Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.


© 1999 by CRC Press LLC
velocity profiles became curved (Figure 11.23b). Increasing the wall–fluid interaction strength further
(Figure 11.23c) caused the first two liquid layers to become locked to the solid wall.
For unequal wall and fluid densities, the flow BC changed dramatically. At the smallest wall–fluid
interaction strengths examined, the velocity profile was linear; however, the no-slip BC was not present.
The magnitude of the liquid velocity at the wall was less than the wall velocity; therefore, slip occurred
(i.e., |V
x
|U < 1) (Figure 11.23a). The magnitude of this slip decreased as the strength of the wall–fluid
interaction increased. For an intermediate value of wall–fluid interaction strength (ε
wf
/ε = 1.8), the first
fluid layer was partially locked to the solid wall. Sufficiently large values of wall-fluid interaction strength
(ε
wf
/ε = 15) led to the locking of the second fluid layer to the wall.
Using a simulation geometry similar to Figure 11.18b, Thompson and Robbins (1990a) examined the
origin of stick-slip motion when an LJ liquid is sheared between two solid walls using MD. Instead of
two solids in contact moving smoothly, the solids may alternatively stick and then slip past one another,
this is known as stick-slip motion. Simulation details are described above (Thompson and Robbins,
1990b), except that the walls in this case were composed of fcc solids with (111) surfaces and the density
of the wall atoms was usually equal to the density of the fluid atoms. The wall separation usually
corresponded to a few layers (i.e., 2σ). To mimic boundary-layer lubrication experiments, the simulations
were performed at constant load by varying the distance between the walls.
The upper wall was coupled through a spring to a stage that advanced at constant velocity. Initially,
the force on the spring was zero when the upper wall was at rest. As the stage moved forward, the spring
stretched and the forced increased. When the film was in a liquid state, the upper wall accelerated until
the force applied by the spring balanced the viscous dissipation. When the film was crystalline in nature,
stick-slip behavior was observed (Figure 11.24). Initially, the film responded elastically, the wall remained
stationary, and the forced increased linearly (Figure 11.24a). When the force exerted by the spring
exceeded the yield stress of the film, the film melted and the tip wall began to slide. The wall accelerated
to catch up with the stage, decreasing the force and creating the sawtooth pattern indicative of stick-slip
motion. The stick-slip behavior was dependent on the velocity of the stage, with the degree of stick-slip
increasing as the velocity decreased. Analysis of the two dimensional structure factor during the course
of the simulation confirmed the existence of the solid–liquid phase transition as the film proceeded from
static to sliding states (Figure 11.24c).
FIGURE 11.24 Force per unit area f, wall displacement
X
w
, and the Debye Waller factor e
–2W
(e
–2W
= 0.6 for a
solid) as a function of time t during stick-slip motion for
a wall velocity of 0.1. The simulation contained 288 wall
atoms and 144 fluid atoms. The walls were fcc solids with
(111) surfaces, and the shear direction was (100). All
quantities have been normalized using the appropriate
variables so that they are dimensionless. (From Thomp-
son, P. A. and Robbins, M. O. (1990). Science 250,
792–794. With permission.)

© 1999 by CRC Press LLC
While simulations with spherical molecules were successful in explaining some experimental phenom-
ena, they were unable to reproduce other features of the experimental data. For instance, the calculated
relaxation times and viscosities remained near bulk fluid values until the films crystallized. Experimen-
tally, these quantities typically increase many orders of magnitude before a yield stress is observed
(Israelachvili et al., 1988; Van Alsten and Granick, 1988). With this in mind, Thompson et al. (1992)
repeated their earlier shearing simulations (Thompson and Robbins, 1990b) using freely jointed, linear-
chain molecules instead of spherical molecules.
The behavior of the viscosity of the films as a function of shear rate was examined for films of different
thicknesses. The relationship between film viscosity and shear rate was examined (Figure 11.25). The
response of films that were six to eight molecular diameters m
l
thick was approximately the same as it
is for bulk systems. When the thickness of the film was reduced, the viscosity of the film increased
dramatically, particularly at low shear rates, consistent with the aforementioned experimental observations.
The structure of liquids confined to smooth pores was compared with the structure of liquids in
irregularly shaped pores by Curry et al. (1994) using grand canonical Monte Carlo and microcanonical
MD methods. Both the confining pore walls were comprised of rigid atoms fixed in a (100) fcc arrange-
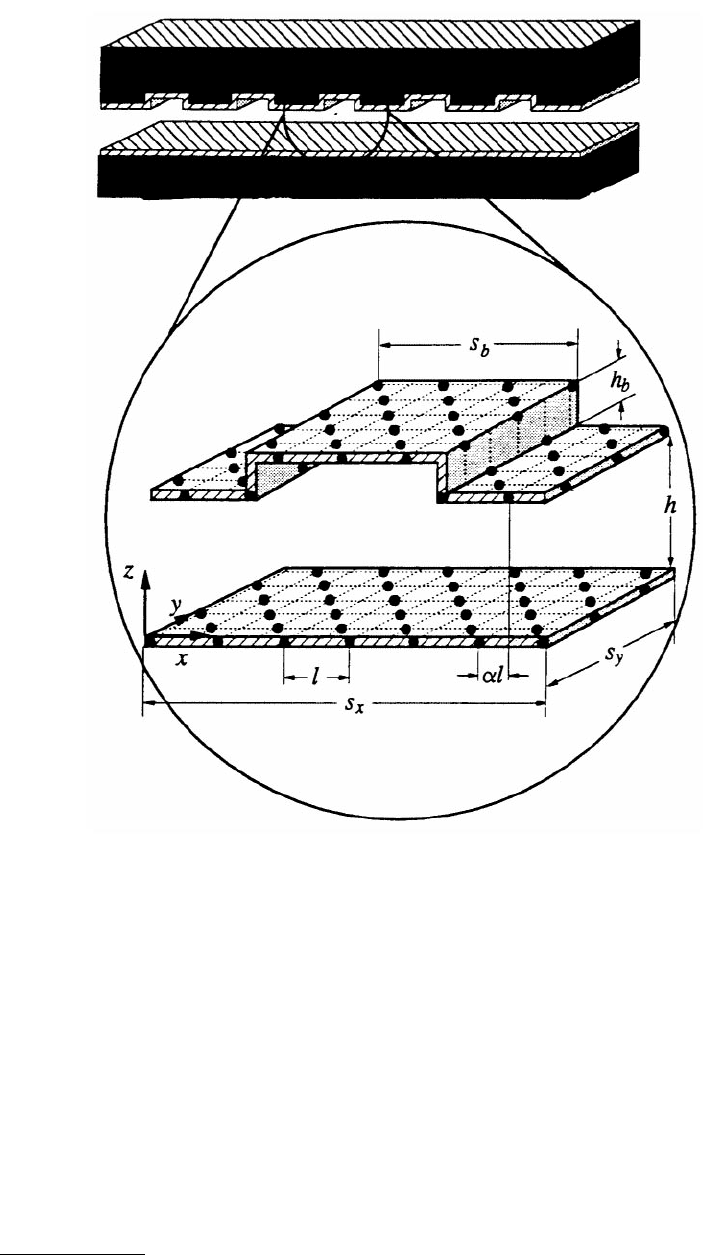
ment. One wall was atomically flat and the other was scored with regularly spaced rectilinear grooves
(i.e., corrugated) (Figure 11.26). The structure of the confined film as a function of groove dimension
and frequency was examined. In each case, the behavior of the film was studied as the registry α was
systematically varied at fixed temperature, chemical potential, and pore width.
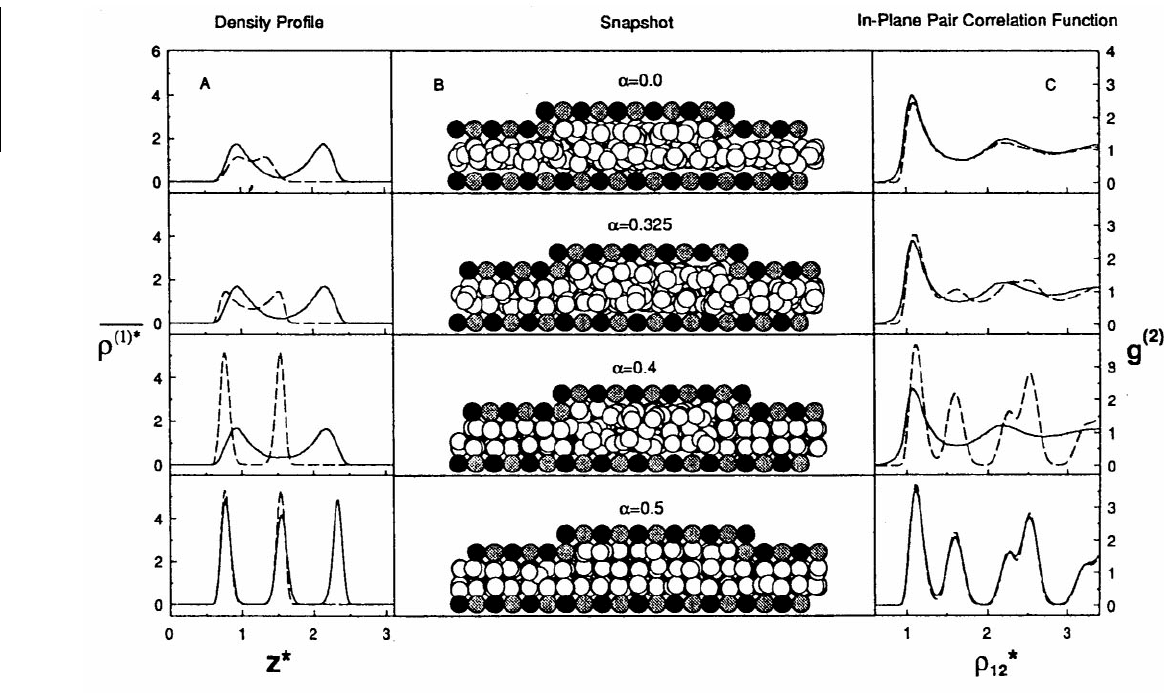
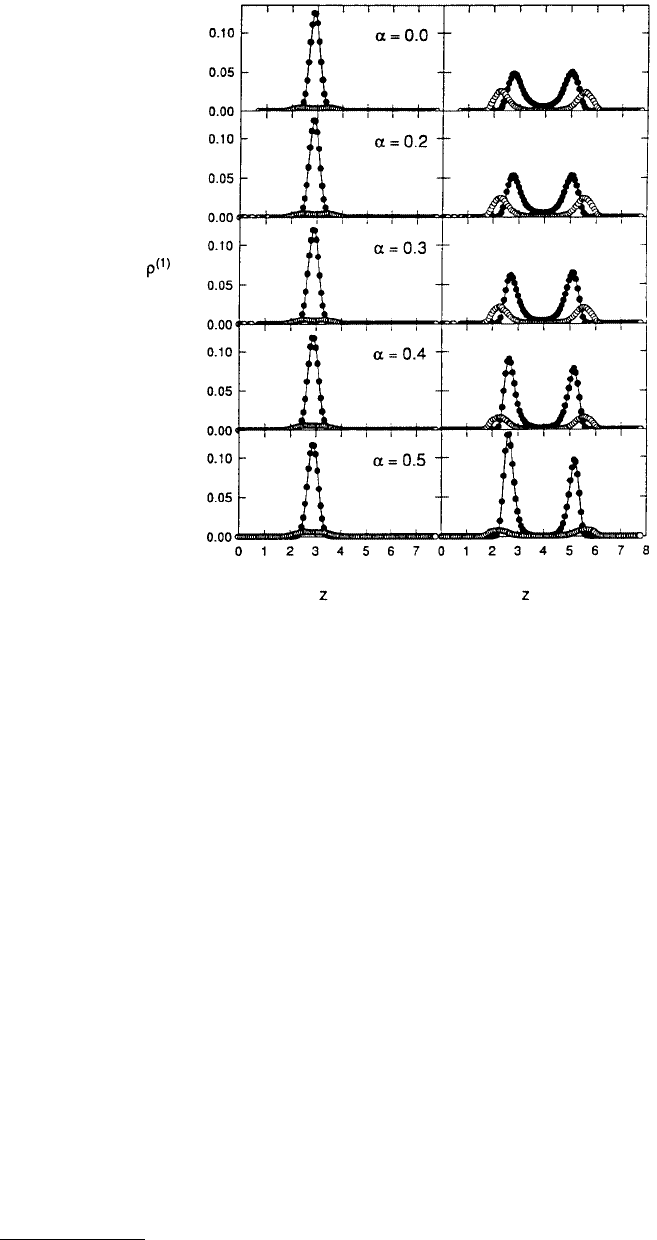
When the groove was five lattice constants wide and the walls of the corrugated pore were in registry
(α = 0.0) the one-layer liquid in the narrow region of the pore was in equilibrium with the two-layer
liquid in the wide region of the pore. Snapshots of the MD simulation taken together with the broad
peaks in the density profile confirmed this conclusion (Figure 11.27). Changing the registry of the pore
walls significantly affected the structure of the liquid. It was clear from examination of the density profiles
that increasing the registry to α = 0.325 caused the liquid in the narrow region of the pore to begin to
separate into two layers, while the liquid in the wide region of the pore remained unaffected. At α = 0.4,
the liquid in the narrow portion of the pore had solidified into two distinct layers while there were still
two liquid layers in the wide region of the pore. Thus, the confined film consisted of parallel, rectilinear
liquid-filled nanocapillaries separated by solid strips. Increasing the registry to α = 0.5 (exactly out of
registry) caused the entire film to solidify and resulted in sharp peaks in the density profile.
The structural character within the film was ascertained from examination of the in-plane pair-
correlation function g
(2)
(Figure 11.27). For registries corresponding to α = 0.0 and α = 0.325, the
configurations were disordered or liquid-like as evidenced by the lack of structure in g
(2)
. The in-plane
pair correlation functions calculated when α = 0.4 clearly showed a different degree of transverse order
in the narrow portion of the pore than in the wide region of the pore. This supported the conclusion
that nanocapillaries had formed. When the registry was increased to α = 0.5 the in-plane pair correlation
functions for both regions of the pore were identical and very structured, indicating total solidification
of the film.
FIGURE 11.25 Dependence of time-averaged liquid viscosity
µ (µ = fh/v, where f is the mean frictional force per unit area,
h is the wall separation, and v is the sliding velocity) on the
shear rate γ (γ = v/h). The variable P
⊥
represents the pressure
on the walls and m
l
corresponds to the number of fluid layers.
The dashed line has a slope of –
⅔. (From Thompson, P. A.
et al. (1992), Phys. Rev. Lett. 68, 3448–3451. With permission.)
© 1999 by CRC Press LLC
Plots of the stress in the film, T*
zx
, as a function of α also yielded insights into the sequence of phase
transitions experienced by the film (Figure 11.28). When α = 0.5 the corrugated-pore film consisted of
two solid layers in the narrow region and three in the wide region. In comparison, the pore with no
groove contained two solid layers. Because the surfaces are symmetrically arranged when α = 0.5, the
shear stress was equal to zero. Pushing the walls into registry (i.e., decreasing α) caused the shear stress
to increase rapidly. Because the corrugated-pore film was, on average, thicker than the film in the
atomically flat pore, the shear stress was less for the corrugated-pore film (Schoen et al., 1994). The shear
stress in the ungrooved pore rose rapidly with increasing α and then dropped precipitously when α fell
below 0.33. This sharp drop was due to abrupt melting and the concomitant loss of nearly an entire layer
of the film. The result was a one-layer liquid film that continued to support substantial shear stresses
FIGURE 11.26 Schematic representation of a corrugated slit pore. The walls consist of atoms rigidly fixed in the
(100) plane of the fcc lattice. The lattice constant corresponds to l (l = 1.5985σ), the cell dimensions are defined by
s
y
and s
x
, the separation of the walls is h in the narrow regions and h + h
b
in the wide regions, and α determines the
alignment of the walls. When α = 0 the walls are in registry. The origin of the coordinate system lies at the intersection
of the vectors labeled x, y, and z. (From Curry, J. B. et al. (1994). J. Chem. Phys. 101, 10824–10832. With permission.)
© 1999 by CRC Press LLC
FIGURE 11.27 Local density profiles ρ
(1
*
)
(z) in (A), snapshots looking down the y-axis of the pore fluid for various registries in (B), and in-plane pair
correlation functions g
(2)
in (C). The dotted and solid lines correspond to the functions calculated separately in the narrow and wide regions of the pore. In
the snapshots black and gray shaded spheres correspond to alternating rows of the fcc lattice and unshaded spheres correspond to film atoms. Starred variables
are reported in reduced units, that is, z
*
= z/σ and ρ
*
= ρ × σ
3
. (From Curry, J. B. et al. (1994), J. Chem. Phys. 101, 10824–10832. With permission.)

© 1999 by CRC Press LLC
down to α = 0.30. In contrast, the corrugated-pore film did not all melt at once as α was varied. First,
when α = 0.45 the solid in the wide region of the pore melted and almost an entire layer of liquid exited
the wide region of the pore. This was accompanied by a drop in the shear stress of the film (Figure 11.28).
The shear stress decreased approximately linearly with decreasing α until approximately equal to 0.35.
Beyond this point the solid in the narrow region of the pore melted and one layer of liquid exited the
pore; consequently, the shear stress decreased significantly. Because the film in the narrow region of the
pore melted gradually, the shear stress in the region of α = 0.35 was rounded (Figure 11.28). The melting
of the film in the narrow region of the pore began at the interface between the narrow and wide regions
and progressed toward the middle of the narrow region with increasing α.
Finally, the range of registry values where the solid (narrow region of pore) and liquid (wide region
of pore) coexist was shown to increase with increasing groove width. In the limit of very large groove
width, the fluid in the wide region of the pore acted like an ungrooved or smooth pore.
Curry and Cushman (1995a,b) also used the grand canonical Monte Carlo method to study binary
mixtures of LJ atoms confined to a corrugated pore that were in thermodynamic equilibrium with their
bulk-phase counterpart. The geometry of the corrugated pore was the same as it was in a previous work
(Curry et al., 1994) (Figure 11.26). The fluid and wall atoms were spherical, nonpolar, LJ atoms charac-
terized by diameters σ
i
and interaction energies ε
i
, where i represents the type of atom. The cutoff distance
for the shifted force LJ potential used in this work depended on σ
ij
, where σ
ij
= ½(σ
i
+ σ
j
). Thus, the
cutoff distance varied depending on the identity of the atoms. The values of σ for the two different-sized
atoms (large and small) were chosen so that the mixture resembled a mixture of octamethyltetracyclosi-
loxane and cyclohexane because this mixture has been used in SFA experiments. Two different pores
were examined: one with the walls composed of large atoms and one with the walls composed of small
atoms. For each pore, two binary mixtures were examined, with either 86% of 15% large atoms. Thus,
four combinations of wall size and fluid atom mixture were studied.
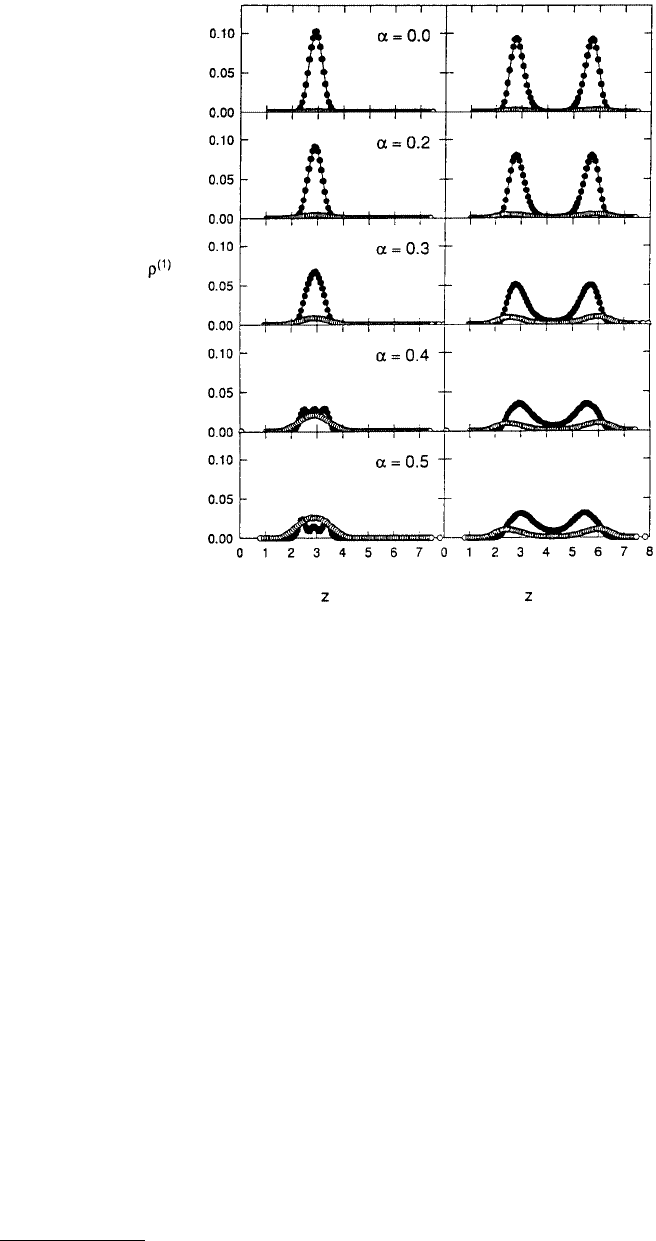
When the wall atoms were large and the bulk fluid was composed of 86% large atoms, the small atoms
were nearly eliminated from both regions of the pore (Figure 11.29) when the walls were in registry (α =
0.0). The pore width was such that one solid-like fcc layer of large atoms fit into the narrow region of
the corrugated pore while the wide region accommodated two solid-like fcc layers of large atoms. Altering
the alignment of the pore walls gradually liquefied the film in both regions of the pore, as evidenced by
the broadening of the local density profiles and the reduced structure in the in-plane pair-correlation
functions. In addition, as the film melted a significant number of small atoms were able to enter both
the narrow and the wide regions of the pore. This effect was most marked when the walls were totally
out of alignment (α = 0.5). The reason for this is that the alignment of the wall atoms effectively decreased
the pore width. This had the effect of excluding large atoms from discrete regions of the pore.
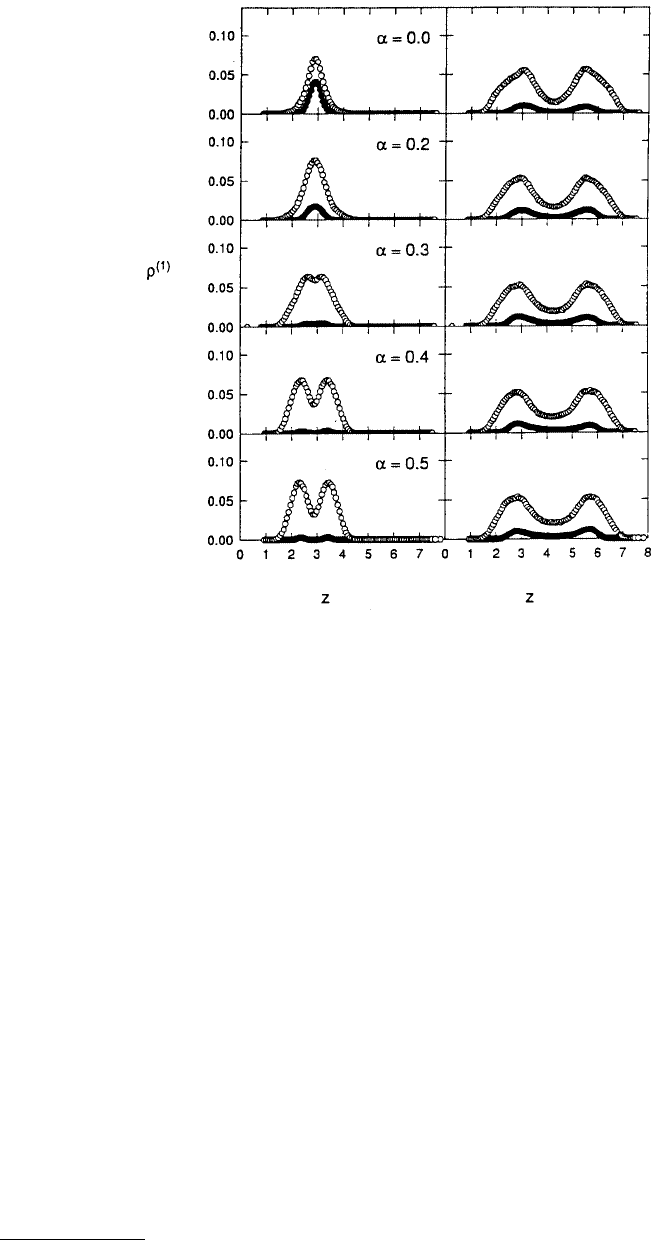
Decreasing the percentage of large atoms (15%) in the bulk dramatically affected the behavior of the
mixture in the pore. For example, when α = 0.0, small atoms were no longer excluded from the pore.
Indeed, in the wide region of the pore a two-layer, liquid-like film composed of small and large atoms
was formed. The liquid-like nature of the film was confirmed using both the density profiles (Figure 11.30)
and the in-plane pair-correlation function. This film was only slightly affected by changing the registry
FIGURE 11.28 Shear stress T
zx
in the x-direction vs.
registry for the ungrooved slit pore (open squares) with
h
*
= 2.3 and for the corrugated pore shown in
Figure 11.27 (filled circles) with h
*
= 2.3 in the narrow
region. Starred variables are reported in reduced units,
that is, T
zx
= T
zx
× σ
3
/ε. (From Curry, J. B. et al. (1994),
J. Chem. Phys. 101, 10824–10832. With permission.)
*
*
© 1999 by CRC Press LLC
of the walls. In the narrow region of the pore, the large atoms formed a one-layer solid and the number
of large atoms participating in the structure was small compared with the number of small atoms.
Decreasing the registry of the surfaces (increasing α) caused the large atoms to be driven from the narrow
region of the pore and resulted in the formation of a two-layer small-atom solid. Examination of the in-
plane correlation function confirmed the existence of the two-layer solid at α = 0.5.
When the wall atoms were small and the mixture was composed of 86% large atoms, the narrow and
the wide regions of the pore contained a one-layer and two-layer liquid, respectively (Figure 11.31). In
the narrow region of the pore the one-layer liquid was composed mostly of large atoms, and changing
the registry of the pore walls had very little effect. In contrast, the two-layer liquid in the wide region of
the pore was composed of both large and small atoms. Altering the registry of the pore walls drove the
small atoms from the wide region of the pore and caused the film to solidify. The two-layer solid in the
wide region was in equilibrium with the one-layer liquid in the narrow region. This freezing in the wide
region while the narrow region remained liquid was not observed in the other cases considered. Indeed,
if the component that froze was the same size as the wall atoms, the atoms in the wide region of the pore
froze only after the atoms in the narrow region were frozen (i.e., after nanocapillaries were formed).
Last, decreasing the percentage of large atoms in the mixture while keeping the size of the wall atoms
small resulted in liquid-like structures, composed of large and small atoms, in both the wide and narrow
regions of the pore. Altering the registry of the walls had little effect on the liquid in the wide region of
FIGURE 11.29 Local density profiles ρ
(1)
for large atoms (filled circles) and small atoms (open circles) calculated
separately for the narrow (left) and wide (right) regions of the pore at various registries α. (The variable z is defined
in Figure 11.27.) The bulk fluid contained 86% large atoms and the period of the corrugation s
x
(Figure 11.26) was
10l, where l is the lattice constant. The value of l used in these simulations was 1.5985σ
w
, where σ
w
corresponds to
the diameter of the wall atoms and was equal to the diameter of the large fluid species. The variables s
y
and s
b
(Figure 11.27) were both set to 5l. (From Curry J. E. and Cushman, J. H. (1995a), J. Chem. Phys. 103, 2132–2189.)
© 1999 by CRC Press LLC
the pore. In contrast, the small atoms, which possessed a one-layer liquidlike structure in the narrow
region of the pore, developed a two-layer structure as the registry was changed.
The corrugated-pore studies of Curry and co-workers (Curry et al., 1994; Curry and Cushman,
1995a,b) provided unique insight into the behavior of mixed LJ liquids confined between nonuniform
surfaces. The behavior of liquids between nonuniform surfaces under shear was also investigated by Gao
et al. (1995) using MD simulations. In that work, Gao et al. (1995) confined hexadecane (n–C
16
H
34
)
between two Au substrates with exposed (111) faces. Topographical nonuniformities (or asperities) were
modeled by flat-topped pyramidal Au structures of height h
a
from the underlying Au surface. The
asperities had a finite length in the sliding direction and an infinite length in the direction perpendicular
to sliding. In this respect, they were very similar to the corrugated slit pore geometry of Curry et al.
(1994). The interactions between Au atoms were modeled by EAM (Foiles et al., 1986), the alkane
molecules were modeled by the united-atom model, and the interactions between the molecular segments
and gold atoms were modeled by an LJ potential.
Sliding was performed by moving the opposing Au surfaces in opposite directions so that the relative
sliding speed was 10 m/s. the simulations typically lasted 1.2 ns and were carried out at 350 K. Three
simulation geometries were examined. The first consisted of 422 hexadecane molecules with the separa-
tion between asperity tops held at 17.5 Å. The second, “near-overlap,” case consisted of 243 hexadecane
molecules with the relative separation between asperity tops set to 4.6 Å. The third, “overlap,” case
consisted of 201 fluid molecules with a relative separation between asperity tops of –6.7 Å. (It should be
noted that the height of the asperities was 9.3 Å in geometries one and two. In the third geometry the
FIGURE 11.30 Local density profiles ρ
(1)
for large atoms (filled circles) and small atoms (open circles) calculated
separately for the narrow (left) and wide (right) regions of the pore at various registries α. The bulk fluid contained
15% large atoms and all other pore parameters were the same as in Figure 11.29. (From Curry, J. E. and Cushman,
J. H. (1995a), J. Chem. Phys. 103, 2132–2139.)
© 1999 by CRC Press LLC
asperities were larger at 14 Å.) Prior to the start of the sliding simulations, each system was equilibrated
with the asperities well separated in the horizontal direction such that steady-state flow was established.
From an examination of the atomic and molecular configurations recorded as a function of time
during the shearing process, Gao et al. (1995) were able to elucidate two main patterns for all three
simulation geometries. First, there was an evolution of layered liquid structures in the region between
the colliding asperities. In particular, for large transverse separations of the asperities, layering of the
liquid in the interasperity region was initially absent and developed dynamically as the asperities
approached each other. Furthermore, the number of layers in the interasperity region evolved in each
case in a quantized manner as successive layers were squeezed out as the asperities drew near. Finally,
severe deformation of the asperities resulted for the near-overlapping and overlapping asperity height
cases.
This interasperity layering phenomenon was also apparent from examination of the time variation of
the shear ( f
x
) and normal ( f
z
) forces. For example, for the near-overlap case, plots of both f
x
and f
z
vs.
time had oscillatory patterns prior to the collision between asperities. This oscillatory behavior of the
forces was reminiscent of solvation-force oscillations observed between smooth solid surfaces under
equilibrium conditions.
Gao et al. (1995) also commented on the crucial importance of the dynamic mechanical response of
the substrate (and the asperities) in determining the evolution and properties of the sheared systems.
FIGURE 11.31 Local density profiles ρ
(1)
for large atoms (filled circles) and small atoms (open circles) calculated
separately for the narrow (left) and wide (right) regions of the pore at various registries α. The bulk fluid contained
86% large atoms and the period of the corrugation s
x
(Figure 11.26) was 14l, where l is the lattice constant. The value
of l equaled 1.5985σ
w
, where σ
w
corresponds to the diameter of the wall atoms and was equal to the diameter of the
small fluid species. The parameters s
y
and s
b
(Figure 11.26) were both set to 7l. (From Curry, J. E. and Cushman,
J. H. (1995a), J. Chem. Phys. 103, 2132–2139.)

© 1999 by CRC Press LLC
When the asperities were separated vertically by large distances (the first simulation geometry), the shear
flow was accompanied by a structuring of the liquid with no distortions of the metal surfaces. In contrast,
for the overlapping and near-overlapping cases, shearing resulted in pressurization of the liquid in the
interasperity region, accompanied by a significant increase in effective viscosity. This resulted in plastic
deformations of the gold asperities.
It is clear, based on the discussion in this section, that fluids confined to areas of atomic-scale
dimensions do not necessarily behave like liquids on the macroscopic scale. In fact, depending on the
condition, they may often behave more like solids in terms of structure and flow. This presents a new
set of concerns for lubricating moving parts at the nanometer scale. However, with the aid of simulations
like the ones mentioned here, plus experimental studies using techniques such as the SFA, general
properties of nanometer-scale fluids are being characterized effectively with profound precision. This, in
turn, will allow scientists and engineers to design new materials and interfaces that will have specific
interactions with confined lubricants, effectively controlling friction (and wear) at the atomic scale.
11.5 Friction
Friction at sliding solid interfaces results from the conversion of the work of sliding into some other,
less-ordered, form. For strongly adhering systems, the work of sliding may be converted into damage
within the bulk. As adhesive forces are decreased between the solid surfaces, the conversion of work
changes to mechanical damage at or near the surface, leading to, for example, the formation of wear
debris and transfer films (Singer, 1991; Singer et al., 1991). For still weaker adhesive forces, friction can
occur through the conversion of work to heat at the interface with no permanent damage to the surface.
The latter regime is the topic of this section.
While the thermodynamic principles of the conversion of work to heat are well known, many of the
detailed mechanisms at sliding interfaces are just beginning to be understood. This understanding is the
by-product of new scientific instrumentation, such as the SFA and the AFM, analytic theories, and
simulations. A detailed understanding of these processes is important for producing interfaces with
specific friction (and wear) properties.
Atomic-scale friction has been investigated using MD simulations for systems composed of a variety
of materials, in a number of geometries. For instance, the atomic-scale friction and wear of diamond
surfaces, both atomically flat and rough, was examined using MD by Harrison et al. (1992c, 1993a,b,c).
In addition, atomic-scale friction between monolayers composed of alkane chains bound to rigid sub-
strates (Glosli and McClelland, 1993), between perfluorocarboxylic acid and hydrocarboxylic LB mono-
layers (Koike and Yoneya, 1996), between contacting Cu surfaces (Hammerberg et al., 1995; Sorensen
et al., 1996), between a silicon tip and a silicon substrate (Landman et al., 1989a,b), and between con-
tacting diamond surfaces in the presence of third-body molecules (Perry and Harrison, 1997) have all
been examined using MD simulations.
Molecular dynamics simulations have also provided the first insights into tribochemical reactions that
might occur when two diamond surfaces are in sliding contact (Harrison and Brenner, 1994).
11.5.1 Solid Lubrication
Harrison et al. (1992c, 1993a,b,c) examined the atomic-scale phenomena that occurred when two (111)
diamond surfaces were placed in sliding contact. The effects of crystallographic sliding direction, tem-
perature, sliding speed, applied normal load, and nature of the surface on atomic-scale friction were
examined. The frictional properties of hydrogen-terminated diamond surfaces were compared with the
frictional properties of alkyl-terminated diamond surfaces to elucidate the effects of surface condition
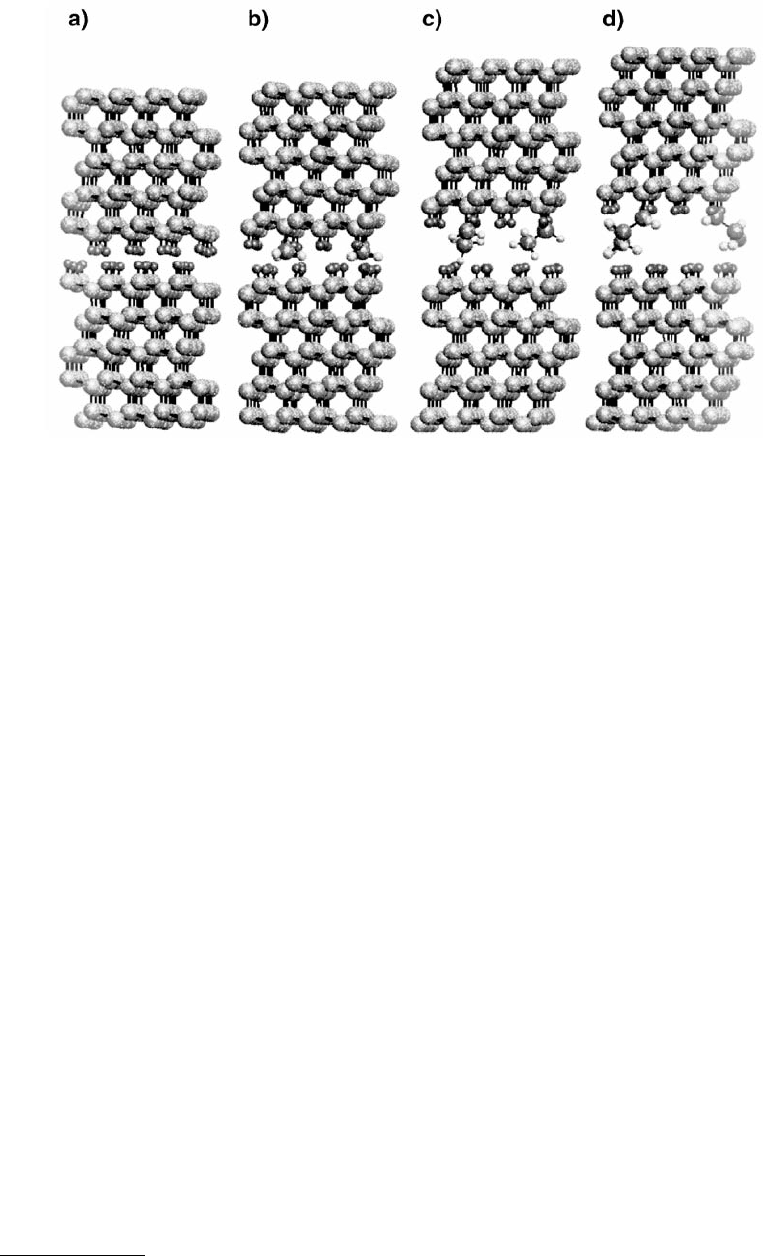
on atomic-scale friction. The alkyl-terminated systems were composed of two diamond lattices, config-
ured so that their (111) surfaces were in contact (Figures 11.32a to d). One eighth of the hydrogen atoms
on the upper surfaces were then randomly replaced with methyl (Figure 11.32b), ethyl (Figure 11.32c),
or n-propyl (Figure 11.32d) groups.
© 1999 by CRC Press LLC
In these simulations, the vertical separation of the atoms in the two outermost layers of the lattices
were held constant. The molecular dynamics friction simulations were carried out by moving the atoms
in the rigid layers of the upper surface at a constant velocity (usually 100 m/s) in the chosen crystallo-
graphic sliding direction. (Similar results were obtained with sliding speeds of 50 m/s.) Both the [1
–
10]
and the [11
–
2] crystallographic directions were examined. (These correspond to moving the upper surface
in Figure 11.32 in the page, or from left to right, respectively.) The remaining atoms were allowed to
dynamically evolve in time according to classical equations of motion. The forces governing their motion
were derived from the Brenner potential (Brenner, 1990). The temperature of the systems was maintained
at 300 K by applying a thermostat (Berendsen et al., 1984) to the middle five layers of each lattice. The
simulations were performed at average normal loads varying between 0 to 15 GPa. These pressures are
well within the range of pressures that can be achieved experimentally using an AFM (Germann et al.,
1993).
A constant value for the relative distance between the rigid layers in the top and bottom slabs was
maintained during these simulations, and this distance was used to define the separation between the
two slabs.
The average frictional forces for sliding in the [11
–
2] crystallographic direction were calculated as a
function of load for the systems shown in Figure 11.32. For an individual sliding simulation, the frictional
force was taken to be the sum of the forces in the sliding direction on the rigid-layer atoms, normalized
by the number of rigid-layer atoms and averaged over the simulation. Because the relative lateral place-
ment of the opposing diamond surfaces affects the frictional force, frictional force (and normal force)
data were also averaged over simulations whose starting configurations differed in lateral registry of the
upper surface. (For precise details, see Harrison et al., 1993c and Perry and Harrison, 1997.)
The frictional force was found to increase with normal load in all cases, usually in an approximately
linear manner (Figure 11.33). This result is entirely reasonable, and was found to originate from the
detailed atomic-scale sliding mechanisms and the associated pathways of energy dissipation. For instance,
FIGURE 11.32 Illustration of atoms of a number of diamond-containing systems viewed along the [1
–
10] crystal-
lographic direction at the start of MD simulations. The (111) faces of two hydrogen-terminated, diamond lattices
are in contact in (a). Two of the upper-surface hydrogen atoms have been replaced by methyl groups in (b), ethyl
groups in (c), and n-propyl groups in (d). Sliding was achieved by moving the top two (rigid) layers of the upper
surfaces in the [11
–
2] crystallographic (right to left in the figure). Large spheres represent carbon atoms and small
spheres represent hydrogen atoms. (From Harrison, J. A. et al. (1993), Mater. Res. Soc. Bull. 18, 50–53. With permission.)
