Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

product is frozen can be of critical importance.
Table 1 illustrates the influence of temperature on
the amount of water frozen out for various foods,
determined experimentally. Similar values can be cal-
culated by thermodynamic analysis provided the ini-
tial water content and freezing point of the product
are known. (See Water Activity: Principles and Meas-
urement; Effect on Food Stability.)
Drying Phase
Sublimation
0008 In practice, sublimation of ice crystals usually begins
at about 20
C in a vacuum of less than 1.33 mbar,
provided sufficient heat is available from the sur-
roundings (benign heat). How long will a product
take to dry by sublimation? Ideally, the rate of sub-
limation is derived from a classical kinetic model of
sublimation which assumes: (1) a dynamic equilib-
rium between the ice surface and its vapor; (2) the
rate of escape of water molecules depends on the
temperature of the ice; and (3) the tendency of mol-
ecules to return to the ice depends on the temperature
and pressure of the vapor. This model has limited
value in practice. Of greater value in understanding
the rate of freeze drying of a food product, is to take
account of the rate of vapor diffusion as the water
molecules migrate through the vacuum space (con-
taining fewer air molecules than vapor molecules)
between the product and the condenser. The migra-
tion rate is described by the following equation:
G
m
¼
MD
e
P
RTl
ln
P p
0
c
P p
0
ð1Þ
where G
m
is the migration rate, M the molecular
weight of water vapor, D
e
the effective diffusion coef-
ficient, P the vacuum pressure, R the gas constant, T
the temperature, l the distance in the vacuum space, p
0
the vapor pressure outside the product surface, and
p
0
c
the vapor pressure outside the condenser surface.
If the freeze drier is considered as a closed system,
then the rates for sublimation (G
s
(ap
s
p
0
)), conden-
sation (G
c
a(p
0
c
p
c
)), and migration (G
m
) are equal,
i.e., G
s
¼ G
c
¼ G
m
, where p
s
is the sublimation vapor
pressure and p
c
the condensation vapor pressure.
Apart from the separate vapor pressure differences
(p
s
p
0
and p
0
c
p
c
), the difference in vapor pressure
overall (p
s
p
c
) is usually regarded as the driving
force for sublimation. This model of freeze drying
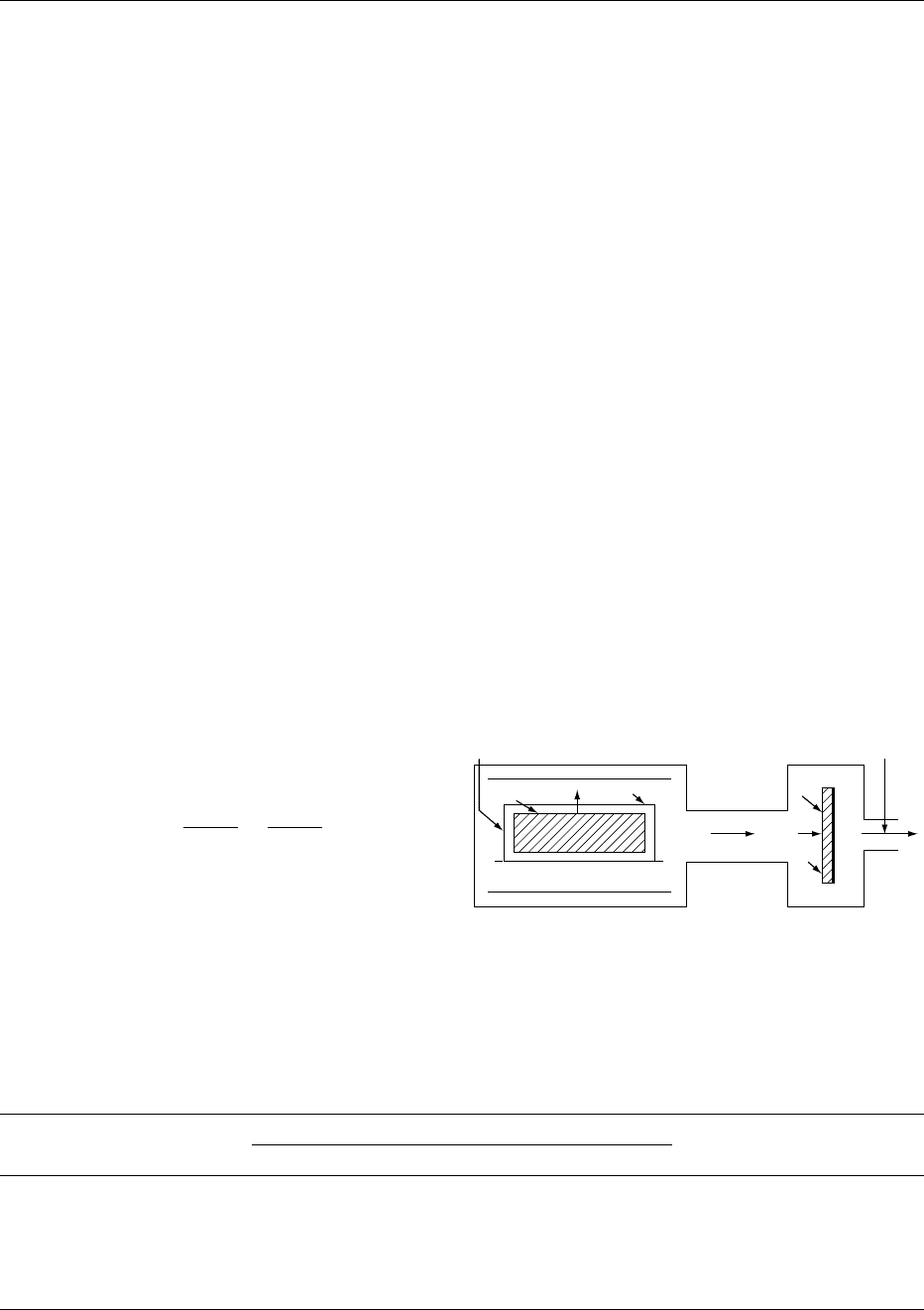
is illustrated in Figure 1.
Vapor Flow
0009As a piece of food freeze dries, the porous dry product
formed around the subliming ice core can cause a
severe restriction to vapor flow, and may prevent
continuation of the drying process. A useful model
of the above conditions assumes the pores in the dry
material resemble a bundle of circular cylinders or
tubes, with a porosity E expressed as a ratio of pore
volume to the intrinsic volume of the layer of dried
product, and a tortuosity factor (L
e
/L)
2
taken as the
square of the ratio of the effective flow path L
e
to the
thickness of the layer L. It can be shown mathematic-
ally that:
D
e
¼ ED= L
e
=LðÞ
2
ð2Þ
where D
e
is the effective diffusion coefficient, and D
is the water vapor diffusion coefficient in air. In the
Partially dry material
P
s
G
s
G
m
G
c
p'
c
p
c
p'
To vacuum
pump
fig0001Figure 1 Representation of a common freeze drier. See text
for notation of the mathematical variables illustrated. Repro-
duced from Freeze Drying, Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
tbl0001 Table 1 Amount of water frozen in various foods as a function of temperature
Food Water content (%) Freezablewater (%) frozen at different temperature (
C) Nonfreezable water (kg kg
1
solids)
5 10 15 20 30
Lean beef 74 83 93 97 99 100 0.35
Cod 80.5 85 94 97 98 100 0.39
Egg 74 90 95 98 99 100 0.20
Fruit juice 88 75 87 93 96 100 0.20
White bread 46 50 87 97 99 100 0.30
Peas 78 68 86 92 96 100 0.2–0.3
Data from Kuprianoff J (1964) In: Rey R (ed.) Advances in Freeze-Drying, p. 48. Paris.
2698 FREEZE-DRYING/The Basic Process
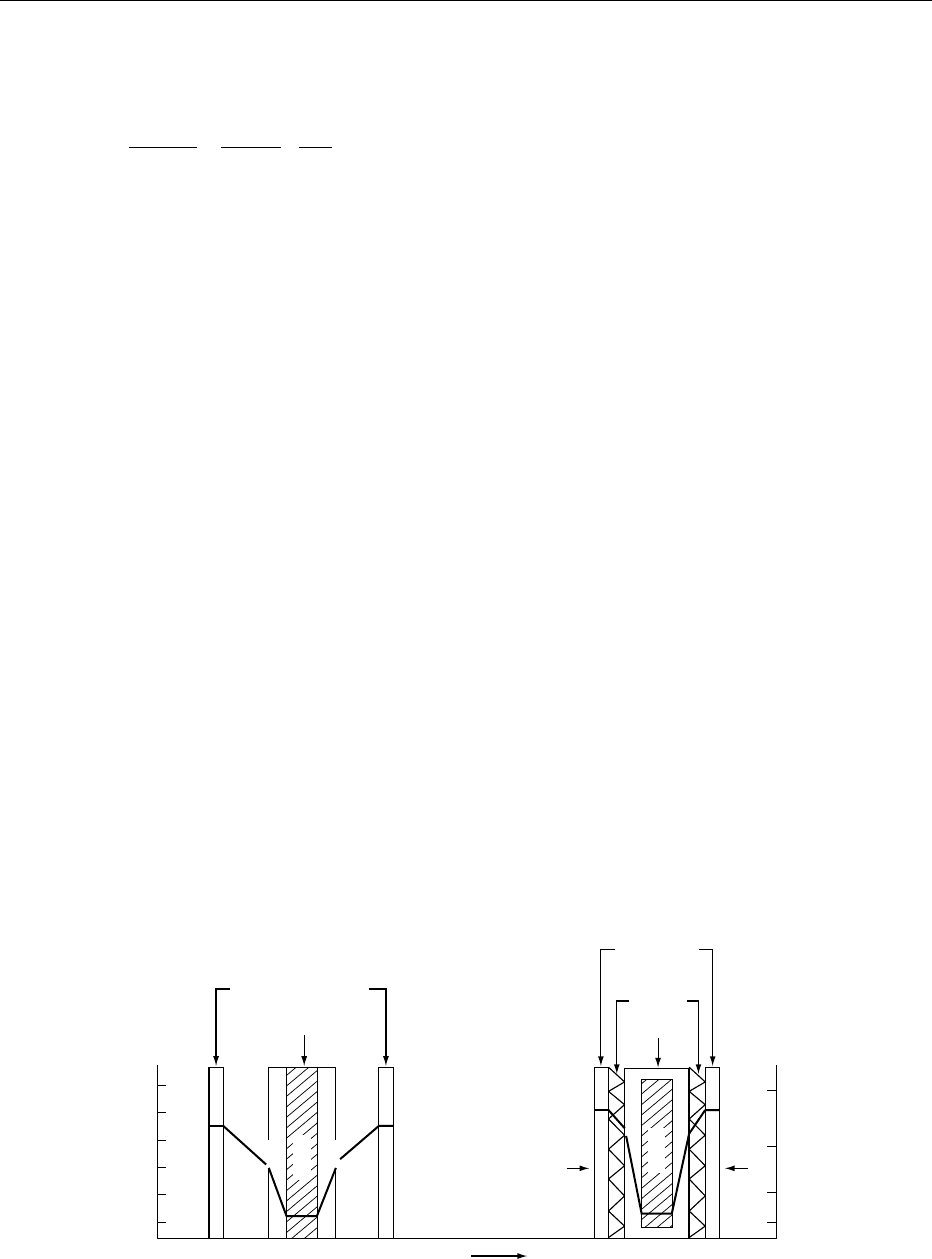
porous layer, D
e
varies inversely with vacuum
pressure P and directly with temperature T to the
1.75 power; therefore:
D
e
¼
E
L
e
=LðÞ
2
D
1013:3
P
T
273
1:75
ð3Þ
0010 The value D
e
increases with decreasing pressure
until the mean free path of the gas molecules at inter-
mediate pressures becomes comparable with the pore
diameter, so that wall collisions begin to compete
with collisions in the gas stream itself in limiting
flow by diffusion. This condition is known as transi-
tion flow and is the dominating vapor flow in the
freeze drying of food.
Heat Flow
0011 In food freeze driers, heating the product is the rate-
controlling variable when it is applied to the inner
core of ice crystals through an outer layer of dry
product. If heat is applied directly through the ice
layer by conduction, as is common in laboratory
and medical freeze driers, then vapor diffusion is the
rate-controlling variable. Food freeze driers usually
apply heat by radiation to the product surface which
proceeds by conduction through the dry layer. How-
ever, the dry product layer insulates the product and
limits the radiated heat supply.
0012 Various attempts have been made to compensate
for this insulating effect by maximizing heat distribu-
tion to the product. These include: (1) the accelerated
freeze drying (AFD) technique, illustrated in Figure 2,
which employs heat-conducting expanded metal
sheets that are hydraulically pressed against the
frozen product’s surface and serve to conduct heat
to the product while also allowing water vapor to
escape, and (2) the tempomatic or ribbed-tray
method which exploits the conducting properties of
a number of ribs that reach into the bulk of the
product. Both these methods have seen extensive
industrial use. The latter has been used extensively
for drying of coffee, berries, and dairy products.
Refrigeration Condensers
0013It is common to condense the escaping water vapor,
produced by sublimation at the product surface, on a
refrigerated coil, where condensation is the reverse
of sublimation, from vapor to solid without passing
through the liquid phase. As ice builds up on a re-
frigerated coil, it insulates the condenser and renders
it less effective. Heat flow through the ice deposit can
be modeled to give an indication of the build-up of
ice with time, and the resulting temperature deficit
(difference between ice surface temperature and coil
temperature).
0014The available surface area of the condenser deter-
mines the thickness of ice that will be condensed on to
it. The surface area of the condenser is, therefore, an
important consideration in designing a food freeze
drier.
0015Condensers have some special characteristics
which have been generally neglected in considering
the basic physical processes in freeze drying.
0016It is one of the phenomena of freeze drying that the
partial pressure of air near the condenser is always
relatively high, this air having been liberated from the
product during sublimation, and having come from
leaks in the vacuum chamber. The larger the space
available to be screened by the condenser, the lower
will be the vapor pressure of air in the rest of the
Radiation Distance
Mechanical press
Va
p
or
p
ressure
(
kPa
)
Accelerated
0.1
1
10
100
Expanded
platens
Heating
metal
Material
Heating platens
Materials
Dry
Dry
Dry
Dry
−25
0
25
Temperature (⬚C)
50
75
100
Frozen
Frozen
fig0002 Figure 2 Temperature and vapor pressure gradients (heavy black line) in freeze drying with heating through the dry layers.
Reproduced from Freeze Drying, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
FREEZE-DRYING/The Basic Process 2699

vacuum chamber, and hence less air pressure will
interfere with the escape of water vapor from the
subliming product.
Desorption (Secondary Drying)
0017 After the disappearance of the ice from the freeze-
dried product, the residual moisture (nonfreezable
water content and, as frequently occurs in practice,
incompletely sublimed ice) can be reduced by a sec-
ondary drying step called desorption. This consists of
heating the product while under a vacuum of between
0.13 and 0.67 mbar. The effect of heating is dimin-
ished by the insulating effect of the vacuum in the
porous dry product.
0018 An endpoint to secondary drying should be deter-
mined to prevent a result in which the product either
retains too much water or becomes overdried and
damaged by secondary heating. The endpoint can be
determined by plotting the ratios of the pressures in
the vacuum line before and after isolating the vacuum
pump from the chamber by means of a valve. The
endpoint is reached when the ratio approaches unity,
though values in the range 1.1–1.2 may be practic-
able. This method assumes the chamber has a low
leak rate.
0019 A more exact endpoint can be determined by trap-
ping a sample of the vapor/air mixture from above the
desorbing product, then condensing the vapor com-
ponent outside the vacuum chamber. As in the previ-
ous method, the ratio of the partial pressure before
and after trapping approaches unity at the endpoint
of drying. Precise calibration can be achieved by ref-
erence to moisture content predicted by a vapor pres-
sure isotherm for the particular product being dried.
Breaking the Vacuum
0020 A dry inert gas, such as nitrogen, is often used to
break the vacuum, and thereby prevent entry of
moisture-laden air (and oxygen) into the dry product.
Supplementary (Conditioning) Phase
0021 A freeze-dried product is dry, light, and porous, and
ideally retains its original shape. It can be stored
for long periods provided its container excludes
moisture and oxygen. Residual moisture should be
evenly distributed and should not exceed 2% (dry-
weight-basis measurement). A conditioning pro-
cedure is often necessary to achieve even distribution
of residual moisture through the entire mass of the
product, since freeze driers with multiple shelves
usually have a temperature gradient between the top
and bottom shelves and, therefore, do not achieve
exactly the same drying conditions on every shelf.
Conditioning is achieved by combining the product
from all shelves and allowing the mixture to stand for
24 h in an airtight drum before packaging into smaller
quantities.
Rehydration
0022Rehydration restores the lyophile (dry product) to its
original physical and organoleptic properties. How-
ever, rehydration by immersion of the product in
water can only proceed with difficulty in some
cases, where pores may have collapsed and protein
chains may have changed positions. Protein can be
partly digested by adding a proteolytic enzyme to the
fluid, and so assist rehydration. The product should
be allowed to stand incompletely immersed in the
fluid during rehydration, thereby minimizing resist-
ance to rehydration by air becoming entrapped in the
porous product.
Technological Improvements
0023Freeze-drying rates and results for certain food prod-
ucts can be improved by variants of the basic process.
Microwave Heating
0024This is best applied to remove residual moisture in the
desorption stage, since it can speed up this final stage
most advantageously. Use of microwave heating in
the primary phase is not recommended, because of
the danger of ionization of the air under vacuum
pressures of less than 1.33 mbar, and possible explo-
sive effects.
Cyclic Pressure
0025If the vacuum pressure is deliberately increased peri-
odically (two-thirds of the cycle), and then reduced
again (one-third of the cycle), a vapor flash occurs at
the product surface, as a result of raising the thermal
conductivity of the gas, and a small increase in ice
interface temperature. The result of this process is a
reduction in overall drying time of up to 34% for
products such as cooked meat, whole egg, and apple.
Adsorbents
0026The adsorption process removes water vapor by
means of a desiccant rather than refrigeration coils.
The desiccant creates a high and well-sustained vapor
pressure driving force, particularly at moderately low
temperatures, since the equilibrium water vapor pres-
sure of the desiccant decreases as the temperature is
lowered.
0027At 20
C and 1.04 mbar vacuum, silica gel
provides a vapor pressure force to ‘drive’ freeze
drying equivalent to refrigeration coils at 40
C
2700 FREEZE-DRYING/The Basic Process
and similar vacuum pressure. An adsorption system
can, therefore, be set at 20
C and many of the
engineering complexities, involving low-temperature
refrigeration, high vacuum, and problems of drying
the vacuum chamber after defrosting the refrigeration
coils, are eliminated.
0028 In an adsorption system, secondary drying can be
achieved at 20
C instead of þ20
C, with a greatly
reduced risk of overheating the product.
See also: Drying: Theory of Air-drying; Freezing:
Operations; Water Activity: Principles and
Measurement; Effect on Food Stability
Further Reading
Bell GA and Mellor JD (1986) Development of the adsorp-
tion freeze-dryer. CSIRO Food Research Quarterly 46:
56–58.
Dalgleish JMcN (1990) Freeze-Drying for the Food Indus-
tries. London: Elsevier Applied Science.
Goldsmith SA, Rey L and Rothmeyer WW (eds) (1975)
Freeze-Drying and Advanced Food Technology.
London: Academic Press.
King CJ (1970) Freeze-drying of foodstuffs. CRC Critical
Reviews in Food Technology 1: 379.
Mellor JD (1978) Fundamentals of Freeze-Drying. London:
Academic Press.
Structural and Flavor (Flavour)
Changes
M Marin, Unite
´
Mixte de Recherche de Ge
´
nie et
Microbiologie des Proce
´
de
´
s Alimentaires (INRA-INA
P-G), Thiverval-Grignon, France
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Freeze-drying is a dehydration process especially
suited to the conservation of biological products. In
comparison with other drying processes, freeze-
drying is considered as a reference for manufacturing
high-quality dehydrated product. The direct transi-
tion of water from solid to vapor (sublimation), with-
out a liquid phase, helps to preserve most of the initial
raw material’s properties such as appearance, shape,
taste, color, and flavor. As an important functional
property, the freeze-dried product has a high rehydra-
tion capacity. The main limit to the industrial devel-
opment is its cost due to the low productivity.
Consequently, except the application for biological
active material (bacteria, vaccine), the use of
freeze-drying is restricted in food industry to high
added-value products like coffee, ingredients for
ready-to-eat foods (fruits and vegetables, meat and
fish), and aromatic herbs.
Freezing
0002Freezing, the first step of the process, determines the
structure of the freeze-dried product. The rate of
freezing and the temperature level, depending on the
nature and the composition of the product, define
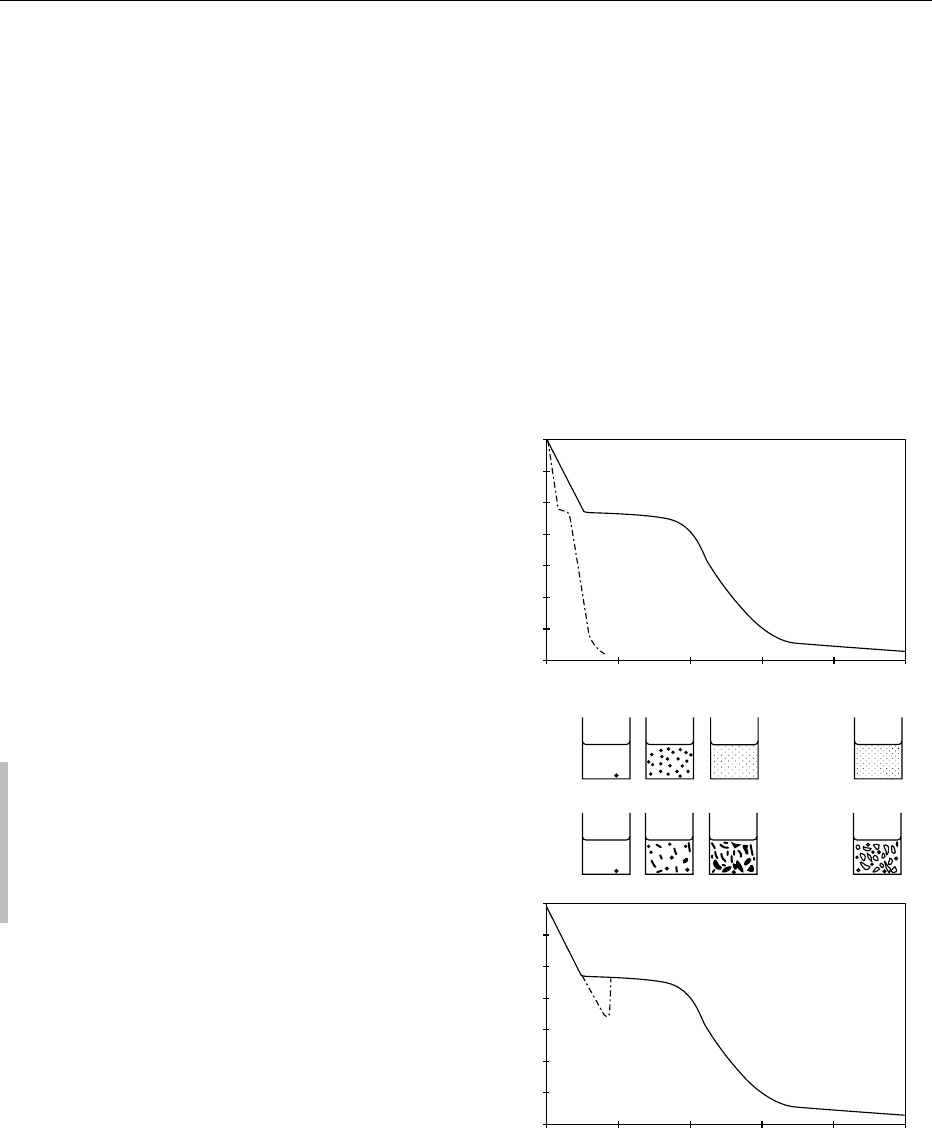
the number (nucleation) and size (growth) of the ice
crystals (Figure 1).
0
−50
−40
−30
−20
−10
0
10
20
12
Time (h)
34
(c)
(d)
3
2
1
1
5
Temperature (⬚C)
(c)
(a) (d)
1 2 3 After freeze-drying
Freezing step
0.00
−50
−40
−30
−20
−10
0
10
20
0.02 0.04
Time (h)
0.06 0.08
(a)
(b)
3
2
1
0.10
Temperature (⬚C)
fig0001Figure 1 Influence of freezing rate on the final structure of
freeze-dried products. (a) Rapid freezing rate; (b) very rapid
freezing rate; (c) slow freezing rate; (d) slow freezing with sub-
cooling. The liquid product is shown schematically with black
points representing ice. Step 1, appearance of the first ice crystal;
step 2, ice nucleation and crystal growth; step 3, end of freezing
step. Porosity of the freeze-dried product is shown in relation to
freezing rate.
FREEZE-DRYING/Structural and Flavor (Flavour) Changes 2701

0003 In the case of rapid freezing (more than several
centimeters per minute), ice crystals remain small,
and the high nucleation rate leads to the creation of
numerous crystals. In food products, water crystal-
lizes simultaneously inside and outside the cell, and
the microcrystals yield small pores after freeze-
drying. This method of freezing preserves the struc-
ture of the material as much as possible, but at the
same time, the absence of a high porous network
tends to decrease the rate of subsequent drying
steps and the rehydration capacity of the freeze-
dried product.
0004 In the case of slow freezing (less than one centi-
meter per hour), ice crystal formation is based on two
mechanisms. If crystallization begins at a temperature
lower than but close to 0
C(1to3
C), the ice
crystal growth is faster than the ice nucleation and
takes place preferentially outside the cell. Owing to
the resulting osmotic effect, water inside the cell tends
to move out, and the large crystals obtained in the
interstitial areas can cause mechanical injury to the
structure. In a few cases, subcooling may occur (sev-
eral degrees under freezing point without ice), and
very rapid crystallization suddenly occurs, yielding
a final structure similar to that obtained by quick
freezing.
0005 In noncellular systems (gel, juices), the result of
slow freezing is a heterogeneous medium with a
large dendritic crystal structure and a highly concen-
trated phase that can form an impermeable layer at
the surface of the product. During subsequent drying,
if the vapor is thus prevented from leaving the prod-
uct, the vapor pressure inside the product will increase
and make the product foam. To a lesser extent, a
nonhomogenous freezing step, based on rapid freez-
ing at the surface (with a refrigerating effect of the
superficial water evaporation in a dry cold atmos-
phere) and a slow freezing inside the product, also
creates a less porous layer on the surface. This layer
constitutes a barrier to the elimination of the water
vapor.
0006 Quenching in liquid nitrogen prevents any crystal-
lization (vitrification) but is not usually carried out
in food processing. Meanwhile, if the temperature
achieved at the end of the freezing process is suffi-
ciently low, often far below 20
C, the frozen
product is in an overall amorphous state. This is a
good condition to maintain the stability of the frozen
structure. Moreover, a lower storage temperature has
to be applied in order to preserve the structure
obtained after a rapid freezing than after a slow
freezing rate.
0007 The freezing step gives the high porosity of the
freeze-dried product but explains the main part of
the texture loss when damaging the cell wall structure.
The volume difference between the fresh and freeze-
dried product is generally considered negligible. A
rapid (homogeneous) freezing will contribute to pre-
serve the structure and overall texture of the material,
while creating the uniform porous network of the
freeze-dried product. Slow freezing is nevertheless
preferred because the interstices and openings are
larger and facilitate mass transfer during drying and
rehydration before eating.
Drying
0008The drying steps (primary – sublimation, and second-
ary – desorption) may also affect the structure of the
product, and the most important factors affecting
the criteria of final product quality are the heating
temperature and the working pressure.
Temperature
0009Large differences between the temperature values
from the frozen part to the dried layer are observed
during freeze-drying.
0010In the frozen core, it is well known that the tem-
perature must remain under the melting point in
order to prevent fusion. Moreover, the structure
resulting from the freezing step is stable if the
temperature also remains under another lower tem-
perature level, which is characteristic of the glass
transition step of the concentrated medium around
the ice crystal (Figure 2). As an example, when the
vitreous region is obtained (area (3) in Figure 2), the
reactions like the recrystallization, also called ‘Ost-
wald maturation,’ are highly reduced. Moreover, if
Temperature (⬚C)
Freezing
temperature
(1)
(2)
0
0
5 100
(4)
(3)
T
g
⬘
C
g
⬘
Glass transition
temperature (
T
g
)
Solute content (%)
fig0002Figure 2 Phase state diagram for a binary mixture. Curves
showing transition temperatures – glass transition and melting
– are plotted against moisture content. There are four regions: (1)
liquid, (2) ice crystal with concentrated liquid, (3) amorphous
solid, and (4) liquid with solute crystal. In the amorphous solid
region, the viscosity of the matrix is higher than 10
12
–10
14
Pa.s.
These diagrams may be experimentally determined for a mater-
ial by differential scanning calorimetry. T
g
0
: Glass transition tem-
perature, of the maximally frozen concentrated solute, C
g
0
.
2702 FREEZE-DRYING/Structural and Flavor (Flavour) Changes

the temperature in the frozen core during the primary
drying phase is below the melting point but above the
glass transition temperature (T
g
0
), the structural
damage may be significant, and the product may
collapse when the frozen ice crystal is sublimated. A
low temperature (under T
g
0
) maintains a high appar-
ent viscosity; shrinkage and, consequently, collapse
are avoided. The product-collapse temperature is
closely related to the glass transition temperature
but sometimes appears higher than T
g
, especially for
cellular material (biological tissue). For foods with a
high sugar content, the collapse temperature is low
(40 to 60
C) and can be raised by the addition of
high-molecular-weight additives (polysaccharides).
0011 The glass transition temperature decreases strongly
with the water content, due to the plasticizing effect
of water (Figure 2). In the dried layer, a higher
temperature, allowing a higher drying rate, can be
applied without product damage. But over a critical
value of 60
C, another kind of damage occurs in the
biological product, i.e., protein degradation. This
results in a change in the product structure, especially
in protein-based foods (meat, fish), and a reduced
tenderness and hydration capacity.
0012 During the freeze-drying process, the passage of the
product from the frozen state to the dried state is a
critical step in which there is a wide variation in water
content as well as the temperature. The probability of
structural changes (i.e., collapse) is important. As a
general rule, to preserve the original structure, the
product temperature should remain under the glass
transition temperature (T
g
), which depends strongly
on the water content.
0013 On a practical point of view, the control of tem-
perature is based on the temperature and energy sup-
plied from the heating source. Among the traditional
techniques, conductive heating is more preferable for
use with thermally sensitive products than a radiation
heat source. With microwave heating, it is more diffi-
cult to control a homogeneous supply of energy inside
the frozen core of the product, and this often leads to
a reduction in final product quality.
Pressure
0014 In the atmosphere of the freeze-drier under vacuum,
the total pressure is generally assumed to be close
to the vapor pressure. Following the equilibrium
rule, the vapor pressure is directly related to the tem-
perature at the sublimation front inside the product
where the vapor is created. Thus, a control of the
total pressure is an indirect way to monitor the prod-
uct temperature at the sublimation front. At first, the
total pressure has to be sufficiently low to avoid the
melting of ice. After a rapid abnormal increase in
pressure in the freeze-drier, the food product foams
and shrinks due to an increase in front temperature
above the melting point. Furthermore, during pri-
mary drying, if the pressure is sufficiently low to
avoid the melting point but higher than the glass
transition, shrinkage and collapse mechanisms can
be observed. In order to maintain the quality of
the product, the total pressure should be sufficiently
low in comparison with the sublimation front
temperature.
0015In conclusion, the main modification of the struc-
ture of the product is a consequence of the freezing
step, assuming that the drying steps are well con-
trolled (no recrystallization and no collapse). During
the subsequent drying steps, low values of pressure
and temperature (levels depending on the nature of
the dry matter of the product) tend to preserve the
shape and texture of the frozen product. Knowing that
an increase in temperature is a good way to reduce the
freeze-drying time, an optimum temperature has to be
defined for industrial applications.
Flavor Losses
0016Freeze-drying is often considered the best drying pro-
cess as far as flavor compound retention is concerned,
due in a first approximation to the low temperature
level.
0017Molecules, responsible for food flavor, usually
have a high relative volatility to water and a low
molecular weight (less than 300 g mol
1
). Owing to
the low concentration of aroma compounds in the
food products (p.p.b. to p.p.m.), food flavor is usually
evaluated using two kinds of tools: analytical meas-
urements (gas chromatography and mass spectrom-
etry analysis) and/or sensory panel. From these
properties, it is difficult to imagine removing water
as a vapor phase, without any other volatiles like
flavor compounds. Also, flavor losses, even in low
proportions, are rapidly considered to be significant.
As a matter of fact, the loss of flavor compounds can
be limited during freeze-drying with regard to water
elimination. The freeze-drying operating conditions
that improve the drying rates give an increased reten-
tion of volatiles. Rapid primary drying is expected
to result in higher levels of volatiles. This can be
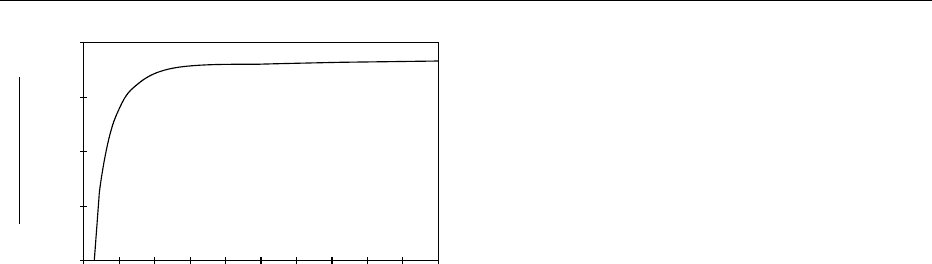
explained from the principle of selective diffusion.
The diffusion coefficient (mobility) of the volatile
decreases more rapidly with the water content inside
the product than the diffusion coefficient of water
itself (Figure 3). The loss of volatile decreases and
stops as soon as a critical moisture is attained. One
way to decrease the first drying duration is to
increase, in a preliminary step, the dry matter (addi-
tives, osmotic dehydration). Based on the techniques
of encapsulation, additives can also decrease the
FREEZE-DRYING/Structural and Flavor (Flavour) Changes 2703
flavor mobility in the matrix during drying. With this
approach, a decrease in flavor losses has been ob-
served in coffee, fruits, and vegetables when slow
freezing is applied.
0018 Using a constant heating temperature source,
retention of flavor decreases when the total pressure
increases. Shrinkage and collapse phenomena (high
pressure and low apparent viscosity of the medium)
are correlated to a poor aroma retention. Considering
flavor compounds (or lipids) encapsulated in the
amorphous part of the freeze-dried product, recrys-
tallization (either water or solutes) above T
g
results
in the release of entrapped compounds. Thus,
crystallization is related to a loss of flavor. Moreover,
exposure of lipids to ambient oxygen is enhanced,
which promotes oxidation and creates off-odors.
Low-fat meats like chicken, turkey, and ham are
easier to freeze-dry. During secondary drying, low
heating temperatures are recommended to obtain a
freeze-dried product without a cooked flavor, thus
preventing new and often undesirable aroma com-
pounds.
0019 Lowering the driving force is another way to
reduce the mass transfer of flavor compounds. Redu-
cing the total pressure under vacuum implies that a
vacuum pump continually extracts the volatiles from
the chamber. Freeze-drying under atmospheric pres-
sure has been proposed to remove less volatiles, but it
is necessary to use a highly selective adsorbent that
depletes only the water vapor in the freeze-drying
atmosphere.
0020 The flavor losses remain low during the freeze-
drying process. Under vacuum freeze-drying, the low
total pressure prevents oxidation. The storage condi-
tion, especially the temperature, can also be detrimen-
tal to flavor sensory components.
Packaging and Storage of the
Freeze-dried Product
0021The moisture of the freeze-dried material associated
with the temperature during the storage has to be
finely controlled in order to preserve the high quality
of the freeze-dried product.
0022The freeze-dried product has a very low water
content (less than 5%), and so it is very hygroscopic
and necessitates the use of packaging film that is
impermeable to water. If there is a gain of a few
percent of water, the product can become rubbery at
ambient temperature (temperature above the glass
transition temperature). Its structure becomes sticky,
and the product collapses into lumps after a few
weeks. In high-sugar products, there is also a risk of
solute crystallization. This loss of structure is associ-
ated with an increase in all kinetic reactions inside the
product.
0023Moreover, because of the high porosity of the
freeze-dried product, the ambient oxygen is very effi-
cient, and the oxidation reaction can be accelerated
(flavor losses, browning).
0024Depending on the product, a vacuum seems to be
better than an inert gas like nitrogen, in order to
prevent off-flavors from developing.
0025A good stability of the freeze-dried product is
achieved with a nonpermeable packaging, referring
to water vapor and oxygen. Packaging under vacuum
and at a low storage temperature (above ambient
temperature) is always favorable to increase the time
of preservation of the original flavor to several
months.
Other Quality Criteria: Nutrients, Color,
and Rehydration
0026Freeze-drying is definitively taken as a reference when
quality criteria should be maintained close to the
original product. Superior retention of nutrients and
a better preservation of the color are also obtained
when freeze-drying operating conditions have been
chosen to maintain the structure and flavor of the
product.
0027Fruits and vegetables are major sources of vitamins
and provitamins, with a reported anticancer activity,
which are highly unstable and oxidizable. Freeze-
drying at low heating temperatures and under an
inert gas gives the best preservation of nutrients com-
pared with other processes (drying, canning). High-
activity enzyme extracts from fruits have also been
obtained with freeze-drying processes.
0028In freeze-dried foods, the color is brighter than that
of products dried by other techniques. Browning due
to Maillard reactions, if compared with other drying
0
10
−4
10
−3
10
−2
10
−1
1
10 20 30 40 50 60 70 80 90 100
Water content (%)
Diffusivity of volatiles
Diffusivity of water
fig0003 Figure 3 Influence of water content inside food products on the
ratio between the diffusion coefficients of volatile compounds and
water vapor.
2704 FREEZE-DRYING/Structural and Flavor (Flavour) Changes

processes, is reduced because of the low temperature
used. Nevertheless, a slow freezing rate gives a more
pronounced color and higher luminance than a
high freezing rate but disappears after rehydration.
Referring to the chemical reaction, slow freezing,
associated with the cryoconcentration effect, tends
to increase the intensity of the red color in freeze-
dried products like strawberries and beef meat. This
risk of enzymatic browning reactions in fruits is
higher with slow freezing. The effect of freeze-drying
pressure has been observed after rapid freezing on
freeze-dried coffee, with a darker product at high
pressure. Secondary drying at more than 60
C will
promote browning (Maillard reactions). Eventually,
freeze-dried products are very sensitive to UV light.
This is particularly true for vegetables like garlic and
carrots, which may become virtually white after a few
weeks’ storage under light. A layer of aluminum is
usually introduced in the packaging film to make it
impermeable to UV radiation.
0029 The rehydration capacity is an interesting func-
tional property for industrial use of the product as
well as for the consumer. The freezing step, associated
with negligible shrinkage, leads to the characteristic-
ally high porosity of the freeze-dried product. A low
freezing rate promotes a high rehydration capacity.
This effect on different freeze-dried food products is
not obvious if recrystallization has taken place during
freeze-drying. The percentage of sublimed water,
which often occupies 80–90% of the volume of bio-
logical materials, coincides with the porosity index of
the freeze-dried product. The freeze-dried structure
explains the instantaneous and high rehydration cap-
acity, which may reach up to 90% of the lost water.
Deformation as well as protein denaturation, due to
high temperatures during freeze-drying, can decrease
this ratio. Nevertheless, it will not achieve the
rehydration ratio observed for traditional drying
processes, i.e. not higher than 10–20%. During rehy-
dration in boiling water, hot liquid rapidly reenters
the pores, and freeze-dried products such as beverages
(coffee or tea), vegetables, and all ready-to-eat meal
preparations can be instantaneously heated and
cooked.
See also: Drying: Dielectric and Osmotic Drying; Physical
and Structural Changes; Chemical Changes; Flavor
(Flavour) Compounds: Structures and Characteristics;
Production Methods; Freezing: Storage of Frozen Foods;
Structural and Flavor (Flavour) Changes; Nutritional
Value of Frozen Foods; Oxidation of Food Components;
Water Activity: Effect on Food Stability
Further Reading
Dalgleish J (1990) Freeze-drying for the Food Industries.
London: Elsevier Science.
Goldblith SA, Rey L and Rothmayr WW (eds) (1975)
Freeze-drying and Advanced Food Technology. London:
Academic Press.
Heldman DR and Lund DB (1992) Handbook of Food
Engineering. New York: Marcel Dekker Inc.
King CJ (1971) Freeze-drying of Foods. Cleveland, OH:
CRC Press.
Mujumdar AS (1987) Handbood of Industrial Drying. New
York: Marcel Dekker.
Simatos D, Blond G, Dauvois PH and Sauvageot F (1974)
La Lyophilisation: Principes et Applications. Paris:
Collection de l’ANRT.
FREEZE-DRYING/Structural and Flavor (Flavour) Changes 2705

FREEZING
Contents
Principles
Operations
Blast and Plate Freezing
Cryogenic Freezing
Storage of Frozen Foods
Structural and Flavor (Flavour) Changes
Nutritional Value of Frozen Foods
Principles
B R Becker and B A Fricke, University of Missouri-
Kansas City, Kansas City, MO, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Preservation of food is one of the most significant
applications of refrigeration. It is known that cooling
and freezing of food effectively reduces the activity of
microorganisms and enzymes, thus retarding deteri-
oration. In addition, crystallization of water reduces
the amount of liquid water in food items and inhibits
microbial growth.
Thermodynamics of the Cooling and
Freezing Process
0002 The cooling and freezing of food is a complex pro-
cess, as illustrated in the cooling/freezing curve shown
in Figure 1. Prior to freezing, sensible heat must be
removed from the food to decrease its temperature
from the initial temperature. As the temperature of
the food is decreased, it will pass through its initial
freezing point to become undercooled prior to the
formation of ice. Then, the formation of ice nuclei
releases energy to bring the temperature of the under-
cooled food back to its initial freezing point. Once ice
nuclei have formed, ice crystals will grow as energy is
removed.
0003 The number, size, and location of the ice crystals
are determined by the rate of energy removal. If the
heat transfer rate is much higher than the rate of
energy release due to crystal growth, the food will
become undercooled, resulting in the formation of
additional ice nuclei, and hence, a large number
of small ice crystals will form within the food. If the
rate of energy release due to crystal growth is more
nearly equal to the heat transfer rate, fewer nuclei will
form, and the existing crystals will grow to a larger
size. In addition, a high freezing rate will not allow
sufficient time for water migration from within the
cells, thus resulting in intracellular ice crystal nucle-
ation and growth. Conversely, a lower heat transfer
rate will provide sufficient time for water migration
through the cell walls as extracellular ice crystals
remove free water external to the cells, thus resulting
in the growth of larger extracellular ice crystals.
Initial Freezing Point
0004Foods do not freeze completely at a single tempera-
ture, but rather they freeze over a range of
temperatures, and some foods that are high in sugar
content may never be completely frozen. Thus, foods
do not have a distinct freezing point but rather an
initial freezing point at which the crystallization
process begins.
0005The initial freezing point is important in the deter-
mination of proper storage conditions and in the
calculation of thermophysical properties. Fresh fruits
and vegetables, for example, should be stored above
their initial freezing point to avoid frost damage, and
since the thermophysical properties of a food change
drastically as it freezes, knowledge of its initial
freezing point is necessary to accurately model its
thermophysical properties.
Freezing Point Depression
0006The initial freezing point is somewhat lower than the
freezing point of pure water because of dissolved
substances in the moisture within the food. At the
initial freezing point, a portion of the water within
the food crystallizes, and the remaining solution
becomes more concentrated. Thus, the freezing
point of the unfrozen portion of the food is reduced
further. As the temperature continues to decrease, the
formation of ice crystals increases the concentration
2706 FREEZING/Principles
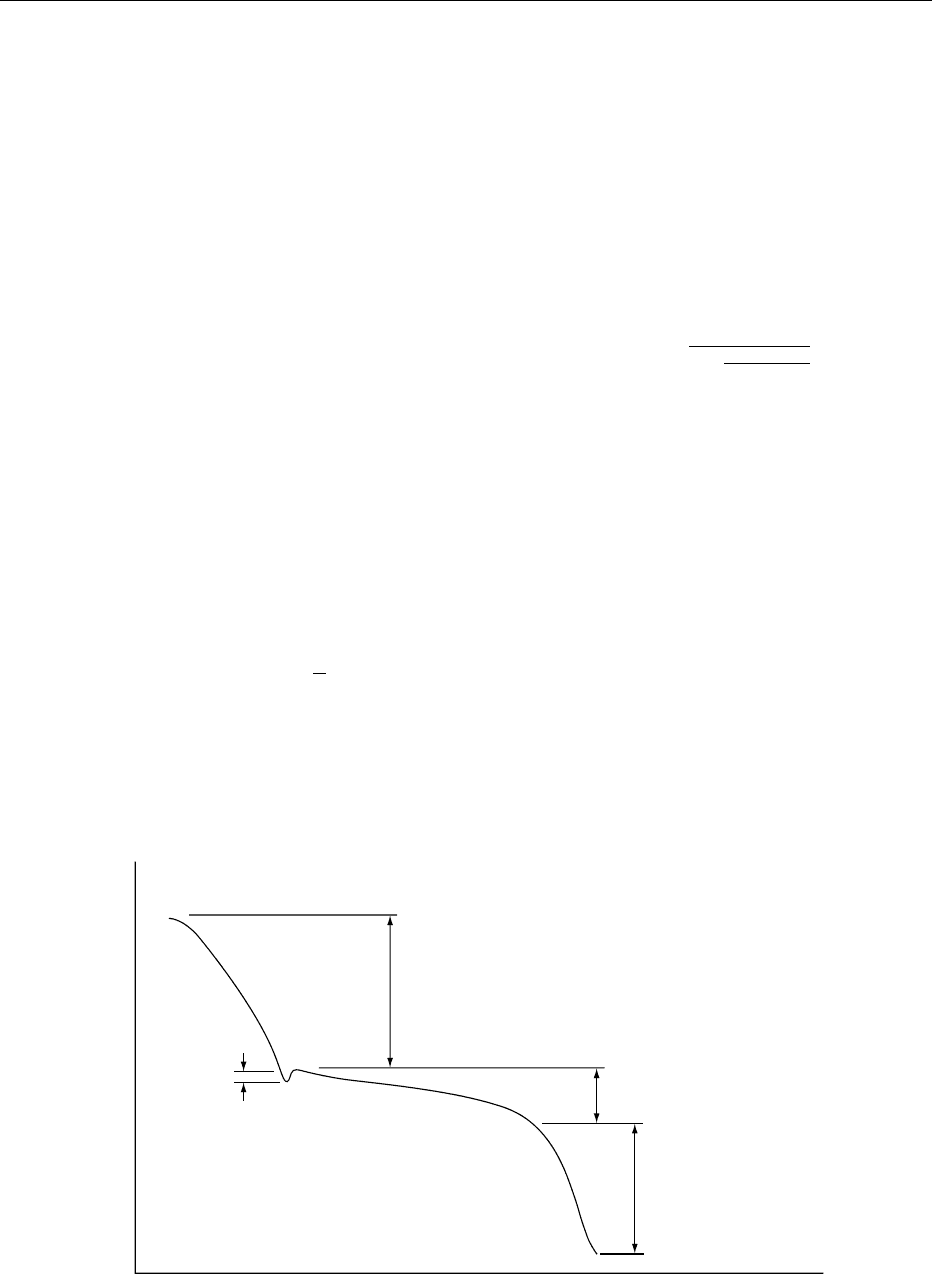
of the solutes in solution and depresses the freezing
point further.
Ice Fraction
0007 To predict the thermophysical properties of frozen
foods, which depend strongly on the ice and water
fractions within the food, it is necessary to determine
the mass fraction of water that has crystallized. For
temperatures below the initial freezing point, this
mass fraction is a function of temperature.
000 8 As the food item is cooled to its initial freezing
temperature, it will contain ice, unfrozen water, sol-
uble solids, and insoluble solids. As the temperature
decreases further, there is an increase in the mass frac-
tion of ice, w
ice
, and a decrease in the mass fraction of
unfrozen water, w
w
, which are related as follows:
w
wo
¼ w
ice
þ w
w
, ð1 Þ
where w
wo
is the total mass fraction of water within
the food.
0009 Assuming that the high moisture content food
items can be modeled as ideal dilute solutions, an
ice fraction equation can be derived from Raoult’s
law:
w
ice
¼ w
wo
w
b
ðÞ1
t
f
t
hi
, ð2 Þ
where t
f
is the initial freezing point of the food (
C),
t is the food temperature (
C) and w
b
is the mass
fraction of water that is bound to solids within the
food, and thus is unavailable for freezing. This bound
water fraction may be estimated as follows:
w
b
¼ 0:4w
p
, ð3Þ
where w
p
is the mass fraction of protein in the food
item.
0010Because eqn (2) underestimates the ice fraction at
temperatures near the initial freezing point and
overestimates the ice fraction at lower temperatures,
the following empirical relationship has been pro-
posed to estimate the mass fraction of ice:
w
ice
¼ w
wo
1:105
1 þ
0:7138
ln t
f
tþ1ðÞ
2
4
3
5
: ð4Þ
Energy Transfer During Freezing
0011In many food cooling and freezing processes, transi-
ent convective heat transfer occurs between a fluid
medium and the food item. Knowledge of the corres-
ponding surface heat transfer coefficient is required in
order to design the equipment in such processes.
Newton’s law of cooling defines the surface heat
transfer coefficient, h, as follows:
q ¼ hAðt
s
t
m
Þ, ð5Þ
where q is the heat transfer rate, t
s
is the surface
temperature of the food, t
m
is the surrounding
fluid temperature, and A is the surface area of the
food through which the heat transfer occurs.
Sensible energy removal
above freezing
Time
Undercooled
region
Temperature
Sensible energy removal
below freezing
Latent energy
removal
fig0001 Figure 1 Cooling/freezing curve.
FREEZING/Principles 2707
