative values of Young’s moduli were not in good agreement with
experimental values.
Classical elasticity theory predicts that the slope of the indentation curve is proportional to the modulus
of the material (Sneddon, 1965). For cubic systems, the Young’s moduli E(111) for a (111) surface and
E(100) for a (100) surface are related to the elastic constants c
ij
of the substrate via the following
relationships:
© 1999 by CRC Press LLC
(11.14)
(11.15)
Substitution of the bulk elastic constants for diamond into Equations 11.14 and 11.15 yields values of
1267 GPa for E(111) and 1172 GPa for E(100). When the elastic constants calculated from Brenner’s
potential (Brenner, 1990; Brenner et al., 1991) were substituted into Equations 11.14 and 11.15 values of
1102 and 488 GPa were obtained for E(111) and E(100), respectively. Because the hydrocarbon potential
was developed principally to model chemical vapor deposition of diamond films, little attention was paid
to fitting of the elastic constants. As a result, the values of E(111) and E(100) calculated from the
hydrocarbon potential differ by 13% and 58%, respectively, from the values calculated using the exper-
imentally determined elastic constants. With this in mind, efforts focused upon extracting qualitative
information from the indentation of diamond (111) surfaces and upon refitting the hydrocarbon potential
energy function.
The plastic indentation of diamond (111) substrates, with and without hydrogen termination, using
a hydrogen-terminated sp
3
-bonded tip, was investigated by Harrison et al. (1992b). The depth at which
the diamond (111) substrate incurred a plastic deformation due to indentation was determined by
examination of the total potential energy of the tip–substrate system as a function of tip–substrate
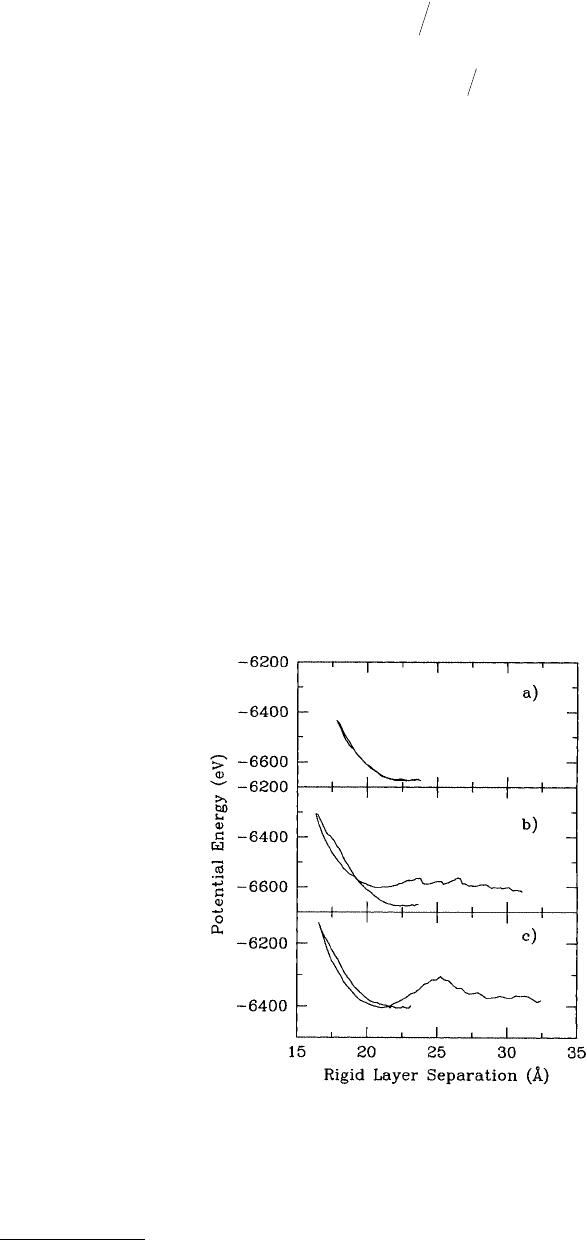
separation (Figure 11.7a to c). No hysteresis in the potential energy vs. distance curve was observed
(Figure 11.7a) when the maximum normal force on the tip holder was 200 nN or less. Thus, the
indentation was nonadhesive and elastic; therefore, the tip–substrate system did not sustain any perma-
nent damage due to the indentation. This was also apparent from a comparison of initial and final
tip–substrate geometries.
In contrast, increasing the maximum normal force on the tip holder to 250 nN prior to retraction
caused plastic deformation of the tip and the substrate. This was apparent from the marked hysteresis
FIGURE 11.7 Potential energy as a function of rigid-layer separation generated from an MD simulation of an elastic
(nonadhesive) indentation (a) and plastic (adhesive) indentation (b) of a hydrogen-terminated diamond (111) surface
using a hydrogen-terminated, sp
3
-hybridized tip. Panel (c) shows the results of the same tip indenting a diamond
(111) surface after the hydrogen termination layer was removed. (Data from Harrison, J. A. et al. (1992), Surf. Sci.
271, 57–67.)
E cccccc111 6 2 2 4
44 11 12 11 12 44
()
=+
()
++
()
Ecccc100 2
11 12 12
2
11
()
=+− .

© 1999 by CRC Press LLC
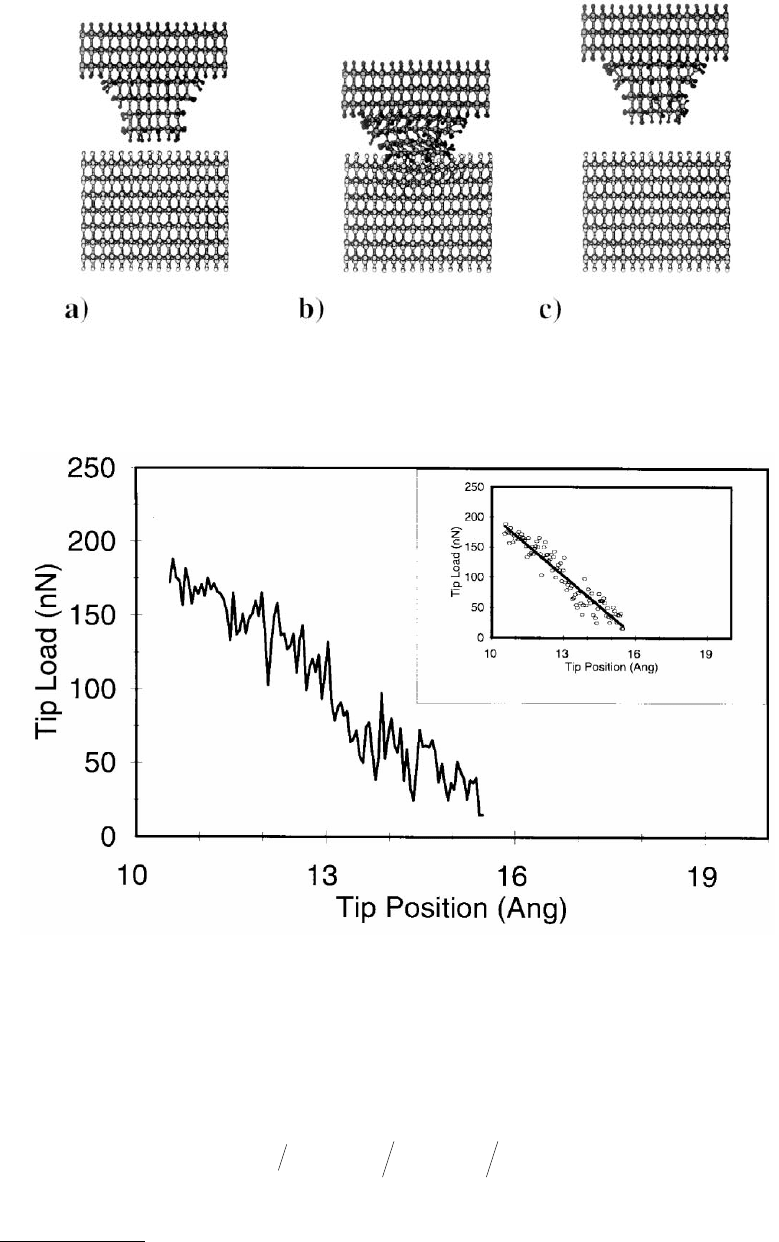
in the plot of potential energy vs. distance (Figure 11.7b) and from the initial and final tip–substrate
geometries (Figures 11.8a to d). Examination of the atomic coordinates as a function of time allowed
for specific, atomic-scale motions to be associated with certain features in the plot of potential energy
as a function of distance. As the tip was withdrawn from the substrate, connective strings of atoms formed
(Figure 11.8c). Increasing the distance between the tip and crystal caused these strings to break one by
one. Each break was accompanied by a sudden drop in the potential energy at large positive values of
tip–substrate separation (Figure 11.7b).
During indentation, the end of the tip twisted to minimize interatomic repulsions between hydrogen
atoms chemisorbed to the tip and those chemisorbed to the substrate. This twisting allowed the tip atoms
to form chemical bonds with the carbon atoms below the first layer of carbon atoms in the substrate. As
a result, the indentation was disordered and ultimately led to the formation of connective strings of
atoms between the substrate and tip as the tip was retracted.
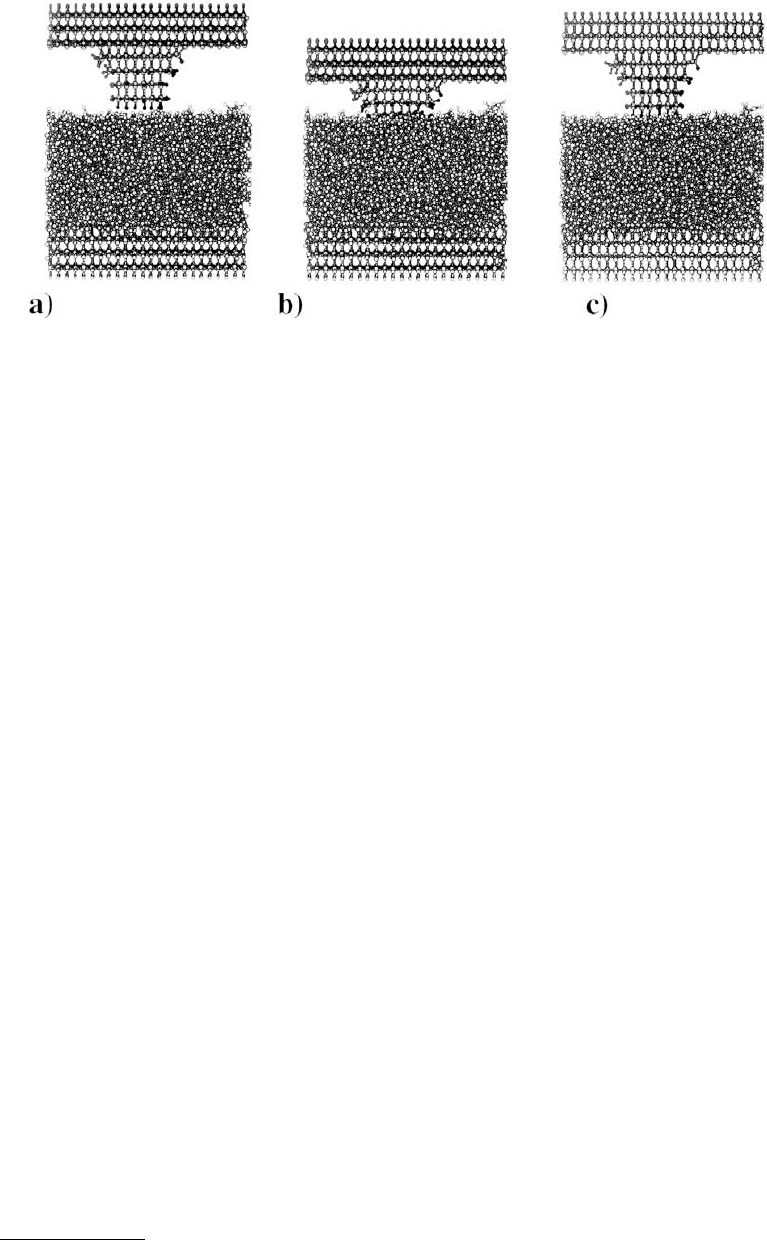
When hydrogen was removed from the substrate surface and indentation to approximately the same
value of maximum force was repeated, plastic deformation was also observed. However, the atomic-scale
details, including the degree of damage, differed. The absence of hydrogen on the surface of the substrate
minimized repulsive interactions during indentation and, therefore, allowed the tip to indent the substrate
without twisting (Harrison, et al., 1992b). Because carbon–carbon bonds were formed between the tip
and the first layer of substrate, the indentation was ordered (i.e., the surface was not disrupted as much
by the tip) and the eventual fracture of the tip during retraction resulted in minimal damage to the
FIGURE 11.8 Illustrations of atoms in the MD simulation of the indentation of a hydrogen-terminated diamond
(111) substrate with a hydrogen-terminated, sp
3
-hybridized tip at selected time intervals. The tip–substrate system
at the start of the simulation (a), at maximum indentation (b), as the tip was withdrawn from the sample (c), and
at the end of the simulation (d). Large and small spheres represent carbon and hydrogen atoms, respectively. (Data
from Harrison, J. A. et al. (1992b), Surf. Sci. 271, 57–67. With permission.)*
* Color reproduction follows page 16.

© 1999 by CRC Press LLC
substrate (Figures 11.9a and b). The concerted fracture of all bonds in the tip gave rise to the single
maximum in the potential vs. distance curve at large distance (Figure 11.7c).
The hydrocarbon potential developed by Brenner (Brenner, 1990; Brenner et al., 1991) has also been
used by Glosli et al. (1995) to examine the evolution of the microstructure of amorphous-carbon films
during indentation. A blunt rigid tip was used to indent films, which were 4 nm thick, at 35 m/s. The
tip interacted with the film via a truncated LJ potential. A thermostat was applied to the middle layers
of the amorphous film to maintain the desired temperature.
The force curves in these simulations had steps that were indicative of plastic events, likely due to
rapid rearrangement of the bonding network during the indentation. The reported hardness of the films
calculated from these simulations was 75 ± 25 GPa.
In an effort to glean quantitative information from the indentation of hydrocarbons, the REBO
potential was recently improved to reproduce the elastic constants of diamond and graphite accurately
while maintaining all of its original properties (Brenner et al., unpublished). The elastic constants for
diamond calculated using this improved potential are in good agreement with experimentally determined
values (Sinnott et al., 1997). Substitution of the elastic constants calculated from the improved potential
into Equations 11.14 and 11.15 yields values of 1367 and 1177 GPa for E(111) and E(100), respectively.
Recently, Sinnott et al. (1997) used an sp
3
-hybridized carbon tip to indent a hydrogen-terminated (111)
face of diamond and a thin film of amorphous carbon on (111) diamond. Comparison of pictures before
and after indentation (Figure 11.10a to c) confirmed a lack of significant adhesion between the tip and
substrate, so this indentation was classified as elastic. Thus, the simulated force curves could be used to
calculate the reduced elastic modulus of the tip–sample system. For an elastic indentation, the loading
portion of the force curve is related to the reduced modulus, although the exact relationship varies
depending upon the geometry of the indenter. For example, classical elasticity theory predicts a linear
relationship for flat-ended indenters and a nonlinear relationship for spherical or conical indenters
(Sneddon, 1965).
For the force curve depicted in Figure 11.11, the force curve in the loading region is linear. The
relationship of the slope to the reduced elastic modulus is given by
(11.16)
FIGURE 11.9 Illustrations of atoms in the MD simulation of the indentation of a non-hydrogen-terminated,
diamond (111) substrate with a hydrogen-terminated, sp
3
-hybridized tip. The tip–substrate system prior to inden-
tation (a) and subsequent to indentation (b). The large, dark gray spheres represent carbon atoms and the small,
white spheres represent hydrogen atoms. (Data from Harrison, J. A. et al. (1992b), Surf. Sci. 271, 57–67.)
FEah
r
= 2,
© 1999 by CRC Press LLC
where F is the force, E
r
is the reduced elastic modulus, a is the radius of the contact area (approximately
6 Å), and h is the penetration depth. The reduced modulus is given by
(11.17)
where ν is Poisson’s ratio and the subscripts 1 and 2 denote tip and substrate, respectively.
FIGURE 11.10 Illustrations of atoms in the MD simulation of a hydrogen-terminated, diamond (111) surface indented
by an sp
3
-hybridized tip. The dark gray, light gray, white, and black spheres represent tip carbon atoms, surface carbon
atoms, surface hydrogen atoms, and tip hydrogen atoms, respectively. Simulation times are 0.0 ps in (a), 6.0 ps in (b),
and 15.0 ps in (c). (From Sinnott, S. B. et al. (1997), J. Vac. Sci. Technol. A 15, 936–940. With permission.)
FIGURE 11.11 Force vs. position of the tip rigid layer for the indentation of a diamond (111) surface by the sp
3
-
hybridized tip using MD simulations. (Only the loading portion of the curve is shown.) The tip first contacts the
surface when the tip position is approximately 14 Å. The inset shows the line of best fit to these data. (From Sinnott,
S. B. et al. (1997), J. Vac. Sci. Technol. A 15, 936–940. With permission.)
11 1
1
2
12
2
2
EEE
r
=−
()
+−
()
νν,
© 1999 by CRC Press LLC
When the slope of 33.5 nN/Å, obtained from the data in Figure 11.11, was substituted into
Equation 11.16 it yielded a reduced modulus of 279 GPa. Using the value of 1367 GPa for Young’s modulus
of the substrate (Equation 11.14) and Equation 11.17, the elastic modulus of the tip was determined to
be 357 GPa. Thus, the sp
3
-hybridized carbon tip, with no extensive stabilizing lattice, was found to be
significantly softer than the diamond (111) substrate.
The indentation of diamond (111) covered by an amorphous-carbon film was performed with the
same sp
3
-hybridized carbon tip. Prior to indentation, the film was composed of approximately 21% sp
3
-
hybridized carbon and approximately 58% sp
2
-hybridized carbon. Less than 2% of the carbon atoms had
two nearest neighbors, and approximately 0.1% had five nearest neighbors. The remaining atoms were
at the film edges (Figure 11.12a to c). For comparison purposes, the amorphous-carbon film was indented
to approximately the same depth as the diamond (111) substrate discussed above. Analysis of the atomic
positions as a function of time indicated that no significant depression was left in the film subsequent
to indentation. In addition, the distribution of carbon-atom hybridizations was approximately the same
as it was at the start of the indentation. Thus, this indentation was considered to be elastic.
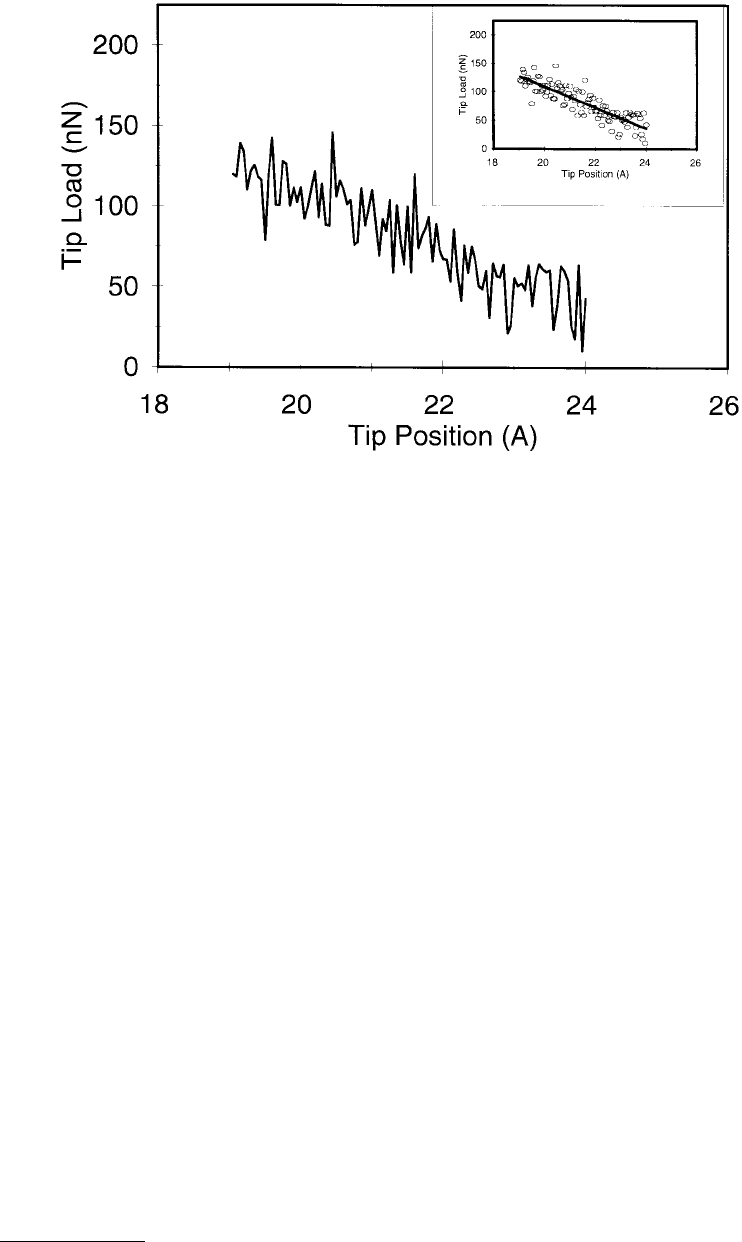
The loading portion of the force curve was again linear and was related to the reduced modulus of
the tip and the film (Figure 11.13). The slope of 18.4 nN/Å, when substituted into Equation 11.16, yielded
a value of 153 GPa for E
r
. By using the E
1
value of 357 GPa determined for the 696-atom tip, the elastic
modulus of the amorphous carbon film was found to be 243 GPa, well within the range of experimentally
determined modulus values (Davanloo et al., 1992; Robertson, 1992; Rossi et al., 1994). These simulations
demonstrated that MD simulations, with an accurate potential, can be used to calculate quantitative
information from indentations.
11.3.4 Cutting of Metals
The fabrication of high-tolerance metal parts involves the precision machining of metal surfaces. Single-
point diamond tools are currently generating components with nanometer tolerances. However, the
mechanisms by which tools wear, tools and substrates interact, and surfaces are cut are not currently
known. With that in mind, Belak and co-workers (Belak and Stowers, 1990; Belak et al., 1993) have
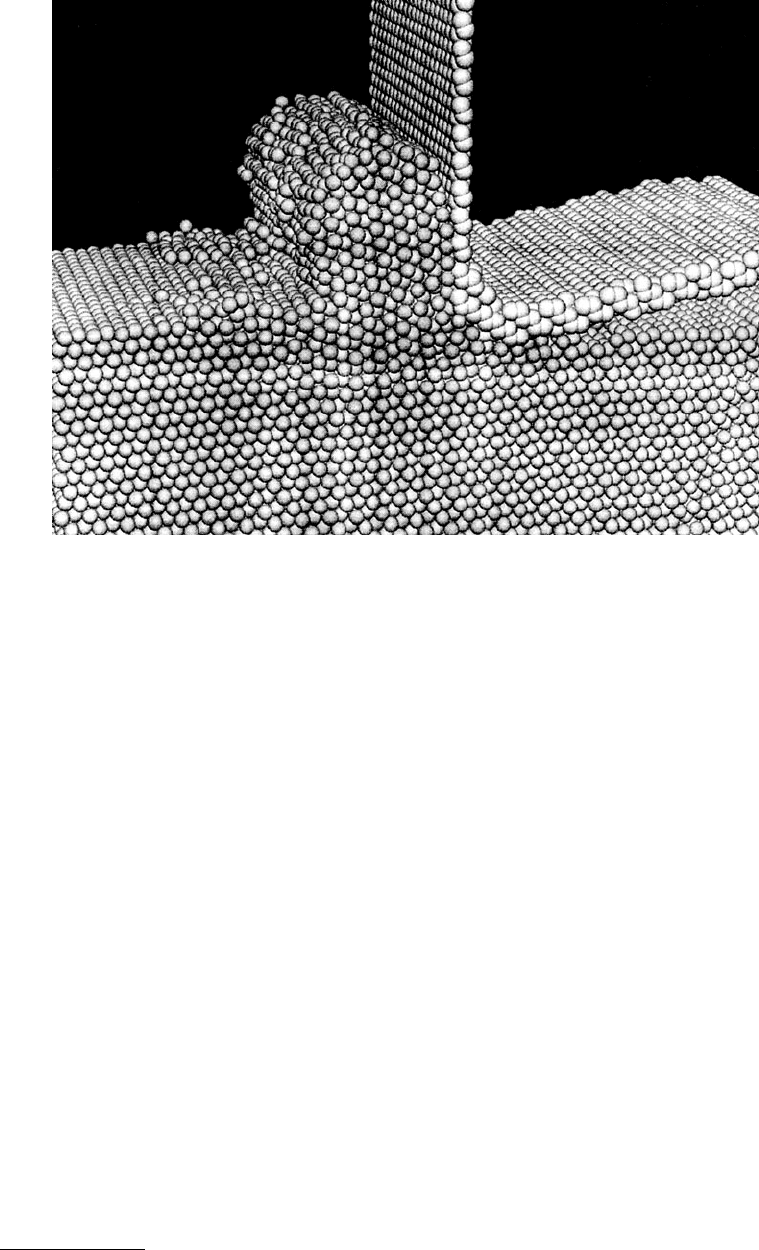
examined the orthogonal cutting of Cu(111) substrates using a rigid cutting tool.
In those simulations, a static diamond-like tip was placed into contact with the Cu surface. Cutting
was performed by continuously moving the tool closer to the plane of the surface while the surface was
moved in a direction perpendicular to the surface normal at 100 m/s. This process formed a Cu chip in
FIGURE 11.12 Illustrations of atoms in the MD simulation of an amorphous carbon film indented by sp
3
-hybridized
tip. The dark gray, light gray, white, and black spheres represent tip carbon atoms, film carbon atoms, surface hydrogen
atoms, and tip hydrogen atoms, respectively. Simulations times are 0.0 ps (a), 8.0 ps (b), and 14.9 ps (c). (From
Sinnott, S. B. et al. (1997), J. Vac. Sci. Technol. A 15, 936–940. With permission.)
© 1999 by CRC Press LLC
front of the tool (Figure 11.14). This chip was crystalline in nature and possessed a different orientation
than the Cu substrate. Additionally, regions of disorder were formed in front of the tool tip and on the
substrate surface in front of the chip. Finally, dislocations originating from the tool contact point were
formed in the substrate.
The cutting force showed a strong dependence on tool edge radius, as it does experimentally. Tools
with large radii require larger forces to achieve the same depth of cut as sharp tools. The specific energy
(work per unit volume of material removed) determined from the simulation (and from micro-diamond-
cutting experiments) has a power dependence on the depth of cut, with a power coefficient of –0.6.
Macroscopic machining yields the same qualitative dependence of specific energy with depth of cut, but
with a coefficient of –0.2. A change in slope in the simulations occurred at length scales of a few microns
and larger. This transition has been interpreted as a change in the mechanism of plastic deformation
from intergranular at macroscopic lengths (moving existing dislocations) to intragranular at the micro-
scopic lengths (creating new dislocations). The simulations, among the largest yet performed, illustrate
that the computer simulations are beginning to invite comparison with both nanoscale and microscale
experiments.
11.3.5 Adhesion
Adhesion can be studied by bringing two materials into contact and then separating them or by separating
two ends of a system already in contact. Pethica and Sutton (1988) performed one of the earliest
theoretical, atomistic examinations of adhesion. Using both a continuum and an atomistic model (based
on LJ pair potentials), they were able to demonstrate the JC phenomenon. The critical separation where
the JC occurred was typically for separations less than 2 Å. When the surfaces were separated, they did
not come apart as the critical jump separation was exceeded, thus, exhibiting hysteresis due to adhesion.
The authors pointed out that one of the consequences of the existence of this region of inaccessible
separation is that there will be significant differences between contact and noncontact AFM experiments.
FIGURE 11.13 Force on the tip vs. position of the tip-rigid layer for the indentation of an amorphous-carbon film
by the sp
3
-hybridized tip using MD simulations. (Only the loading portion of the curve is shown.) The tip first
contacts the film when the tip position is approximately 23 Å. The inset shows the line of best fit to these data. (From
Sinnott, S. B. et al. (1997), J. Vac. Sci. Technol. A 15, 936–940. With permission.)
© 1999 by CRC Press LLC
Smith et al. (1989) also studied the JC phenomenon, using equivalent crystal theory (Smith and
Banerjea, 1987) as the basis for their investigation. This method, based on a perturbation theory approach,
had been demonstrated to give accurate surface energies and relaxed atomic positions for a number of
transition metal surfaces. In a 1989 work of theirs, the JC or the “avalanche in adhesion” between two
Ni(100) crystals was examined. The critical distance where the “avalanche” occurred was found to depend
on the balance between the favorable energy of attraction between surface layers on opposing substrates
and the unfavorable energy involved in pulling these surface layers away from their corresponding
substrates. The avalanche process itself was rapid, on the order of 100 fs. The authors pointed out that
an avalanche was not inevitable; its occurrence depends on film thickness, film stiffness, and the strength
of the adhesive force. In some cases, the adhesive forces may be to weak relative to the restoring forces
for these forces to ever be equal at some separation.
Subsequently, Lupkowski and Maguire, (1992) investigated the nature of the avalanche using MD
simulations of two-dimensional, LJ solids. These simulations demonstrated that the structure of the
surfaces after contact (after the avalanche) depends on temperature. At low temperatures, the avalanche
was qualitatively similar to that predicted by energy minimization calculations (Pethica and Sutton, 1988).
At higher temperatures, there were qualitative changes in the nature of the avalanche. As the approach
was made in steps, individual atoms were stripped off, creating a bridge between the surfaces. The
formation of the bridge took place prior to the contacting of the surfaces. Eventually, the two surfaces
collapsed to form a strained solid with no defects. Approaches at even higher temperatures caused the
bridging to become more pronounced and occurred at larger separations. Subsequent structures had a
number of defects, including vacancies and dislocations in the interior and near what was previously the
surface of the slab. Thus, the authors concluded that the structure of the surfaces at contact varied
qualitatively with temperature.
FIGURE 11.14 Illustrations of atoms from an MD simulation of the orthogonal cutting of a Cu(111) substrate
with a rigid cutting tool. (From Belak, J. and Stowers, I. F. (1992), in Fundamentals of Friction: Macroscopic and
Microscopic Processes (I. L. Singer and H. M. Pollock, eds.), 511–520, Kluwer, Dordrecht. With permission.)
