Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.


124 5. PULP BLEACHING
tive stage or else the oxidant will undo what the
reductive compound accomplished.
Dithionite, hydrosulfite bleaching
Hydrosulfite (the conmion name in the indus-
try, but dithionite is the preferred name) bleaching
is carried out with 0.5-1.0% dithionite on wood.
Previously, zinc dithionite was used because it is
very stable. However, zinc is toxic to fish, there-
fore,
the sodium form has replaced the zinc form.
Zinc dithionite was prepared in the pulp mill from
zinc and sulfur dioxide as follows: Zn -f- 2SO2 -*
ZnS204. Bleaching is carried out at pH 5-6 with
chelating agents such as ethylenediaminetetraacetic
acid (EDTA) or sodium tripolyphosphate (0.1-
0.2% on pulp) to prevent metal ions such as
iron(III) from coloring the pulp. Bleaching is
often carried out in the refiners. The reaction
time is on the order of 10-30 minutes. The bright-
ness gain is only 5-8%.
Dithionite reacts with oxygen, so bleaching
with it is carried out at 4% consistency; consisten-
cy below this is unnecessarily dilute, so reaction
with dissolved oxygen consumes dithionite. Con-
sistency above 4% leads to entrained air that
consumes dithionite. [The solubility of oxygen in
the atmosphere is only a few milliliters of gas per
liter of water at 25°C (77°F), and decreases with
increasing temperature; thus, entrained oxygen is
more significant than dissolved oxygen at high
consistencies for dithionite degradation.] Using
temperatures as high as 70°C (158°F) reduces the
oxygen solubility in water. Fig. 11-1 shows the
solubility of oxygen (from air) in water as a
function of temperature.
Sometimes dithionite is added between the
refiner plates where temperatures above 100°C
(212°F) cause steam to displace air. Dithionite
ion reduces lignin and is itself oxidized to sulfite
ion. If hydrogen peroxide and dithionite are used
in a two-stage process, the dithionite must be the
second stage or hydrogen peroxide will reoxidize
those moieties reduced by the dithionite. The
reaction of dithionite is shown below.
SA'" + 2H2O -> 2HS03- + 2H^ -h 2 e-
Peroxide bleaching
Some metal ions, such as Fe^"*",
Mrf"*",
and
Ctf^, catalytically decompose hydrogen peroxide,
so peroxide bleaching is carried out with agents
that deactivate these metal ions.
Fe^^
H,0
2^2
H2O + 1/2 O,
Chelating agents, such as EDTA, have the added
gain of preventing pulp discoloration by binding
with ferric ion that would otherwise form a col-
ored complex with the phenolic lignin structure.
Sodium silicate (5% on wood) is also used
(usually after the addition of magnesium
ion).
The
mechanism for inactivating the ions by sodium
silicate is not clear; it may precipitate the ions,
but, strictly speaking, it is not a chelating agent.
Buffering action is required to keep the pH high
even as organic acids are produced as a result of
some carbohydrate degradation. Sodium silicate
acts as a buffering agent.
Bleaching conditions are 0.5-3% peroxide
and 0.05% magnesium ion (to mitigate carbo-
hydrate degradation by oxygen under alkaline
conditions) on
pulp,
temperature of 40-60°C (104-
140°F) (about 20°C lower than with chemical
pulps since lignin removal is not the goal), pH of
10.5-11,
consistency of 10-20%, 1-3 hour reten-
tion
time,
with a brightness gain of 6-20%.
Hydrogen peroxide with sodium hydroxide
and/or sodium peroxide (NaOOH) is used to pro-
duce the high pH that is necessary to produce the
active perhydroxyl ion, HOO". The formation of
the perhydroxyl ion is as follows:
HA ^ HOO- H- H+ pJ^, = 11.65 @ 25°C
Some carbohydrate degradation occurs and is
responsible for about half of the peroxide con-
sumed. Pine and fir are difficult to brighten.
Color reduction occurs by altering chromophoric
groups such as orthoquinones. The pulp is some-
times subsequently treated with SO2 to neutralize
OH" and reduce any residual peroxide.
5.3 MEASUREMENT OF LIGNIN CONTENT
General
considerations
The measurement of lignin in chemical pulps
is a vital tool to monitor the degree of cook (ex-
tent of delignification during pulping) or to mea-
sure residual lignin before bleaching and between
BLEACHING MECHANICAL PULPS 125
various stages of bleaching to monitor the process,
although pulp brightness between bleaching stages
is often used to control the bleaching operation.
Since most of the lignin remains in mechanical
pulps and bleached mechanical pulps, it is not
measured in these pulps.
Lignin is easily measured indirectly by mea-
suring the amount of an oxidant (such as chlorine
or potassium permanganate) consumed by lignin in
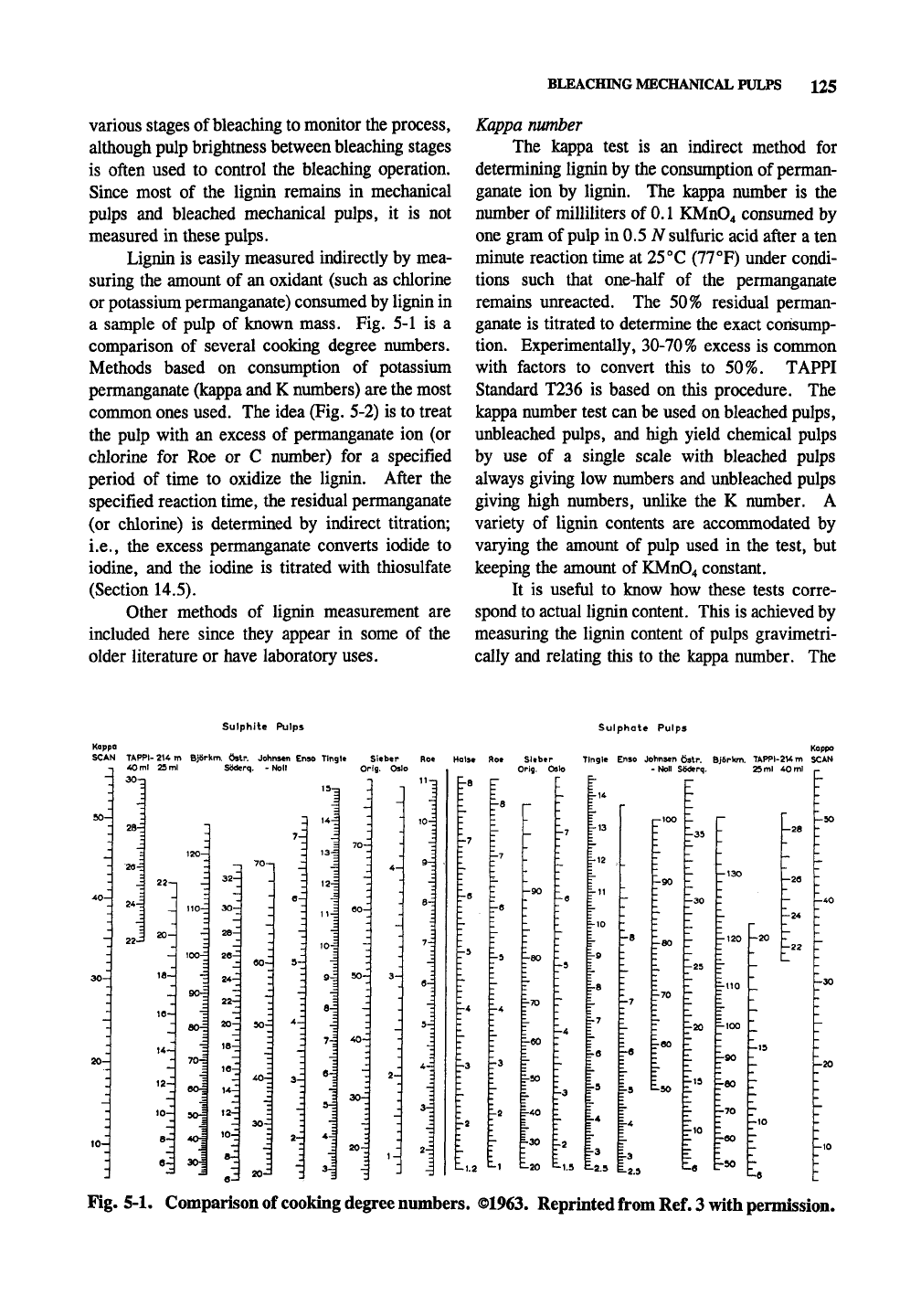
a sample of pulp of known mass. Fig. 5-1 is a
comparison of several cooking degree nimibers.
Methods based on consumption of potassium
permanganate (kappa and K numbers) are the most
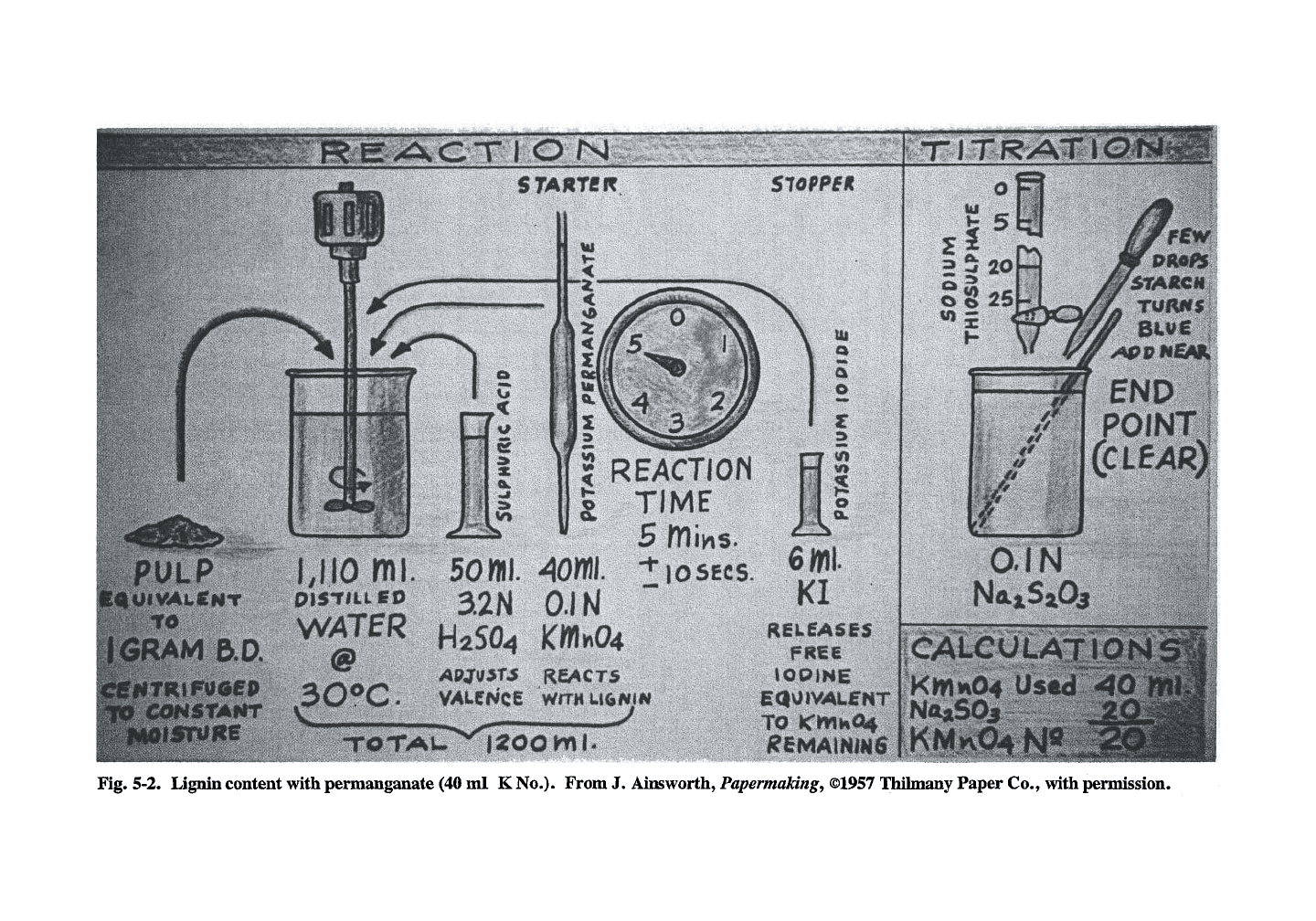
conmion ones used. The idea (Fig. 5-2) is to treat
the pulp with an excess of permanganate ion (or
chlorine for Roe or C number) for a specified
period of time to oxidize the lignin. After the
specified reaction time, the residual permanganate
(or chlorine) is determined by indirect titration;
i.e., the excess permanganate converts iodide to
iodine, and the iodine is titrated with thiosulfate
(Section 14.5).
Other methods of lignin measurement are
included here since they appear in some of the
older literature or have laboratory uses.
Kappa number
The kappa test is an indirect method for
determining lignin by the consumption of perman-
ganate ion by lignin. The kappa number is the
number of milliliters of 0.1 KMn04 consumed by
one gram of pulp in 0.5 N sulftiric acid after a ten
minute reaction time at 25°C (77°F) under condi-
tions such that one-half of the permanganate
remains unreacted. The 50% residual perman-
ganate is titrated to determine the exact consump-
tion. Experimentally, 30-70% excess is common
with factors to convert this to 50%. TAPPI
Standard T236 is based on this procedure. The
kappa number test can be used on bleached pulps,
unbleached pulps, and high yield chemical pulps
by use of a single scale with bleached pulps
always giving low numbers and unbleached pulps
giving high numbers, unlike the K number. A
variety of lignin contents are accommodated by
varying the amount of pulp used in the test, but
keeping the amount of KMn04 constant.
It is useful to know how these tests corre-
spond to actual lignin content. This is achieved by
measuring the lignin content of pulps gravimetri-
cally and relating this to the kappa number. The
Sulphite Pulps
Kappa
SCAN
Sulphate Pulps
TAPPI-2Km BjSrkm. Oslr. Johnsen Enso Tingle Sieber Roe Holse Roe Sieber Tingle Enso Johnsen
6SIP.
Bj6rknf«. TAPPI-2Km SCAN
«OmI 25ml ^—- '• " -. -. _. _. ........ „ . .. .
Sdderq.
- Noll
Orig.
Oslo
20^
'2-1
10
8
e-1
26-1
24-
22-
20-^
IB-
le^
H-
12-
10-3
6
Grig.
Oslo - Noil S6derq.
•30 g.2 I
•20
E"3 E
'^l.S E.2.5
E-3
E.2.5
•20
F-lOO
F-90
15 |eo
E- E-70
P-10 E
t-50 tr
Fig. 5-1. Comparison of cooking degree numbers. ©1963. Reprinted from Ref. 3 with permission.
~rQ
C~
Q~
C~
C~
C~
~D
@
0
Q
t,J,m
ID

BLEACHING MECHANICAL PULPS 127
Klason lignin prcx:edure is described below.
Klason lignin is considered to be essentially the
same as the actual lignin content. Alander,
Palenius, and Kyrklund (1963) give the following
relationship for sulfite and kraft chemical hard-
wood pulps:
Klason lignin, % = 0.15 x kappa number
This relationship is approximately correct for
softwoods as well. Chiang et al (1987) found the
coefficient of 0.159 for Douglas-fir and 0.168 for
western hemlock. The authors related kappa
number to the sum of Klason lignin and acid solu-
ble lignin (which are defined below).
Permanganate
number,
K
number
The permanganate (or K) number, is really
four different tests. A constant amount of
pulp
is
used with either 25 ml (for bleached pulp), 40 ml
(Fig. 5-2), 75 ml, or 100 ml (for high yield pulps)
of permanganate. Results of
the
100 ml K number
test are not easily compared to the results of the
75 ml (or any other) K number test, so there is no
continuum or results for all types of
pulps
as with
the kappa test. Guillory (1982) gives the relation-
ship in the equation:
log (kappa no.) =
0.837
+ 0.0323 (40 ml K no.)
Therefore, a 40 ml K number of
10
corresponds to
a kappa no. of 14.5, 20 (40 ml K number) corre-
sponds to 30.4 kappa number, and 30 (40 ml K
number) corresponds to 64.1 kappa number.
Roe
number
The Roe number is a measure of lignin
content by the number of grams of gaseous Clj
consumed by 100 grams dry pulp at 25 °C (77 °F)
in 15 minutes. TAPPI Standard 202 (now with-
drawn) was one method. Alander, Palenius, and
Kyrklund (1963) give the following relationship
for hardwood pulps:
Roe number = 0.158 x kappa - 0.2 (kraft)
Roe number = 0.199 x kappa + 0.1 (sulfite)
Chlorine
number,
C, hypo number
The chlorine number is a test method similar
to that of
Roe,
except the CIO2 is generated
in
situ
by acidification of sodium hypochlorite. TAPPI
Standard T 253 uses this method to determine a
hypo number. The following empirical equation
relates the chlorine number to the Roe number.
Chlorine number = 0.90 x Roe number
Klason
lignin,
acid
insoluble lignin
Klason lignin is the residue obtained after
total acid hydrolysis of
the
carbohydrate portion of
wood. It is a gravimetric method for determining
lignin directly in woody materials, for example, by
TAPPI Standard T 222. This method is not used
for routine quality control in the mill, but has uses
in the laboratory. Wood meal or pulp is treated
with 72% sulfiiric acid at 20°C (68°F) for 2.0
hours followed by dilution to
3%
sulfiiric acid and
refluxing for 4 hours. The lignin is filtered in a
tared crucible, washed, dried, and weighed. The
isolated lignin in this manner is degraded consid-
erably; nevertheless, it corresponds (by weight)
closely to the original amount of lignin in the
sample. Some of the lignin, especially in sulfite
or hardwood pulps, remains soluble (called acid
soluble lignin) and can be estimated spectrophoto-
metrically in the UV region.
In the wood chemistry literature many modi-
fications of
the
hydrolysis conditions exist, but the
difference in the amount of lignin isolated is
probably not appreciable. For example, the
primary hydrolysis may be carried out for 1.5 h at
25^C (77^F) or
1
h at 30°C (86°F); the secondary
hydrolysis is often carried out at 4 or 6% H2SO4
for 3 to 4 hours, but TAPPI Standards seldom
offer such flexibility.
5.4 BLEACHING CHEMICAL PULPS
Cellulose
viscosity
The cellulose
degree
of polymerization in low
yield pulps and bleached pulps is very important
since these processes lower the degree of poly-
merization of cellulose to the point where the
paper strength properties are adversely affected.
Cellulose viscosity of mechanical pulps and high
yield pulps are not measured since it is usually
quite high and not a factor in the strength proper-
ties of
papers
derived from these pulps. Cellulose
viscosity is measured by dissolving the pulp in
128
5. PULP BLEACHING
o
LiJ
STAGE 3
/ STAGE 2
/ STAGE 1
V
CHEMICAL ON PULP
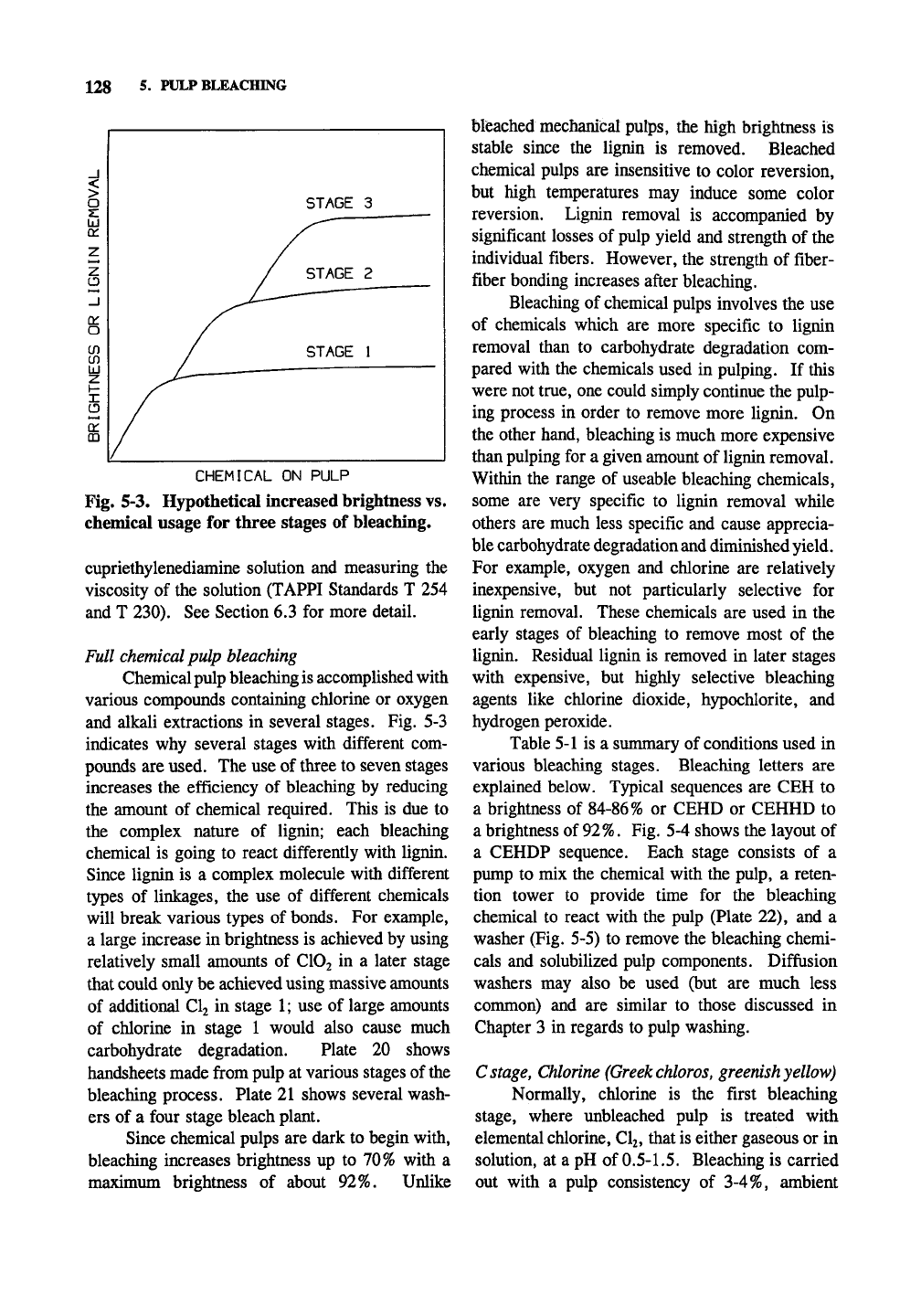
Fig. 5-3. Hypothetical increased brightness vs.
chemical usage for three stages of bleaching.
cupriethylenediamine solution and measuring the
viscosity of the solution (TAPPI Standards T 254
and T 230). See Section 6.3 for more detail.
Full
chemical
pulp bleaching
Chemical pulp bleaching
is
accomplished with
various compounds containing chlorine or oxygen
and alkali extractions in several stages. Fig. 5-3
indicates why several stages with different com-
pounds are used. The use of three to seven stages
increases the efficiency of bleaching by reducing
the amount of chemical required. This is due to
the complex nature of lignin; each bleaching
chemical is going to react differently with lignin.
Since lignin is a complex molecule with different
types of linkages, the use of different chemicals
will break various types of bonds. For example,
a large increase in brightness is achieved by using
relatively small amounts of ClOj in a later stage
that could only be achieved using massive amounts
of additional Clj in stage 1; use of large amounts
of chlorine in stage 1 would also cause much
carbohydrate degradation. Plate 20 shows
handsheets made from pulp at various stages of the
bleaching process. Plate 21 shows several wash-
ers of a four stage bleach plant.
Since chemical pulps are dark to begin with,
bleaching increases brightness up to 70% with a
maximum brightness of about 92%. Unlike
bleached mechanical pulps, the high brightness is
stable since the lignin is removed. Bleached
chemical pulps are insensitive to color reversion,
but high temperatures may induce some color
reversion. Lignin removal is accompanied by
significant losses of pulp yield and strength of the
individual fibers. However, the strength of fiber-
fiber bonding increases after bleaching.
Bleaching of chemical pulps involves the use
of chemicals which are more specific to lignin
removal than to carbohydrate degradation com-
pared with the chemicals used in pulping. If this
were not true, one could simply continue the pulp-
ing process in order to remove more lignin. On
the other hand, bleaching is much more expensive
than pulping for a given amount of lignin removal.
Within the range of useable bleaching chemicals,
some are very specific to lignin removal while
others are much less specific and cause apprecia-
ble carbohydrate degradation and diminished yield.
For example, oxygen and chlorine are relatively
inexpensive, but not particularly selective for
lignin removal. These chemicals are used in the
early stages of bleaching to remove most of the
lignin. Residual lignin is removed in later stages
with expensive, but highly selective bleaching
agents like chlorine dioxide, hypochlorite, and
hydrogen peroxide.
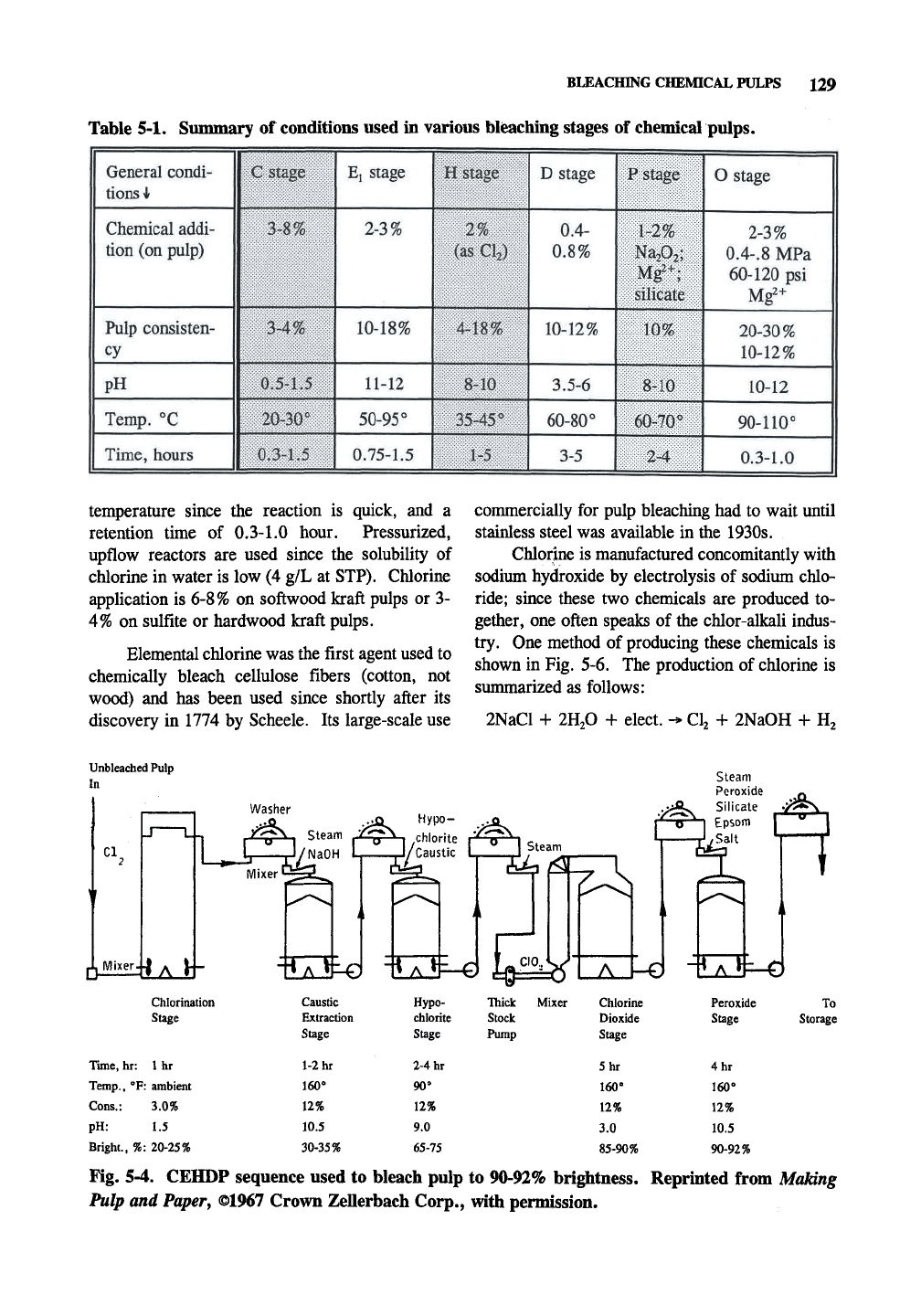
Table 5-1 is a summary of conditions used in
various bleaching stages. Bleaching letters are
explained below. Typical sequences are CEH to
a brightness of 84-86% or CEHD or CEHHD to
a brightness of
92%.
Fig. 5-4 shows the layout of
a CEHDP sequence. Each stage consists of a
pump to mix the chemical with the pulp, a reten-
tion tower to provide time for the bleaching
chemical to react with the pulp (Plate 22), and a
washer (Fig. 5-5) to remove the bleaching chemi-
cals and solubilized pulp components. Diffusion
washers may also be used (but are much less
common) and are similar to those discussed in
Chapter 3 in regards to pulp washing.
C stage,
Chlorine (Greek
chloros,
greenish yellow)
Normally, chlorine is the first bleaching
stage, where unbleached pulp is treated with
elemental chlorine, CI2, that is either gaseous or in
solution, at a pH of 0.5-1.5. Bleaching is carried
out with a pulp consistency of 3-4%, ambient
BLEACHING CHEMICAL PULPS 129
Table 5-1. Summary of conditions used in various bleaching stages of chemical pulps.
General condi-
tions
i
Chemical addi-
tion (on pulp)
Pulp consisten-
cy
|pH
1
Temp.
"C
1
Time, hours
C stage
1
3-8%
3-4%
1 0,5-1.5
1 20-30"
1 0,3-1.5
E, stage
2-3%
10-18%
11-12
50-95°
0.75-1.5
MM^mm
iiiiiiiiii
iiilillii
mmmmmi
iiiiiiiiii
llliilill
iiii»iii:i
D stage
0.4-
0.8%
10-12%
3.5-6
60-80°
3-5
P stage
1-2%
NajOj;
Mg^+;
silicate
10%
8-10
60-70°
2-4
O stage
2-3%
0.4-.8
MPa
60-120 psi
Mg2+ 1
20-30%
10-12%
1
10-12
1
90-110°
1
0.3-1.0
1
temperature since the reaction is quick, and a
retention time of
0.3-1.0
hour. Pressurized,
upflow reactors are used since the solubiUty of
chlorine in water is low (4 g/L at STP). Chlorine
application is 6-8% on softwood kraft pulps or 3-
4%
on sulfite or hardwood kraft pulps.
Elemental chlorine was the first agent used to
chemically bleach cellulose fibers (cotton, not
wood) and has been used since shortly after its
discovery in 1774 by Scheele. Its large-scale use
commercially for pulp bleaching had to wait until
stainless steel was available in the 1930s.
Chlorine is manufactured concomitantly with
sodium hydroxide by electrolysis of sodium chlo-
ride;
since these two chemicals are produced to-
gether, one often speaks of the chlor-alkali indus-
try. One method of producing these chemicals is
shown in Fig. 5-6. The production of chlorine is
summarized as follows:
2NaCl + 2H,0 + elect. -> CL + 2NaOH + H,
Unbleached Pulp
In
CI
D
]!M-< A H-
Chlorination
Stage
Time, hr: 1 hr
Temp.,
'F: ambient
Cons.:
3.0%
pH: 1.5
Bright., %: 20-25%
Washer
Mixer
Steam -
NaOH
I
^x
IZf^
Hypo-
chlorite
Caustic
^RTIE
.J:^
:2^
^
I Steam
j^
Caustic
Extraction
Stage
1-2
hr
160*
12%
10.5
30-35%
Hypo-
chlorite
Stage
2-4 hr
90"
12%
9.0
65-75
Thick
Stock
Pump
Mixer
Steam
Peroxide
Silicate
Epsom
Salt
'TZJ-o
Chlorine
Dioxide
Stage
5hr
160»
12%
3.0
85-90%
Peroxide
Stage
4hr
160'
12%
10.5
90-92%
To
Storage
Fig. 5-4. CEHDP sequence used to bleach pulp to 90-92% brightness. Reprinted from Making
Pulp and
Paper,
©1967 Crown Zellerbach Corp., with permission.
130 5. PULP BLEACHING
Fig. 5-5. An open, bleach washer of the vabuum drum type. Modem washers are enclosed.
Chlorine is not overly specific to lignin, and
much carbohydrate degradation occurs through its
use.
The chlorine reacts with lignin by substitu-
tion of hydrogen atoms for chlorine atoms (partic-
ularly on the aromatic ring), oxidation of lignin
moieties to carboxylic acid groups, and, to a small
extent, addition of chlorine across carbon-carbon
double bonds (Fig. 5-7). The substitution reac-
tions (Fig. 5-8) are probably the most important in
the ultimate lignin removal.
It has been known for a long time that chlo-
rine is first rapidly depleted by the pulp and then
Direct ^
Currentc;,«/)>jCl2
2^^*2H20
Asbestos Paper
Diaphragm >
Perforated Iron .
Cathode Plate X
j:
Glass ^' '^ ^
Fig. 5-6. A method of NaOH and Clj manufac-
ture.
Redrawn from J. Ainsworth, Papermaking,
®1957
Thilmany Paper Co., with permission.
is depleted much more slowly. This indicates
several types of reactions are occurring. Fig. 5-9
shows how chlorine reacts with pulp as a function
of time. The substitution and addition reactions
are much faster than the oxidation reactions and do
not leave CI" in solution, whereas oxidation reac-
tions do. This is the basis for determining the
type of reaction occurring.
Oxidation includes reactions with both lignin
and carbohydrates. Oxidation of the carbohydrates
leads to a decreased cellulose viscosity and de-
creased pulp strength. The species HCIO (Fig.
17.1 shows its formation versus pH) especially
contributes to oxidative degradation of the carbo-
hydrates; therefore, chlorination is carried out
above pH 0.5 (to avoid acid hydrolysis of cellu-
lose) and below pH 1.5 (to avoid carbohydrate
degradation by oxidation). Also sodium hypochlo-
rite bleaching is carried out with excess NaOH to
avoid carbohydrate oxidation by HCIO. Accord-
ing to Giertz (1951) the actual species causing
oxidation may be a complex of hypochlorous acid
and hypochlorite ion as the pH of maximum
degradation is 6.5 to 7.5 and not 4-5, where
hypochlorous is at a maximum (Fig. 17-1).
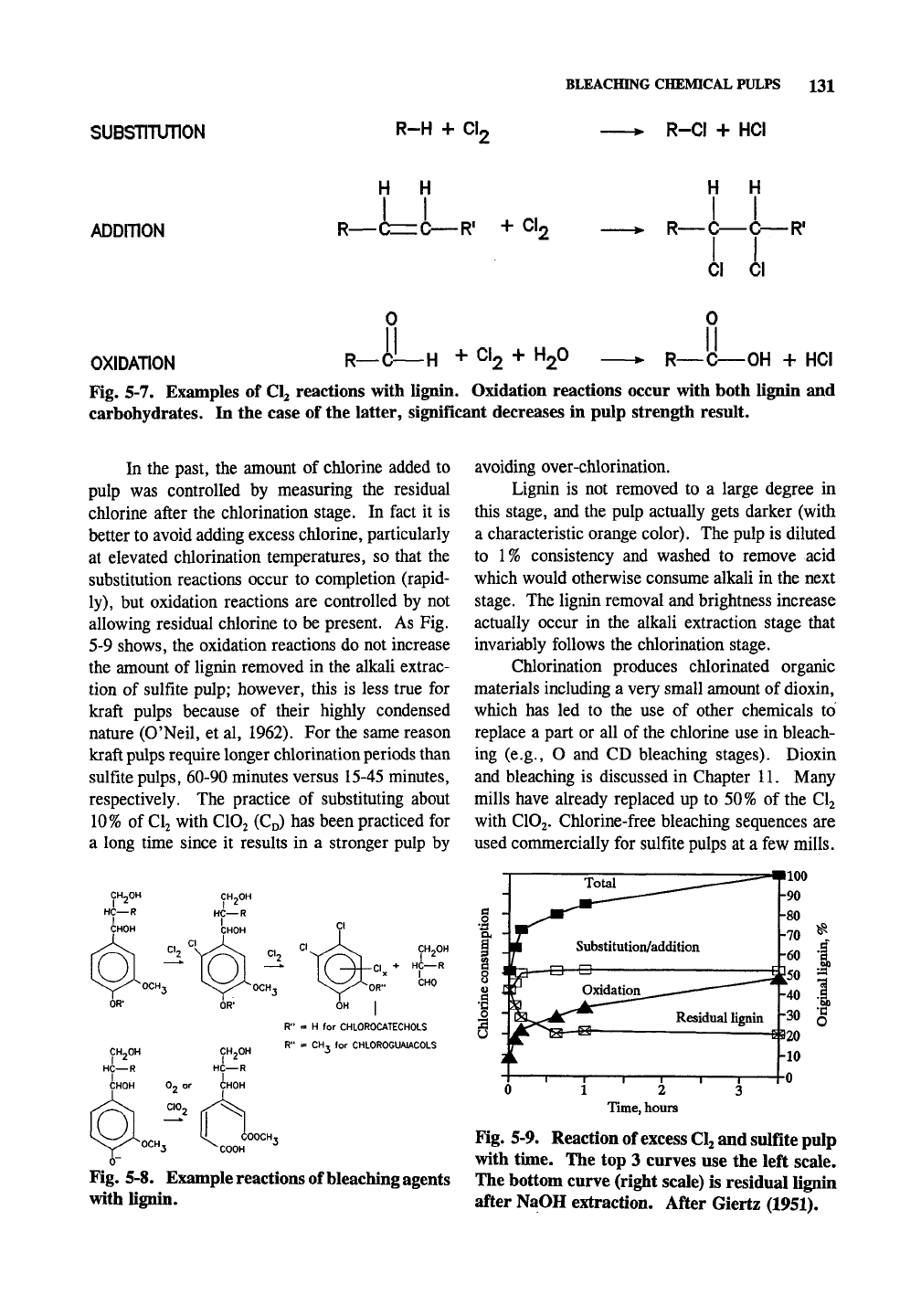
suBsmunoN
R-H
+
CI,
BLEACHING CHEMICAL PULPS 131
> R-CI + HCI
ADOmON
+ CI,
H H
-
R—C—(
CI
'i
OXIDATION
R—
+ CI2 + H2O
-OH + HCI
Fig. 5-7. Examples of Clj reactions with lignin. Oxidation reactions occur with both lignin and
carbohydrates. In the case of the latter, significant decreases in pulp strength result.
In the past, the amount of chlorine added to
pulp was controlled by measuring the residual
chlorine after the chlorination stage. In fact it is
better to avoid adding excess chlorine, particularly
at elevated chlorination temperatures, so that the
substitution reactions occur to completion (rapid-
ly),
but oxidation reactions are controlled by not
allowing residual chlorine to be present. As Fig.
5-9 shows, the oxidation reactions do not increase
the amount of lignin removed in the alkali extrac-
tion of sulfite pulp; however, this is less true for
kraft pulps because of their highly condensed
nature (O'Neil, et al, 1962). For the same reason
kraft pulps require longer chlorination periods than
sulfite pulps, 60-90 minutes versus 15-45 minutes,
respectively. The practice of substituting about
10%
of
CI2
with CIO2
(CD)
has been practiced for
a long time since it results in a stronger pulp by
R" = H for CHLOROCATECHOLS
R" = CH, for CHLOROGUAIACOLS
avoiding over-chlorination.
Lignin is not removed to a large degree in
this stage, and the pulp actually gets darker (with
a characteristic orange color). The pulp is diluted
to 1% consistency and washed to remove acid
which would otherwise consume alkali in the next
stage. The lignin removal and brightness increase
actually occur in the alkali extraction stage that
invariably follows the chlorination stage.
Chlorination produces chlorinated organic
materials including a very small amount of dioxin,
which has led to the use of other chemicals to
replace a part or all of the chlorine use in bleach-
ing (e.g., O and CD bleaching stages). Dioxin
and bleaching is discussed in Chapter 11. Many
mills have already replaced up to 50% of the CI2
with CIO2. Chlorine-free bleaching sequences are
used commercially for sulfite pulps at a few mills.
Fig. 5-8. Example reactions of bleaching agents
with lignin.
Time, hours
Fig. 5-9. Reaction of excess
CI2
and sulfite pulp
with time. The top 3 curves use the left scale.
The bottom curve (right scale) is residual lignin
after NaOH extraction. After Giertz (1951).
132 5. PULP BLEACHING
Papers made from these pulps are promoted as
"environmentally
friendly"
or words to that effect.
It is difficult to make bright grades of kraft pulps
without the use of chlorine, but it is possible if a
system is designed specifically for this purpose.
The future will bring many changes in this area.
CD stage
The CD or Co stage is a modification of C
stage bleaching, where some of the chlorine is
replaced with
ClOj.
CIO2 acts as a scavenger of
chlorine radicals in this stage. Substitution of
10%
of the chlorine with chlorine dioxide is used
to prevent over chlorination. Substitution of 50%
or more of chlorine with chlorine dioxide at many
mills is becoming common to reduce production of
dioxins and other chlorinated organic chemicals.
One problem is that since CI2 and NaOH are both
derived from electrolysis of NaCl, a slackening in
the CI2 market will mean much higher NaOH
prices. Other industries have taken advantage of
excess CI2 supplies, such as the polymer industry
in the manufacture of polyvinyl chloride and other
chlorinated polymers, which has mitigated this
effect.
E stage
The E stage is
extraction
of degraded lignin
compounds, which would otherwise increase the
chemical usage in subsequent bleaching stages,
with caustic (NaOH) solution. It follows the C
stage and sometimes other stages of bleaching.
When it follows the C stage (Ei), it is used at
2-3
%
on
pulp,
often with a downflow tower due to
the high consistency of pulp (10-18%), with a
temperature of 50-95°C (120-200°F), and a reac-
tion time of 0.75 to 1.5 hours. High temperatures
and alkali loading up to 5% are used to remove
hemicelluloses for dissolving pulps or absorbent
pulps.
In later E stages, alkali is used at less than
1%
on pulp. The alkali displaces chlorine and
makes the lignin soluble by reactions such as:
Lignin-Cl + NaOH
-^
Lignin-OH + NaCl
The lignin in the Ej effluent gives a dark color
that is ultimately responsible for much of
the
color
of
the
final
mill effluent. Recently oxygen gas has
been incorporated into this stage
(0.5%
on pulp)
at
many mills and the term
EQ
applies.
H stage
The H stage consists of bleaching with hypo-
chlorite solution, usually as the sodium salt
NaClO; this is the same chemical found in house-
hold liquid bleach. This stage is carried out at
4-18% consistency, 35-45°C (95-113°F), 1-5
hours, and pH 10. The process is often controlled
by measuring the oxidation-reduction
potential.
It
is important to maintain the pH above 8 because
below this pH hypochlorite is in equilibrium with
significant amounts of hypochlorous acid (Fig. 17-
1),
which is a powerful oxidant of carbohydrates,
with a E° of 1.63 V. Since the pH is high, lignin
is continuously extracted as it is depolymerized.
Hypochlorite reacts principally by oxidation.
About
1 %
on wood (based on chlorine) is used.
This chemical is more selective than elemental
chlorine, but less selective than chlorine dioxide;
consequently, the use of hypochlorite has de-
creased since the advent of chlorine dioxide.
Cellulose is oxidized along its chain, forming
aldehyde groups and making random cleavage
more likely. Calcium hypochlorite was discovered
in 1798 by dissolving CI2 in an aqueous slurry of
calcium hydroxide
and
was the principal bleaching
agent of the industry for a century. Sodium
hypochlorite, which is now used since it leads to
less scaling, is made from chlorine as follows:
CI2 + 2NaOH -> NaOCl + NaCl + H2O
D stage
The D stage involves bleaching with chlorine
dioxide. Chlorine dioxide (first studied for pulp
bleaching in 1921 by Schmidt and used for com-
mercial pulp bleaching in the mid 1940s) is rela-
tively expensive, but highly selective for lignin.
This makes it very useful for the latter bleaching
stages where lignin is present in very low concen-
trations. It is explosive at concentrations above 10
kPa (1.5 psi, or 0.1 atm); hence, it cannot be
transported and must be manufactured on site. Its
solubility is 6 g/L at 25 °C with a partial pressure
of 70 mm Hg. It is used at consistencies of
10-12%,
60-80°C (140-176°F), for 3-5 hours at a
pH of 3.5-6. It is used at 0.4-0.8% on pulp.
Downflow towers are used to decrease the risk of
gas accumulation. The D stage is useful for
reducing shive contents.

BLEACHING CHEMICAL PULPS
133
CIO2 -* y2Cl2 4- O2 (undesirable breakdown
above 100 torr pressure)
2CIO2 + H2O ->
HCIO3 + HCIO2
(slow, undesired de-
composition in soln.)
Chlorine dioxide may react in two steps. In
the first step C102' is formed; this then reacts
under acidic conditions to form CI". Thus, CIO2
-^
ClOj"
-> CI". It reacts by oxidation; one reac-
tion is shown in Fig. 5-8.
P stage
Bleaching with hydrogen peroxide, H2O2, is
not common for chemical pulps. (But this is
changing somewhat as mills look for chlorine-free
systems.) It is usually used for brightening me-
chanical pulps, but when it is used to bleach
chemical pulps it appears as the last stage of a
sequence such as C-E-H-P or C-E-H-D-P. It is
used at 10% consistency, 60-70°C (140-160°F),
pH of 8-10, for 2-4 hours. It is an expensive
bleaching agent, but may be used more frequently
as the use of elemental chlorine decreases. Perox-
ide oxidizes carbonyl groups of carbohydrates
(produced by oxidants such as hypochlorite) to
carboxylic acid groups. Its use with chemical
pulps is fairly similar to that with mechanical
pulps,
except for a higher temperature, as de-
scribed in Section 5.2.
O stage,
oxygen pulping
and
bleaching
Oxygen bleaching or pulping is the delignifi-
cation of pulp using oxygen under pressure (550-
700 kPa or 80-100 psi) and NaOH (3-4% on
pulp).
Oxygen bleaching has been used commer-
cially since the late 1960s. This is an odorless,
relatively pollution-free process used prior to
chlorination at high consistencies (20-30%) or
medium consistencies (10-15%). Delignification
is carried out at 90-130°C (195-266°F) for 20-60
minutes. The key to the use of
O2
delignification
was the discovery that small amounts of magne-
sium ion (0.05-0.1
%
on pulp) must be present to
protect the carbohydrates from extensive degrada-
tion.
This is the most inexpensive bleaching
chemical to use, but also the least specific for
lignin removal. A considerable decrease in cellu-
lose viscosity accompanies this process.
Bleaching may be thought of as extended
delignification, that is, an extension of
the
pulping
process. Many mills are considering an oxygen
delignification step after pulping but before the
traditional chlorination first step of bleaching.
Some call it oxygen delignification; others call it
oxygen bleaching. Many mills have added oxygen
to the first alkali extract step, designated as the
E^,
step,
because it is not a major change to the
process and saves bleaching chemicals in subse-
quent stages. Oxygen is even less specific at
lignin removal than chlorination so it is used to
decrease the softwood pulp kappa number from
30-35 after pulping (20-24 for hardwoods) to 14-
18 after the oxygen bleaching stage. Bleaching to
kappa numbers below this leads to unacceptable
losses of the cellulose viscosity.
The effluent of oxygen delignification can be
used in the brown stock washers or otherwise
ultimately
sent to
the recovery boiler because there
is no chloride ion present that would lead to high
dead loads and corrosion in the recovery boiler.
Reducing the amount of lignin removed by the
chlorination step by half reduces by half
the
color
and BOD from the bleach mill water discharge
after secondary treatment. Environmental consid-
erations are the main reason for using oxygen
bleaching. The bleach plant produces mush of the
color of the final mill discharge.
There are two main methods of oxygen
bleaching: medium and high consistency. The
high consistency
process
is
at
30%
consistency and
90-110°C (195-230°F) in a pressurized reactor.
In the
medium consistency process
pulp
at
10-15 %
consistency (the normal consistency
as
it comes off
the drum washers) is used. While high consisten-
cy oxygen bleaching is more common, there are
some difficulties with it. The gas in the reactor
contains high concentrations of oxygen
and
volatile
organic chemicals such as methanol, ethanol, and
acetone that are potentially explosive; it is diffi-
cult to evenly distribute NaOH and O2; and the
pulp is saturated with oxygen which
tends
to cause
foaming on the pulp washers.
Studies indicate that the temperature of
oxygen bleaching is important to the selectivity of
the process with better selectivity at 100°C
(212°F) than 130°(266^F). Partial pressure of
oxygen above 200 kPa (30 psig) leads to little
increase in the rate of delignification.
