Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


equivalent sphere is then used to calculate the freezing
time of the food item.
0038 In the equivalent sphere diameter technique, the
volume–surface diameter, D
vs
, is defined as the diam-
eter of a sphere having the same volume-to-surface-
area ratio as the irregular shape:
D
vs
¼
6V
A
s
ð18Þ
where V is the volume of the irregular shape and A
s
is
the surface area of the irregular shape. In addition, the
volume diameter, D
v
, is defined as the diameter of a
sphere having the same volume as the irregular shape:
D
v
¼
6V
1=3
ð19Þ
Then, the equivalent sphere diameter, D
eq, s
, is defined
as follows:
D
eq;s
¼
1
2
þ 1
D
v
þ
2
2
þ 1
D
vs
ð20Þ
Thus, the prediction of the freezing time of the irregu-
larly shaped food item is reduced to predicting the
freezing time of a spherical food item with diameter
D
eq, s
.
See also: Freezing: Blast and Plate Freezing
Further Reading
Becker BR and Fricke BA (1999a) Evaluation of semi-
analytical/empirical freezing time estimation methods,
part I: regularly shaped food items. International Jour-
nal of Heating, Ventilating, Air-Conditioning, and Re-
frigerating Research 5: 151–169.
Becker BR and Fricke BA (1999b) Evaluation of semi-
analytical/empirical freezing time estimation methods,
part II: irregularly shaped food items. International Jour-
nal of Heating, Ventilating, Air-Conditioning, and Re-
frigerating Research 5: 171–187.
Becker BR and Fricke BA (1999c) Freezing times of regu-
larly shaped food items. International Communications
in Heat and Mass Transfer 26: 617–626.
Becker BR and Fricke BA (1999d) Food thermophysical
property models. International Communications in
Heat and Mass Transfer 26: 627–636.
Cleland AC and Earle RL (1977) A comparison of analyt-
ical and numerical methods of predicting the freezing
times of foods. Journal of Food Science 42: 1390–1395.
Cleland AC and Earle RL (1979) A comparison of methods
for predicting the freezing times of cylindrical and spher-
ical foodstuffs. Journal of Food Science 44: 958–963,
970.
Cleland AC and Earle RL (1982) Freezing time prediction
for foods – a simplified procedure. International Journal
of Refrigeration 5: 134–140.
Cleland DJ, Cleland AC and Earle RL (1987a) Prediction of
freezing and thawing times for multi-dimensional shapes
by simple formulae – part 1: regular shapes. Inter-
national Journal of Refrigeration 10: 156–164.
Cleland DJ, Cleland AC and Earle RL (1987b) Prediction of
freezing and thawing times for multi-dimensional shapes
by simple formulae – part 2: irregular shapes. Inter-
national Journal of Refrigeration 10: 234–240.
Fricke BA and Becker BR (2001) Evaluation of thermophy-
sical property models for foods. International Journal of
Heating, Ventilating, Air-Conditioning and Refrigerat-
ing Research 7: 311–330.
Ilicali C and Engez ST (1990) A simplified approach for
predicting the freezing or thawing times of foods having
brick or finite cylinder shape. In: Engineering and Food,
Speiss WEL and Schubert H (eds) vol. 2, pp. 442–451.
London: Elsevier Applied Science.
Ilicali C and Hocalar M (1990) A simplified approach for
predicting the freezing times of foodstuffs of anomalous
shape. In: Speiss WEL and Schubert H (eds) Engineering
and Food, vol. 2, pp. 418–425. London: Elsevier
Applied Science.
Pham QT (1984) An extension to Plank’s equation for
predicting freezing times for foodstuffs of simple shapes.
International Journal of Refrigeration 7: 377–383.
Pham QT (1985) Analytical method for predicting freezing
times of rectangular blocks of foodstuffs. International
Journal of Refrigeration 8: 43–47.
Plank R (1913) Die Gefrierdauer von Eisblocken. Zei-
tschrift fu
¨
r die gesamte Kalte-Industrie 20: 109–114.
Plank R (1941) Beitrage zur Berechnung und Bewertung der
Gefriergesch Windikeit von Lebensmitteln. Beiheft zur
Zeitschrift fu
¨
r die gesamte Kalte-Industrie 10: 1–16.
Blast and Plate Freezing
M F G Boast, MBA Consulting Engineers, Markyate,
UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001The provision of low temperature for freezing is
obtained from closed-circuit compression–expansion
refrigeration systems, or by a cryogenic process of
exposing the product to a cold atmosphere of liquid
nitrogen or liquid carbon dioxide.
0002Various refrigerants have been used for the compres-
sion–expansion system, which forms the major source
of food freezing, probably 90% of all installations.
0003Ammonia and sulfur dioxide are excellent media
for the refrigeration process, but they carry the
hazard of being inflammable and the risk of explo-
sion. This led to the development during the 1930s of
2718 FREEZING/Blast and Plate Freezing

the first chlorofluorocarbon (CFC) refrigerants, R11
and R12. These refrigerants replaced SO
2
in domestic
refrigeration and NH
3
for small freezing systems. The
later development of R22 (a hydrochlorofluoro-
carbon, or HCFC) and R502 (an azeotrope), dis-
placed NH
3
from all but the largest of industrial
freezing systems. Table 1 shows the characteristics
of common refrigerants.
0004 During the 1980s, the environmental effect of
CFCs and HCFCs, in terms of ozone depletion poten-
tial and contribution to the greenhouse effect, became
apparent. This has led to the restriction in the use of
some refrigerants under the Montreal Protocol, and
the development of new refrigerants called hydro-
fluorocarbons (HFCs).
Refrigerants and the Environment
Ozone Depletion
0005 The ozone-depleting effect of refrigerants is import-
ant, because the ozone layer filters out ultraviolet
(UV) radiation, which might otherwise reach the sur-
face of the earth. This radiation could have seriously
damaging effects on germinating crops and on the
photoplankton of the oceans. In addition, increased
doses of UV light can cause malignant skin cancer in
fair-skinned races.
Greenhouse Effect
0006 Ecological systems of the world are finely dependent
on temperature. Small changes in average temperature
can affect the distribution of crops and animals.
There is evidence that the average global temperature
has been increasing since the mid-nineteenth cen-
tury because of carbon dioxide from the burning
of fossil fuels. If it is not halted, mean sea levels will
rise.
0007The consequences of even a small rise in sea level in
certain areas of the world, where strong seasonal
winds might coincide with high tides, would be disas-
trous. It is impossible to predict the likely rise of mean
sea level, but it could be significant. In the remote
past, the earth had no polar ice caps, presumably
because of high concentrations of carbon dioxide in
the atmosphere, which had not by then been absorbed
by forests and oceans.
0008Chlorofluorocarbons are greenhouse chemicals
more potent than the present greenhouse chem-
icals (carbon dioxide and methane) and should there-
fore not be released into the atmosphere.
Recommendations
0009Refrigerants known as CFCs are typically R11, R12,
R115, R13B1, and R502 and have now been phased
out, although some installations may still exist using
the above refrigerants. New systems are not being
constructed from the refrigerants.
0010The destruction of CFCs present problems in terms
of safety and the need for environmental protection.
Therefore, any system being decommissioned con-
taining CFCs must be addressed in a controlled
manner by personnel with the appropriate experience
and qualifications.
tbl0001 Table 1 Characteristics of common refrigerants
Substance Type Formula MontrealProtocol Ozone depletion potential
(R11 ¼1)
Greenhouse potential
(CO
2
¼1)
Flammability
R11 CFC CC1
2
F Yes 1 3 300 No
R12 CFC CC1
2
F
2
Yes 2 10 000 No
R22 HCFC CCHClF
2
No 0.05 1 100 No
R113 CFC CC1
2
FCC1F
2
Yes 0.8 4 500 No
R114 CFC CC1F
2
CC1F
2
Yes 1.0 1 300 No
R115 CFC CClF
2
CF
3
Yes 0.6 25 000 No
R123 HCFC CHC1
2
CF
3
No 0.02 50 No
R124 HCFC CHClFCF
3
No 0.02 300 No
R125 HFC CHF
2
CF
3
No 0 1 900 No
R134a HFC CF
3
CH
2
F No 0 900 No
R141b HCFC CH
3
CCl
2
F No 0.08 300 Slight
R142b HCFC CH
3
CClF
2
No 0.06 1 200 Slight
R152a HFC CH
3
CHF
2
No 0 100 Moderate
R500 Azeotrope R12/152a Yes 0.74 7 400 No
R502 Azeotrope R22/115 Yes 0.33 13 300 No
R717 Ammonia NH
3
No 0 0 Moderate
R407C Azeotrope R32/125/134a No 0 1 610 No
R404A Azeotrope R125/143a/134a No 0 No
R410A Azeotrope R32/125 No 0 1 890 No
FREEZING/Blast and Plate Freezing 2719

0011 The construction of new systems for small to
medium-sized uses refrigerant R404A. Large indus-
trial systems use refrigerant R717 (ammonia).
0012 Refrigerant R717 should only be used by com-
panies and specialists with knowledge of this refriger-
ant owing to its pungent smell and hazard potential.
Maintenance
0013 Plants should be constructed to the appropriate codes
of practice and standards. Routine leak testing must
be conscientiously carried out. In addition to being
environmentally correct, this will produce cost
savings and improve plant reliability.
0014 Leak detectors should be fitted to all systems with a
large refrigerant charge and should report to a central
monitoring station.
Substitutes for CFCs
0015 The substitute for refrigerant 12 is refrigerant
R134A. The substitute for refrigerant R502 is
refrigerant R404A. These refrigerants are not suitable
for use with mineral oils, and polyester lubricants
have been developed. Changing the system from re-
frigerant R12 or refrigerant R502 requires specialist
knowledge and should only be carried out by suitably
experienced companies and personnel. Changing
the system refrigerant from R12 or R502 requires
system flushing and thoroughly cleaning to insure
that all traces of the previous refrigerant have been
removed.
0016 At the present time, systems using refrigerant R22
can continue to operate and can be serviced with new
virgin refrigerant until the year 2005.
Freezing Considerations
Freezing Times
0017 Freezing times vary, and each product has an associ-
ated freezing time that will depend on its compos-
ition, dimensions, and packaging. Dimensions and
packaging have a direct effect on the freezing time,
as heat can only leave the product through its surface.
Products that are unpacked and have a large surface
area-to-weight ratio, such as peas, will freeze in a
minimum time, whereas a dense block of meat prod-
ucts, with heavy packaging or boxing, may take days
to be fully frozen to the core.
0018 The influence of freezing time is more apparent on
some products than others. For strawberries, the drip
loss is reduced from 20% to nearly 0% with shorter
freezing times. A difference in drip loss from 20% for
strawberries frozen in 12 h, to 8% for strawberries
frozen in 15 min, is significant. Cryogenic systems
perform the same freezing function in 8 min or less,
reducing the drip loss to less than 5%.
0019A modern mechanical freezer or a cryogenic freezer
can crust products very rapidly, minimizing the loss of
natural juices and locking in the qualities that make
the product more marketable.
Packaging
0020Packaging must be of good quality, odor- and taint-
free, and it must totally enclose the product. The
container should not be capable of being opened
without recognizable damage. It should also protect
the product against normal transit and storage
hazards and inhibit dehydration by incorporating a
moisture vapor barrier. All packaging should carry
clear identification and should be coded so that
stock rotation can be carried out. The inner carton
should be marked with an appropriate identification
code that would enable the producer concerned to
establish the date of production and the location of
the producing factory, and gain easy access to daily
production records.
0021As the product is wrapped, the operator must
insure that the inner layer is in good contact with
the actual food and that all air has been excluded.
Unless this is established, reasonable cooling rates
will not be obtained. The packages should then be
placed and arranged to insure good contact with the
freezing surface in the case of a plate freezer, or be in
the air flow of an air blast freezer.
0022Cooked food must be chilled to around 10
Cor
lower before it enters the freezer. The placing of a hot
product in the freezer will considerably slow the
freezing process as the outer surface will quickly
freeze, leaving the hot interior to give up initially
larger amounts of sensible heat than would have
been necessary had the product been allowed to
stabilize and cool prior to freezing.
0023Food cooked in a domestic kitchen should be first
allowed to cool close to ambient temperature and
then transferred to the refrigerator to chill down
before being placed in the freezer. The use of an
immersion thermometer to check core temperatures
prior to freezing is recommended. (See Packaging:
Packaging of Liquids; Packaging of Solids.)
Weight Loss and Economics
0024Freezing equipment will represent the single largest
investment in a food production line, yet its operating
costs will normally only be 3–5% of the total product
cost. Packaging costs vary widely but are normally
several times greater than the total cost of freezing.
0025An important factor to consider when selecting
freezing equipment is the weight loss of the product
2720 FREEZING/Blast and Plate Freezing

that occurs during freezing. This loss may be about
the same as the operating costs of the freezing facility.
This applies to inexpensive products like peas and is
even more significant for expensive products, such as
seafoods and soft fruits.
0026 Weight loss during freezing may be caused by
mechanical losses, downgrading, and dehydration.
Mechanical loss refers to products dropping to the
floor, sticking to conveyor belts or dripping juice. A
modern freezer should have almost no loss in these
categories. Downgrading losses refer to damaged
products, breakages, and similar occurrences that
render the product unsaleable at the top quality
price. Dehydration losses will always be present in
any freezing system. The evaporation of water vapor
from unpacked products during freezing can be seen
as frost on the refrigeration plant freezing surfaces.
This frost is also caused by excessive infiltration of
warm, moist air into the freezing chamber. Still air
inside the vapor-proof product carton can create
larger dehydration losses than an unpacked product
frozen in a cryogenic quick freezer. The heat transfer
is poor because no circulation of air occurs within the
package. The result is evaporation of moisture, which
can be significant, with the frost staying inside the
carton.
0027 A poorly designed freezer or freezing tunnel can
have dehydration losses as high as 5%, as against a
well-designed freezing system in which losses are
maintained below 1.5%. Cryogenic liquid nitrogen
tunnels normally operate with a dehydration loss of
between 0.25 and 1.25%. These losses usually occur
when the nitrogen gas circulates over the product at
the in-feed end of the freezer. It may be necessary
at the in-feed end to temper the product so as to use
the heat capacity of the nitrogen more efficiently.
Nitrogen immersion freezing tends to have even
lower dehydration losses but uses more liquid nitro-
gen. Carbon dioxide freezing operations use jet im-
pingement, and dehydration losses are similar to that
experienced with liquid nitrogen.
Freezing Methods
0028 Freezing equipment can be divided into groups with
regard to the basic method of extracting heat from the
product to be frozen:
1.
0029 Air blast freezing: Air at high velocity (between 4
and 7 m s
1
) is circulated over the product. The air
picks up heat, which is then recooled by means of
an air-to-refrigerant heat exchanger before the air
is recirculated.
2.
0030 Contact freezing: Food (packed or unpacked) is
placed between metal plates or surfaces, and the
heat is extracted by direct conduction through the
metal surface, which is refrigerated by the circula-
tion of a cooling medium.
3.
0031Immersion freezing: Food is immersed in a low-
temperature, nonfreezing solution that is cooled
by evaporators in a conventional refrigeration
system.
4.
0032Cryogenic freezing: Food is exposed to an atmos-
phere below 60
C, which is achieved by
spraying liquid nitrogen (LN) or liquid carbon
dioxide into the freezing chamber.
All of the above methods are used in food freezing
processes. However, the more favored systems are
those that can be operated in line with the proceeding
processing and preparation operations and the subse-
quent packing functions.
Types of Freezing Equipment
Storage Rooms
0033The storage room must not be considered as freezing
equipment, although it may sometimes be used for
this purpose. Freezing in a storage room has many
disadvantages and should only be used in exceptional
cases. The freezing process is slow, and the quality of
almost all products suffers. If products are already
being stored in the room, their quality is jeopardized,
because flavors may be transferred from the warm
products yet to be frozen.
0034Because the storage room is not designed for freez-
ing, the cooling coils may frost up quickly, and total
refrigeration capacity may be reduced. The tempera-
ture of the products already frozen may rise consider-
ably, affecting their quality. Without doubt, freezing
in a storage room leads to a lower product quality.
Blast Freezer
0035A blast freezing room is usually equipped with more
forced-air cooling units or larger coolers than would
normally be found. These coolers are often of suffi-
cient size to warrant floor mounting. The air coolers
are equipped with fans that create considerable air
turbulence. Products may be laid in trays that are then
loaded into a freezing trolley or rack; this is moved in
and out of the blast freezer by hand or with a forklift
truck.
0036The racks must be designed to provide air space
between the trays, which is equal to approximately
50% of the product thickness. Because there is no
control over air circulation, the resulting heat transfer
of the product to air may be less effective. Blast
freezer rooms offer acceptable conditions for a
limited range of products with the freezing cycle and
FREEZING/Blast and Plate Freezing 2721
the quality of freezing dependent on the experience of
the operator.
Stationary Freezing Tunnels
0037 A stationary freezing tunnel is the simplest type of
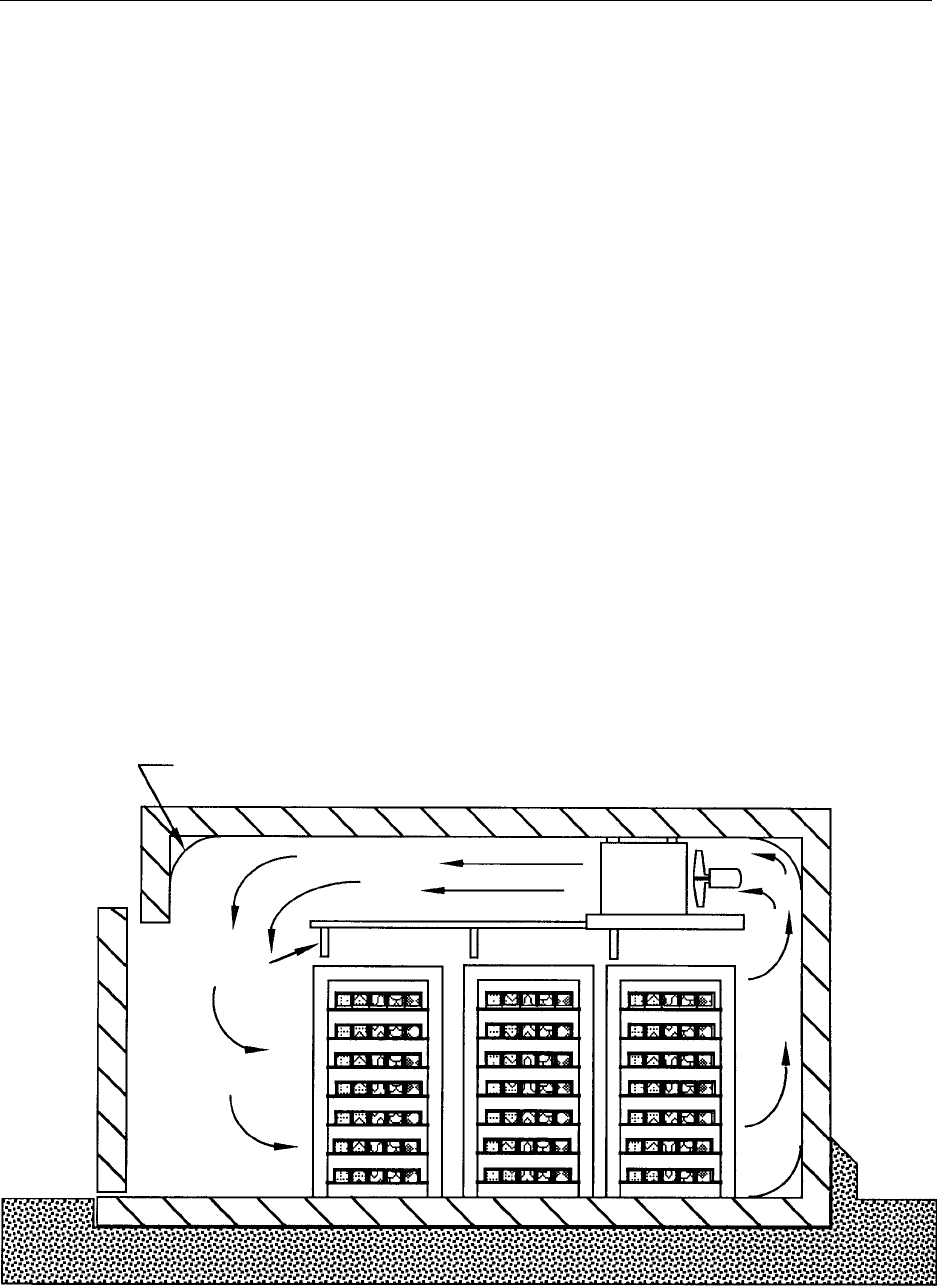
freezer (Figure 1), designed to produce satisfactory
results for the majority of products. It consists of an
insulated enclosure, equipped with refrigeration coils
and fans that circulate air in a controlled pattern
over the product. The air circulation system design
influences the freezing rate and the product weight
loss.
0038 Products are placed on trays that are then placed in
a rack. The racks are arranged to provide an air space
between each level of trays and are moved in and out
of the tunnel manually. The human element becomes
important when positioning the racks inside the
tunnel to prevent air bypass; this is essential for effi-
cient freezing processes.
0039 Most products can be frozen in a stationary freez-
ing tunnel. Whole, sliced, or diced vegetables may be
frozen either in cartons or unpacked, provided that
the layers are limited to 30–40 mm in depth on the
trays. Spinach, broccoli, meat pate
´
s, fish fillets, and
prepared foods are often frozen in packages, using
this type of equipment. By using different rack
designs, thick packages and whole meat carcasses
can also be frozen. The system capacity depends on
the product thickness and composition, as well as on
the existence of packaging.
0040This type of freezer is reasonably flexible in use,
making it suitable for the initial development stage of
the new frozen food product, but does require heavy
outlay for manpower and may result in considerable
weight loss, if not properly used.
Push-through Tunnels
0041Some simple mechanization is achieved in a push-
through tunnel (Figure 2). Tracks are provided in
the floor, and the racks are fitted with casters or
wheels. The mechanization system is often hydraulic-
ally powered with a special stroking facility that
feeds the racks through the freezer. The time of the
hydraulic stroking operation can be adjusted to vary
the time the product is in the freezer.
0042This type of equipment may require open ends to
allow product entry and exit. If doors are provided,
they have to be interlocked with the hydraulic track
system, so that they open and close, permitting a rack
to be drawn into the tunnel and also ejected at the
other end. This type of freezer has a number of ad-
vantages over a stationary tunnel: there is improved
Air deflectors
Rubber flaps
Air flow
Cooler
Fan
fig0001 Figure 1 Stationary freezing tunnel (Michael Boast Associates). Reproduced from Blast and Plate Freezing, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
2722 FREEZING/Blast and Plate Freezing
air circulation over the product as it moves at a steady
rate through the tunnel; labor costs are considerably
decreased; and there is added flexibility with the
facility of varying the freezing time.
0043 Some tunnels may have three or four lines, each
with independent hydraulic stroking facilities, and
this makes different product freezing times available
in a single installation.
Belt Freezers
0044 Modern belt freezers use vertical air-flow patterns
and require uniform distribution of product over the
entire belt surface for effective freezing.
0045 The single-belt freezer, multiple-belt freezer, and
spiral-belt freezer are the main types available. The
simplest is the single-belt freezer, consisting of the
single belt exposed to an updraught of air. This is
suitable for deep-fried or relatively dry products that
do not tend to freeze to each other or form clumps,
i.e., fish sticks, French fried potatoes, and bakery
products.
0046 Multiple-belt freezers are suitable for individual
freezing of fried fish sticks, fish portions, bakery
items and other products.
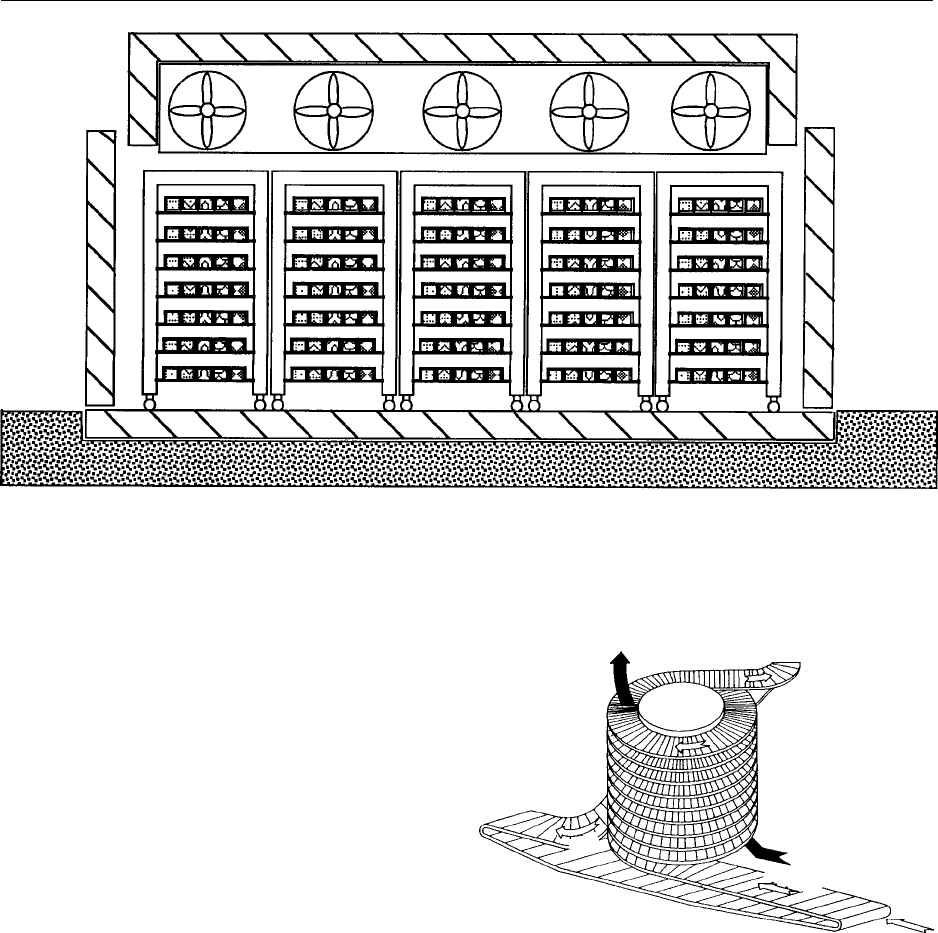
004 7 The spiral-belt freezer (Figures 3 and 4) offers a
design that maximizes the belt surface area in a given
floor space. It is achieved by using a product belt
that can bend around a rotating drum; by stacking
up to 40 tiers of belt on a drum, floor space occupied
is kept to a minimum. The continuous belt eliminates
product transfer points internal to the system. The
product is transferred only at the in-feed and out-feed
ends of the freezer. Spiral-belt freezers are suitable for
unpacked meat, fish, and poultry products, including
meat pate
´
s, meat balls, fish fillets and chicken por-
tions.
Fluidized Bed Freezers
0048Fluidization is defined as a method to keep solid
particles floating in an upward direction (Figure 5)
fig0002 Figure 2 Push-through tunnel (Michael Boast Associates). Reproduced from Blast and Plate Freezing, Encyclopaedia of Food Sci-
ence, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
Air
Product
Air flow
Product
Belt
fig0003Figure 3 Spiral-belt freezer (Michael Boast Associates). Re-
produced from Blast and Plate Freezing, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK
and Sadler MJ (eds), 1993, Academic Press.
FREEZING/Blast and Plate Freezing 2723
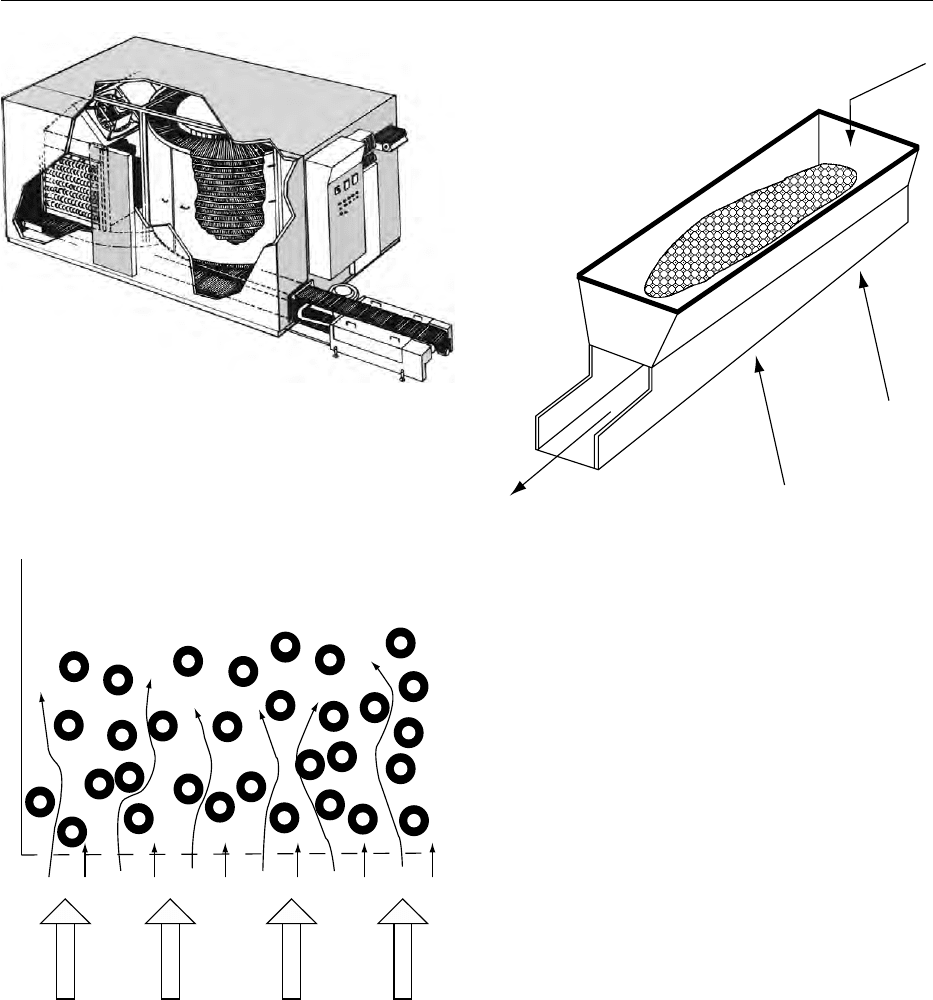
in a flow of gas or liquid. In freezing, fluidization
occurs when particles of a similar shape and size are
subjected to an upward stream of low-temperature
air. At certain air velocities, particles float in the
airstream, with each particle be separated from the
other but surrounded by air and free to move. In this
state, the particle mass assumes the properties of a
liquid or a fluid. By utilizing low-temperature air to
achieve fluidization, the product is simultaneously
frozen and conveyed by the same air without the aid
of a mechanical belt.
0049Using the fluidization principle for freezing pro-
vides several advantages over the conventional belt.
Most products that tend to stick together, i.e., sliced
green beans, sliced carrots, and sliced cucumbers, are
individually quick-frozen. The fluidized bed freezer
(Figure 6) is effective and reliable in freezing wet
products, as it can accept products with a high surface
water content. Another advantage of the fluidized
bed freezer is its complete independence from product
variations and flow. Even when running at reduced
capacity, the same evenly distributed air pattern is
maintained.
Plate Freezers
0050In a plate freezer (Figure 7), the product is pressed
firmly between top and bottom metal plates. The
refrigerant is circulated in channels, housed inside
the plates. This insures good heat transfer and
reasonable freezing times.
0051Because heat transfer at the surface is gradually
reduced with increasing product thickness, package
Air flow
fig0005 Figure 5 Fluidization principle (Michael Boast Associates).
Reproduced from Blast and Plate Freezing, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
fig0004 Figure 4 Diagrammatic arrangement of a spiral-belt freezer.
Reproduced from Blast and Plate Freezing, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK
and Sadler MJ (eds), 1993, Academic Press.
Product inflow
Product outflow
Air flow
fig0006Figure 6 Fluidized bed freezer (Michael Boast Associates).
Reproduced from Blast and Plate Freezing, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
2724 FREEZING/Blast and Plate Freezing
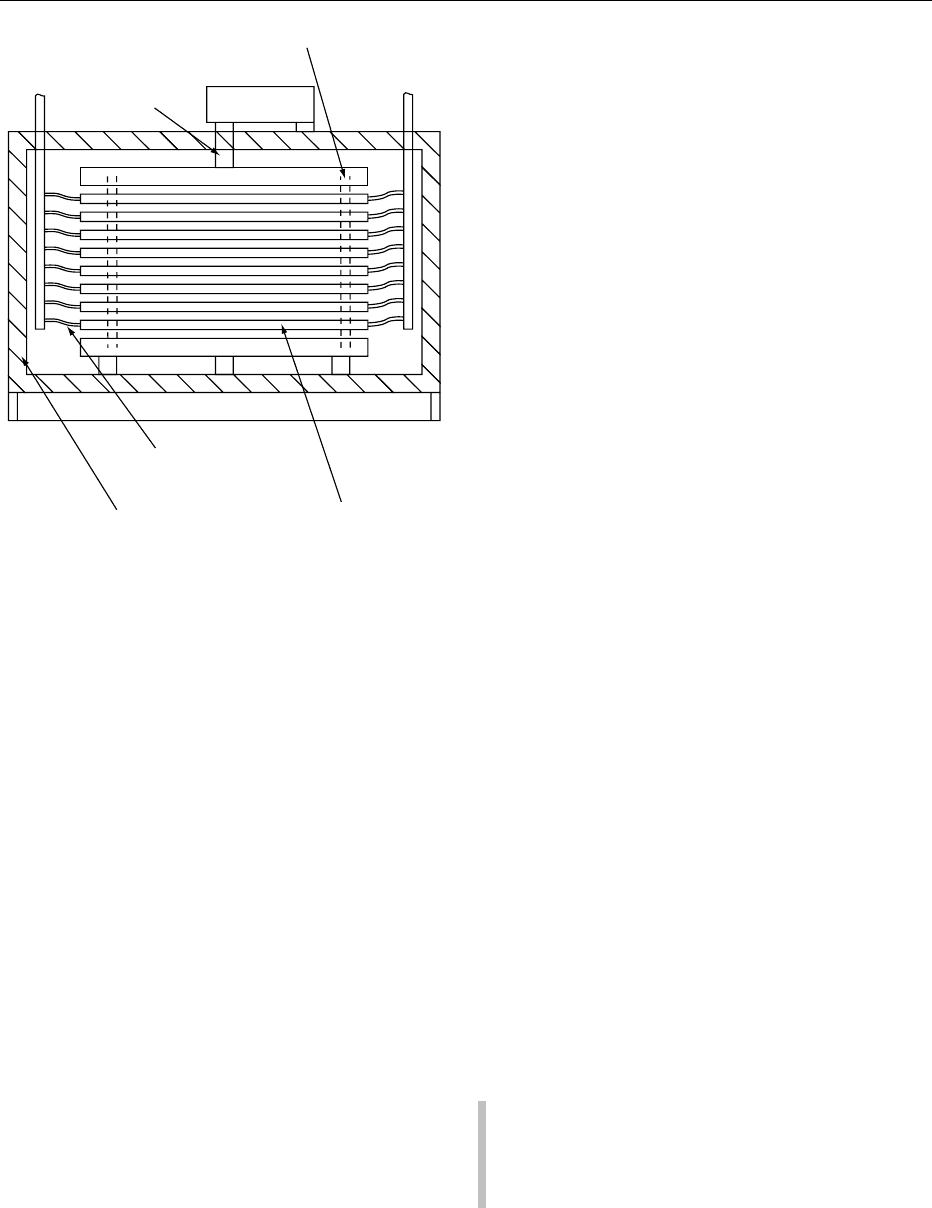
thickness has to be limited to a maximum of 50 mm.
Pressure from the plates has the additional advantage
that it eliminates the bulging of the packages during
the freezing cycle, so that the packages are discharged
with straight sides and within close tolerances.
0052 The two main types of plate freezers are horizontal
or vertical in design; either type can be manual or
automatic. In general, manual horizontal plate
freezers have 15–20 plates. The product is placed on
metal trays at the end of a packaging line, loaded in a
rack or trolley, and transported to the freezer. The
trays are loaded manually between the plates.
0053 The vertical plate freezer was developed specific-
ally for the freezing of fish at sea; it comprises a series
of vertical freezing plates that form partitions in the
container. Products are fed from the top, and the
finished block of frozen products are discharged to
the side, top, or bottom. Usually, this operation is
mechanized. Whole unpacked fish are frozen, but
fillets may also be handled in a vertical plate freezer.
The block thickness varies from 25 to 100 mm.
Drum Freezer
0054 An alternative to the flat plate freezer is the drum
freezer, consisting of a rotating drum containing a
refrigerant at low temperature, resulting in a cold
surface for freezing. The product to be frozen is
placed on the stainless steel refrigerated drum,
which rotates at a preset speed depending on freezing
time required. The product must be frozen, within
one rotation. This rotation will last for less than
1 min if the product is only 3 mm thick, such as
chopped spinach paste. A 6-min rotation would be
required for a 10-mm-thick plaice fish fillet and
16-min rotation for a 20-mm-thick cod fillet.
Immersion Freezing
0055For irregularly shaped products such as chickens and
turkeys, high heat transfer can be achieved in an
immersion freezer, which normally consists of a tank
that houses a refrigerated brine. The brine is most
often of glycol or sodium chloride solution. The prod-
uct is immersed in the brine or sprayed while it is
conveyed through the tank.
0056Immersion freezers are most commonly used for
plastic-bagged chickens or turkeys. The product must
be protected from contact with the brine by using a
high-quality packaging that gives an absolute tight
seal. The brine residues on the packages are normally
washed off with water at the freezer exit.
See also: Freezing: Principles; Operations; Cryogenic
Freezing; Storage of Frozen Foods
Further Reading
ASHRAE (1998) Refrigeration Systems and Applications.
Atlanta, GA: American Society of Heating Refrigeration
and Air Conditioning Engineers.
ASHRAE (2001) Refrigeration Fundamentals. Atlanta, GA:
American Society of Heating Refrigeration and Air Con-
ditioning Engineers.
Boast MFG (1986) Refrigeration and Air Conditioning.
Oxford: Butterworth Heinemann.
Boast MFG (1991) Refrigeration Note Book. Oxford:
Butterworth Heinemann.
Robinson RK (ed.) (1985) Microbiology of Frozen Foods.
Barking, UK: Elsevier Science.
Cryogenic Freezing
C James and S James, University of Bristol,
Langford, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Freezing methods can be divided into two main
classes: (1) those involving direct contact between
Refrigerant
supply
Refrigerant
return
Hydraulic ram
Flexible refrigerant lines
Refrigerated platesInsulated cabinet
Plate guides
Hydraulic power
and control
fig0007 Figure 7 Horizontal plate freezer (Michael Boast Associates).
Reproduced from Blast and Plate Freezing, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK
and Sadler MJ (eds), 1993, Academic Press.
FREEZING/Cryogenic Freezing 2725
the refrigerant and the food; and (2) those involving
the use of a secondary medium, e.g., air, brine, or
metal plate, which is cooled by the refrigerant.
0002 Cryogenic freezing uses refrigerants, such as liquid
nitrogen or solid carbon dioxide, directly. The boiling-
off of the refrigerant when it comes in contact with
the product brings about cooling. As well as using the
latent heat absorbed by the boiling liquid, sensible
heat is absorbed by the resulting cold gas.
0003 Most cryogenic systems use total loss refrigerants,
i.e., the refrigerant is released to the atmosphere and
not recovered. Due to environmental and economic
factors, total loss refrigerants must be both readily
available and harmless, which limits the choice to
atmospheric air and its components.
History
0004 Cryogenic freezing was first carried out commercially
using liquid air, in the 1930s. However, liquid air
contains a high proportion of liquid oxygen, which
is a powerful oxidizing agent. Theoretically it can be
produced on site, eliminating the need to purchase
and store ‘gas.’ Although companies have promoted
the use of liquid air, in practice it has been superseded
by less harmful liquid nitrogen and liquid, or solid,
carbon dioxide.
0005 Food freezing using liquid nitrogen or carbon
dioxide became established in Europe from the early
1960s onwards as manufacturers looked for uses of
these byproducts. Liquid nitrogen is the byproduct
produced when liquid oxygen is made by air dis-
tillation, while carbon dioxide is a byproduct of
fermentation processes and a constituent of nat-
ural gas. By the early 1990s an estimated 10% of
all frozen food in the UK was produced using
cryogens.
0006 An alternative to the air-based total loss cryogens,
liquid dichlorodifluoromethane (food grade R 12,
freon, CCl
2
F
2
), was introduced commercially in
1968 by Du Pont. R 12 had the advantage over
other cryogens of being easily recoverable and having
a higher operating temperature (29.8
C), reducing
the problems of thermal shock. It was claimed that
such gentle handling allows ‘true’ individual quick
freezing (IQF) of products such as soft berries, meat
patties, raw shrimps, breaded scampi, fried onion
rings, etc. in less than 6 min. However, its use was
not permitted in all countries and it was not widely
used.
Physical Properties
0007 The major problem with most cryogenic materials
is that they are extremely cold (Table 1). Direct
immersion can be inadvisable since an extremely
high temperature gradient can be imposed on the
food, which is often sufficient to cause it to disinte-
grate. Research studies have concluded that the ideal
cryogen would have a boiling point of 50
Canda
latent heat of evaporation as high as possible.
0008Liquid nitrogen is produced by liquefaction of air,
either as a principal product or as a byproduct of liquid
oxygen. Nitrogen is the main constituent of atmos-
pheric air and at atmospheric pressure liquefies at a
temperature of 196
C. It is usually supplied and
stored at a pressure of 3–6 bar, with corresponding
boiling points of 185
Cto177
C. A useful rule of
thumbisthat1 t h
1
ofliquidnitrogenisapproximately
equivalent to 100 kWof mechanical refrigeration.
0009Since liquid nitrogen is made from air, its costs are
entirely those of manufacture, i.e., the capital cost of
the plant, electricity, and transportation. The energy
required to produce 1 kg of liquid nitrogen is around
3000 kJ, or eight times the consequent stored refriger-
ating effect.
0010Carbon dioxide’s physical properties are unusual,
in that it does not exist in liquid form at atmospheric
pressure. If stored as a pressurized liquid and released
into the atmosphere, the liquid changes partly to gas
and partly to a frozen solid at 78.5
C, which
sublimes directly into gas without going through a
liquid phase.
0011Liquid carbon dioxide is generally supplied either
at ambient temperature (e.g., 25
C and 65 bar),
giving a refrigerating capacity of 199 kJ kg
1
,orat
16
C and 22 bar, giving a refrigerating capacity of
311 kJ kg
1
. At the point of use, spray nozzles reduce
the pressure of the liquid, generating a mixture of
cold vapor and solid carbon dioxide ‘snow.’
0012The majority of the ‘refrigeration effect’ stored in
solid CO
2
is latent, whereas in liquid nitrogen almost
half the effect is due to sensible heat transfer to the
cold gas. The available refrigerating effect from 1 kg
tbl0001Table 1 Properties of cryogenic materials
Material Boiling point
Cat1atm Latentheatof
vaporization
(kcal kg
1
)
Helium 268.8 108.8
Nitrogen 195.8 47.3
Carbon monoxide 190.6 53.5
Argon 184.4 37.0
Methane 161.1 136.0
Ethane 88.9 109.0
Nitrous oxide 88.9 90.0
Propane 42.2 100.6
Carbon dioxide 57.6 (5 atm) 75 (approx.)
Carbon dioxide (solid) 79 (sublimation point) 135.4
2726 FREEZING/Cryogenic Freezing
of both liquid and solid carbon dioxide and liquid
nitrogen is shown in Figure 1.
Comparison of Cryogenic Freezing
Systems with Mechanical Freezing
Systems
0013 The generally perceived advantages of cryogenic com-
pared with conventional refrigeration are given in
Table 2.
Capital Cost
0014 The low capital cost and simplicity of design permit
cryogenic systems to be used in a standby or back-up
role in emergencies or for ‘peak lopping’ during
periods of exceptional freezing demand.
0015 The overall economics can be favorable for short
road deliveries. For static standby installations, or
those with very low loading factors, low capital cost
can be a major attraction.
Running Costs
0016 In overall energy or fuel terms, there is no way in
which cryogenic refrigerants can compete with the
best mechanical refrigeration systems. Generally
speaking, cryogenic refrigeration offers lower capital
and maintenance costs but incurs higher running
costs. Many cryogenic freezers are leased from the
gas suppliers, reducing capital cost further.
0017The consumption of the cryogen (Table 3) con-
tributes the major proportion of the freezing costs
(about 75%). Refrigerant consumption is measured
as a ‘consumption ratio’ equal to weight of refrigerant
used divided by the weight of product frozen. Table 4
shows some approximate quantities of liquid nitro-
gen and carbon dioxide required to freeze a range of
products. High refrigerant consumption can be
Liquid CO
2
1 kg
−18 ⬚C, 20.7 bar
Soild CO
2
1 kg
−78.5 ⬚C
Liquid N
2
1 kg
−196 ⬚C, 1.01 bar
575 kJ
24 kJ 45 kJ 180 kJ
268 kJ
313 kJ
313 kJ 620 kJ 378 kJ
198 kJ
Gas
−78.5 ⬚C
Gas
−78.5 ⬚C
−18 ⬚C
−18 ⬚C −18 ⬚C
−18 ⬚C
1 kg
Gas
−78.5 ⬚C
0.534 kg
Gas
−195 ⬚C
1 kg
Solid
0.466 kg
−78.5 ⬚C
fig0001 Figure 1 Available refrigeration effect from 1 kg of total loss refrigerant.
tbl0002Table 2 Advantages and disadvantages of total loss
refrigerants in comparison with mechanical refrigeration
Advantages Disadvantages
Low capital investment High operating cost
High refrigerating capacity High refrigerating capacity
Compact (small ‘footprint’) Poor temperature control
Flexible Limited duration without filling
Low weight when out of use High weight at start of use
No residual weight (dry ice) Reduced humidity
Silent operation Suffocation hazard
Advantageous storage Limited availability
atmosphere (N
2
)
Bacteriostatic affect (CO
2
)
Low maintenance requirements
Long life
Foolproof once installed (dry ice)
FREEZING/Cryogenic Freezing 2727
