Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0026 The hypothalamus is the main regulator of
sympathetic nervous system function. Impulses
from the posterior and lateral hypothalamus result
in generalized discharge of the sympathetic nervous
system, including the adrenal medulla, although
they can also be activated separately. The sym-
pathoadrenal system is characterized by speed and
integration, in that catechol-mediated events can
take place within seconds, and can coordinate vascu-
lar, metabolic, and hormonal components. As dis-
cussed above, these responses occur to a variety of
noxious, threatening, or stressful stimuli. In addition,
the sympathetic nervous system is instrumental in
maintaining an appropriate circulating volume and
cardiac output during changes of posture from supine
to upright. These feedback systems are mediated by
sensors in the carotid sinuses, aorta, and medulla,
which detect changes in circulatory volume and blood
pressure. Although of different embryological origins,
and operating via different regulatory mechanisms,
the hypothalamic–pituitary–adrenocortical system
and the sympathoadrenal system complement each
other in the maintenance of homeostasis and a
stable metabolic milieu in response to many forms of
stress.
See also: Amino Acids: Metabolism; Fatty Acids:
Metabolism; Renal Function and Disorders: Kidney:
Structure and Function; Stress and Nutrition
Further Reading
Baxter J and Tyrrell J (1987) The adrenal cortex. In: Felig P
(ed.) Endocrinology and Metabolism. New York:
McGraw-Hill.
DeQuattro V, Myers M and Campese V (1989) Anatomy
and biochemistry of the sympathetic nervous system.
In: DeGroot L (ed.) Endocrinology. Philadelphia: WB
Saunders.
Ganong W (1991) Review of Medical Physiology. The
Adrenals. Norwalk, CT: Appleton & Lange.
Guyton A (1986) The adrenocortical hormones. In:
Textbook of Medical Physiology. Philadelphia: WB
Saunders.
HO
HO
OH
CH
CH
2
NHCH
3
HO
HO
OH
CH
CO
CO
CO
CO
OH
HO
HO
OH
CH
CH
2
NH
2
HO
HO
CH
2
CH
2
NH
2
Epinephrine
COMT
COMT
Norepinephrine
Dihydroxymandelic
acid
HO
HO
CH
2
OH
Dihydroxyphenyl
acetic acid
HO
CH
3
O
CH
2
CH
2
NH
2
3-Methoxytyramine
HO
CH
3
O
CH
2
OH
Homovanillic
acid
HO
CH
3
O
OH
CH
CH
2
NHCH
3
Metanephrine
HO
CH
3
O
OH
CH
CH
2
NH
2
Normetanephrine
HO
CH
3
O
OH
CH
OH
3-Methoxy-4-hydroxy
mandelic acid
Dopamine
MAO
MAO
COMT
MAO
MAO
MAO
MAO
COMT
COMT
fig0010 Figure 10 Metabolism of catecholamines by catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). Reproduced
from Goldfien A (1986) The adrenal medulla. In: Greenspan F and Forsham P (eds) Basic and Clinical Endocrinology. Los Altos, CA:
Lange Medical Publications, with permission.
HORMONES/Adrenal Hormones 3139
Hale A and Rees L (1989) ACTH and related peptides.
In: DeGroot (ed.) Endocrinology. Philadelphia: WB
Saunders.
Loriaux DL (1990) The adrenal glands. In: Becker K (ed.)
Principles and Practice of Endocrinology and Metabol-
ism. Philadelphia: JB Lippincott.
Orth D and Kovacs W (1998) The adrenal cortex. In:
Williams Textbook of Endocrinology. Philadelphia:
WB Saunders.
Parker L (1989) Adrenal androgens: normal physiology. In:
Adrenal Androgens in Clinical Medicine. San Diego:
Academic Press.
Thyroid Hormones
J Vanderpas, Faculte
´
Me
´
decine, FUNDP, Namur,
Belgium
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Iodinated Thyroid Hormones
Thyroid Follicles and their Development
0001 The thyroid gland displays a peculiar, highly organ-
ized architecture characterized by the presence of
spheroidal structures – follicles – that are composed
of a single layer of epithelial cells (thyroid follicular
cells) surrounding a closed cavity (follicular lumen)
filled with colloid, a concentrated solution of thyro-
globulin (Tg). The follicle has been defined as the
morphologic and functional unit of the thyroid.
Notably, during intrauterine life, the onset of thyroid
function (around 10 weeks in humans) coincides with
the appearance of differentiated follicles. It is the
follicular organization, together with the polarity of
the follicular cells, that allows the several biochemical
steps required for thyroid hormone biosynthesis: (1)
secretion of a peculiar protein with iodinated amino
acids in the follicular lumen as exocrine cells; (2)
reabsorption of this peculiar protein, with hydrolysis
of its iodinated amino acids; (3) release of iodo-
thyronines into blood by endocrine secretion.
0002 The follicle cell divides the follicular lumen (where
hormone synthesis begins) and the blood stream,
from where iodine has to be uploaded and where
hormones will be released at the end of the process.
0003 The surface of a polarized thyroid follicular cell is
divided into two functionally distinct, but physically
contiguous regions: an apical and a basolateral
domain. Junctional complexes between cells separate
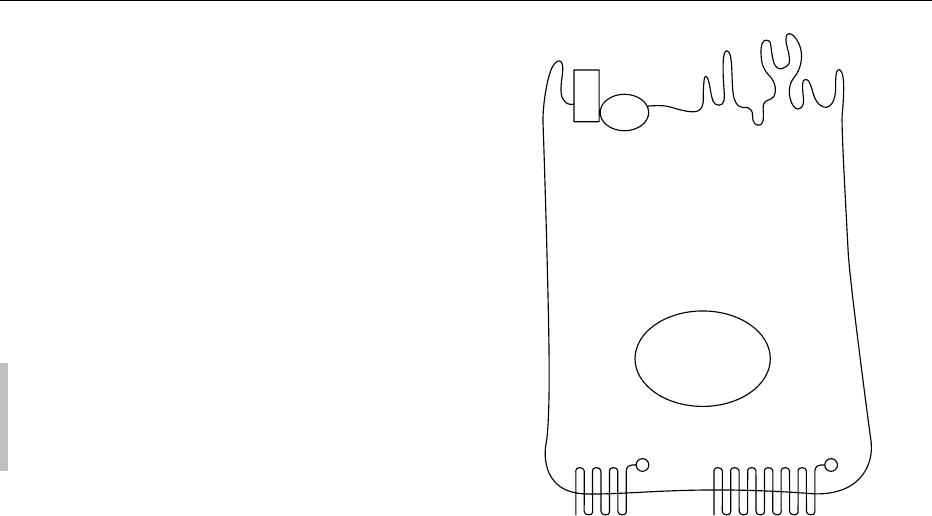
these two domains and prevent the mixing of
asymmetrically distributed proteins (Figure 1). The
apical domain displays a differentiated tissue-specific
organization characterized by the presence of apical
microvilli and pseudopods, and by the localization of
thyroperoxidase (TPO). Na
þ
/I
symporter (NIS),
epidermal growth factor, and thyroid-stimulating
hormone (TSH) receptors are located in the basal
domain. Thyroid hormone synthesis requires basal-
to-apical transport of iodide and Tg. Conversely, hor-
mone secretion is based on apical-to-basal transport
of Tg and hormones; in addition, a bidirectional ion
transport system controls follicular size.
0004The thyroid primordium of the human embryo is
first visible at 20 embryonic days, as a midline
enodermal thickening in the floor of the primitive
pharynx. It migrates caudally to form a transient
thyroglossal duct, and reaches its final position at
35 embryonic days. Tg is detectable at 60 embryonic
days, and this step corresponds to the occurrence of
fetal thyroid hormone biosynthesis. The early stages
of folliculogenesis are independent of thyrotropin. At
midgestation (18–20 weeks), the hypothalamopitui-
tary–thyroid axis begins to develop, and thyrotropin
is absolutely necessary for thyroid growth and
function.
Biosynthesis of Thyroid Hormones
0005Figure 2 summarizes the process of thyroid hormone
synthesis, which includes active concentration of
B
TSH-R NIS
TTF-1
TTF-2
Pax8
Tg
TPO
NADPH
Oxidase
fig0001Figure 1 Schematic structural representation of thyroid follicu-
lar cell with its polarized architecture. TSH-R, thyroid-stimulating
hormone receptor; NIS, Na/I symporter; Tg, thyroglobulin; TPO,
thyroperoxidase; NADPH, reduced form of nicotinamide adenine
dinucleotide phosphate; TTF-1.
3140 HORMONES/Thyroid Hormones
iodine, incorporation of iodine in thyroglobulin, and
breaking down thyroglobulin to release tetraiodo-
thyronine (T
4
) and triiodothyronine (T
3
).
0006 NIS An active transport mechanism allows thyroid
cells to concentrate iodide (I
) some 20- to 40-fold its
level in extracellular space. This transport system is
sensitive to other inhibiting anions (thiocyanate, per-
chlorate). It is found in other tissues (salivary glands,
mammary gland). In the thyroid, it is restriced to the
basolateral cell membrane. The key protein in the
transport mechanism is the human Na/I symporter
(hNIS). Its synthesis is stimulated by TSH. It is a
12-loop protein, of 65 kDa. It has homology with
the Na/glucose transporter.
0007 hTg Human Tg (hTg) is a 660-kDa protein. It con-
tains 66 tyrosils, and half of them – in maximal
conditions – are able to incorporate iodine in a cova-
lent binding, resulting in mono- or diiodotyrosine
(MIT, DIT) residues. Some iodothyrosine residues
fuse, to form T
4
with its four iodine atoms and T
3
with its three iodine atoms. In normal physiological
conditions, Tg contains five residues each of MIT and
DIT, 2.5 of T
4
and 0.7 of T
3
. These proportions
depend on iodine supply, and on Tg accumulation in
follicles. Its synthesis and release of thyroid hormones
is under thyrotropin control. Iodine by itself,
independently of thyrotropin, has an autoregulatory
function on Tg synthesis and metabolism.
0008 hTPO Human hTPO is a 103-kDa protein localized
in the apical membrane of the follicular cell. It con-
tains heme and, in the presence of hydrogen peroxide,
it iodinates tyrosil residues of hTg present in the
lumen of the follicles. The iodination process of pro-
teins is not specific to thyroid cells: macrophages
and leukocytes iodinate bacteria proteins through a
similar process, involving myeloperoxidase in place
of hTPO. However, the further step of coupling and
thyroid hormone synthesis is quite specific to the
thyroid. The same hTPO enzyme is responsible for
the coupling of DIT and MIT in T
3
and T
4
.Itis
proposed that the loops of hTg polypeptide make
some DIT and MIT residues close, and the TPO
enzyme and hydrogen peroxide interact to produce
free radicals which form a diphenyl ether across the
OH
.
free radicals.
0009hThOX Reduced nicotinamide adenine dinucleo-
tide phosphate (NADPH) oxidase is essential to pro-
duce hydrogen peroxide at the apical membrane of
the follicular cells. It has recently been identified as a
specific thyroid oxidase, with NADPH and calcium
as substrates. It has a cytochrome moiety.
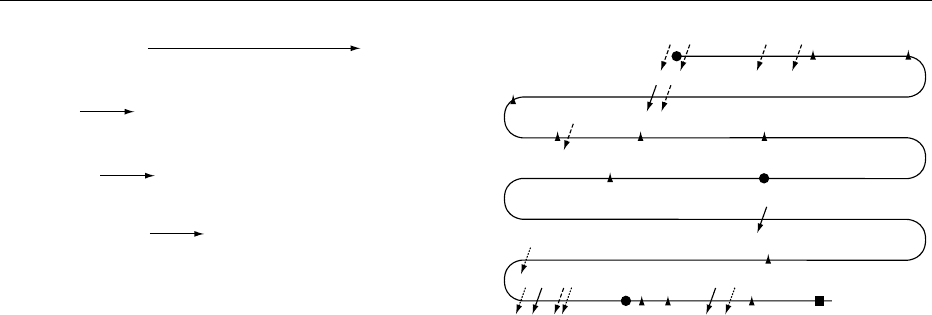
0010The sites of tyrosine iodination, as well as the sites
of iodotyrosine coupling on Tg molecule are not sto-
chastic, but are hierarchically defined. The number
of iodinated tyrosine residues depends closely on
the iodine supply and on thyrotropin stimulation.
Figure 3 depicts the general scheme of preferential
sites of iodination and coupling. The thyroid stores
its hormones as colloid in the follicular lumen, still
part of the Tg peptide structure. This process guaran-
tees a stable storage of both iodine and iodinated
thyroid hormones in variable-iodine-supply condi-
tions. A normal human adult thyroid accumulates
10–20 mg iodine, which represents a storage for at
least 3 months (required daily administration of
iodine: + 100 mg). This storage depot allows the thy-
roid to dole out thyroid hormones as the body needs
it, and to mobilize it rapidly when called for. Such
Hydrogen peroxide production
Oxidation of iodide in iodine or in iodide free radical (still debated)
Organification of specific tyrosil residues in thyroglobulin
Coupling of iodotyrosines in thyroid hormones
1. NADPH + O
2
+ Ca
2+
H
2
O
2
+ NADP
ThOX (thyroid-specific NADPH oxidase)
2. H
2
O
2
+ I
−
I
0
TPO
3. I
0
+ [Tg]−Tyr [Tg]−DIT and [Tg]−MIT
TPO
4. [Tg]−DIT + [Tg]−MIT [Tg]−T
4
+ [Tg]−T
3
TPO
fig0002 Figure 2 Oxidation of iodide and iodination of thyroglobulin
(Tg) to produce thyroxine. DIT, diiodotyrosine; NADP(H), (re-
duced) and oxidized nicotinamide adenine dinucleotide phos-
phate; TPO, thyroperoxidase; ThOX, thyroid-specific NADP(H)
oxidase; MIT, monoiodotyrosine.
COOH
C
B
A
H
2
N
D
2746
2677
26572643
2597
2567
2553
2490
2487
2468
2452
2389
1835
1290
1447
766
795
847 972
532551685
5
86 124
130 239
2164
fig0003Figure 3 General linear picture of human thyroglobulin poly-
peptide chain. Numbers refer to cDNA sequence. A, B, D, sites of
thyroxine formation; D, site of triiodothyronine formation. Cleav-
age sites of various cathepsins are symbolized by arrows.
Reproduced from Dunn JT (2001) Biosynthesis and secretion
of thyroid hormones. In: DeGroot LJ and Jameson JL (eds) Endo-
crinology WB Saunders, with permission.
HORMONES/Thyroid Hormones 3141

mobilization is accomplished by bringing stored Tg
back from the follicular lumen into the cell, passing it
through endosomal and lysosomal digestive systems,
and delivering free hormone to the basal membrane
for secretion into the circulation. About 70% of Tg’s
iodine is in the form of the inactive precursors MIT
and DIT, which the thyrocyte deiodinates and then
recycles the iodine. The most important enzymes
for Tg degradation are the lysosomal proteases. The
best characterized are the aspartic endopeptidase
cathepsin D and the cysteine endopeptidases cathe-
psin B and L. Peptides produced by the individual
enzymes purified from human thyroids were isolated
and the sites of cleavage identified by analogy to the
cDNA sequence of hTg. Figure 3 summarizes, also,
the major cleavage sites for each enzyme. Cathepsin L
(dotted broken arrows in Figure 3) attacked near
hydrophobic residues, particularly leucine, and its
major cleavage sites were within the C-terminal 400
residues of Tg. Cathepsin D (solid arrow in Figure 3)
preferentially cleaved peptide bonds between hydro-
phobic residues, particularly aromatic ones, similar to
its activity in other tissues. Cathepsin B (dashed
broken arrows) had its principal cleavage sites in
both the N-terminal and C-terminal regions. The
presence of major hormonogenic sites at Tg’s extreme
N- and C-termini may favor selective release of thy-
roid hormone during proteolysis. The endopeptidases
cleave Tg into large peptides that must then be further
degraded by exopeptidases. The several exopepti-
dases identified in the thyroid include dipeptidyl-
peptidases I and II, lysosomal dipeptidase I, and the
carbaxyl exopeptidase N-acetyl-l-pbenolalanyl-l-
tyrosine hydrolase. Also, cathepsin B has exopeptidase
activity, in addition to its role as an endopeptidase.
The combination of cathepsin B and lysosomal
dipeptidase I is sufficient to release T
4
from its most
important site at residue 5.
0011 The thyroid stimulated by TSH shows prompt and
vigorous engulfment of material from the lumen to
form colloid droplets, which are then internalized
into the thyrocyte and passed through its lysosomal-
degradative pathway. However, under more usual
physiologic conditions, colloid resorption takes place
by micropinocytosis, and endocytotic vesicles are
formed and pass successively through an endosomal
compartment and then into lysosomes. The initial
event in micropinocytosis appears to involve the
entrance of Tg into coated pits, probably without
the intervention of receptors; the amount processed
depends on the availability of endocytotic vesicles
and the amount of Tg in the lumen.
0012 Tg’s structure, specifically, the completion of its
carbohydrate side chains, is important for its traffick-
ing at the apical membrane. Immature Tg molecules
are deficient in both iodine and glycosylation, so they
have exposed N-acetylglucosamine residues. These
residues are recognized by membrane receptors that
apparently direct these immature molecules back to
the Golgi for glycosylation and subsequent iodin-
ation. These observations suggest that Tg molecules
are sorted in the endosomes, perhaps by iodine con-
tent, with immature molecules being recycled and
mature ones readied for degradation and hormone
release.
0013Some proteolytic processing may take place in
endosomes before Tg enters lysosomes. Lysosomal
enzymes are also present in endosomes, where the
acidic pH would favor their activity. Some proteolytic
processing may occur even before endocytosis. For
example, cathepsin B activity has been detected at
the cell surface of cultured thyrocytes. In the same
experimental model, activation of cysteine proteases
permitted T
4
release at extralysosomal sites, as well as
in the lysosomes, although T
3
release appeared to be
restricted to lysosomes.
0014Specific cleavages in the peptide chain of Tg are
also associated with its iodination. Such breaks
occurred in the presence of protease inhibitors, thus
suggesting that proteolytic enzymes were not in-
volved. However, other specific proteolytic cleavages
may also occur as early events in Tg degradation.
Limited digestion with trypsin has identified several
susceptible regions in the Tg molecule, most notably a
region after residue 500 and another one around
residue 1800.
0015T
4
emerges from the lysosomal-degradative path-
way, probably as the free hormone or perhaps in
small peptides. Most is secreted into the circulation.
A specific transport mechanism across the basal
membrane has not been identified. While still in
the thyroid, some T
4
is converted to T
3
by type 1
selenium-dependent 5
0
-iodothyronine deiodinase.
This enzyme is the same as that in other tissues,
such as the liver and kidney, produces T
3
, the active
form of the hormone, from circulating T
4
.
0016About 70% of Tg’s iodine is in MIT and DIT.
Although small amounts of MIT and DITare secreted
into the circulation, most is deiodinated within the
thyroid and its iodide is returned to the general pool
for recycling. This mechanism is important for iodine
conservation, as shown by the functional iodine defi-
ciency of patients who have the rare defect that blocks
this step. The iodotyrosine deiodinase responsible for
this activity is an NADPH-dependent flavoprotein (it
is not a selenium-containing enzyme, and it should
not be confound with iodothyronine deiodinase). Its
partial purification showed a molecular weight of
about 42 000, and it consisted of two possibly
identical subunits.
3142 HORMONES/Thyroid Hormones

Circulating Thyroid Hormone and Free Thyroid
Hormone Metabolism
0017 Iodothyronines produced by the thyroid are highly
hydrophobic, and need to be bound to specific
proteins for transport through the general blood cir-
culation to their target tissues. In blood, thyroid hor-
mones are not covalently bound to specific thyroid
hormone-binding proteins (thyroid hormone-binding
globulin (TBG) and transthyretin (TTR), previously
known as prealbumin). They are also transported
aspecifically by albumin. Table 1 shows the proper-
ties of the major human thyroid hormone-binding
proteins.
0018 Free fraction of thyroid hormones describes the
percentage of total hormone that is unbound. About
one molecule of T
4
in 4000 and one molecule of T
3
in 400 is free; the remaining molecules are bound to
the specific and aspecific proteins. By contrast to
corticoid-binding proteins, none of the thyroid
hormone-binding proteins has a role in the delivery
of thyroid hormones to target tissues. Only free hor-
mones are delivered to target tissues: so, clinical thy-
roid status is closely dependent on free thyroid
hormone concentration, and the general equilibrium
is as follows:
Free T
4
þ TBG ,½TBG ...T
4
0019 The normal free T
4
concentration and TBG con-
centration are such that about half of TBG sites avail-
able to thyroid hormone binding is occupied by T
4
.
This involves that within a certain range; free hor-
mone concentration is linearly associated with total
hormone concentration. So, TBG stabilizes the tissue
distribution of T
4
.
0020 Modern immunoassays measure the serum free T
4
and free T
3
concentrations, which are directly related
to clinical thyroid status.
0021 Table 2 presents the kinetic parameters of circulat-
ing total and free thyroid hormones in a 70-kg adult
human.
0022Within some tissues (mainly, liver and kidney),
circulating T
4
is deiodinated in circulating T
3
by a
selenium-dependent type 1 iodothyronine deiodi-
nase. In normal iodine supply conditions, half of
circulating T
3
derives directly from thyroid (break-
down of Tg), and half from liver conversion of T
4
to
T
3
. The same deiodinase is also able to convert T
4
to reverse T
3
(metabolically inactive). In some
physiological conditions such as the neonatal period
(cord blood) or during illness, the preferential liver
conversion of T
4
to reverse T
3
explains the paradox-
ical serum profile of low T
3
syndrome without
hypothyroidism. The exact mechanism of preferen-
tial conversion of T
4
to reverse T
3
in place of T
3
is
still unexplained.
Thyroid Hormone Action at the Cellular Level
0023Free thyroid hormones T
4
and T
3
diffuse through the
cell membrane of their target tissues. Up to now, no
specific membrane receptors have been identified,
and it is considered that this delivery to cells is
through a passive diffusion mechanism.
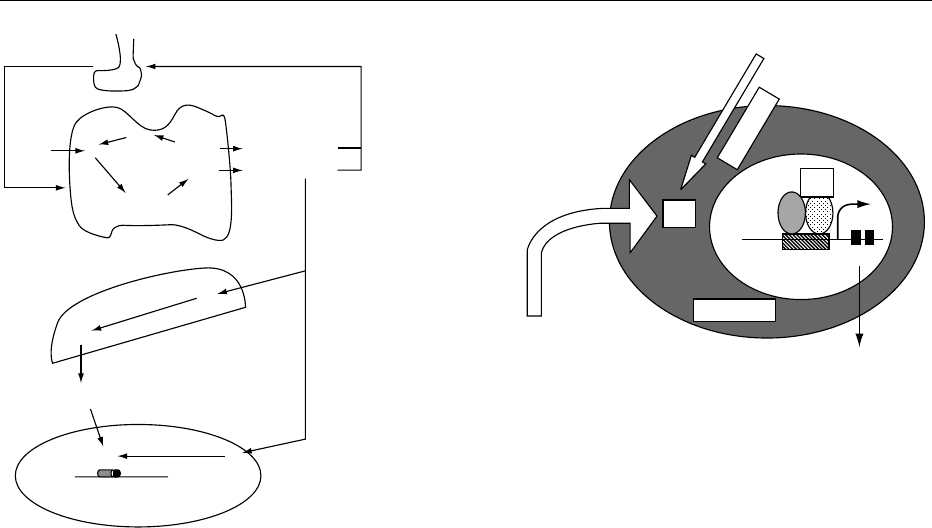
0024Within the cell, iodothyronine deiodinases act to
convert intracellular T
4
to intracellular T
3
or reverse
T
3
(Figure 4).
0025The differential intracellular conversion of T
4
to T
3
in peripheral tissues (thyroid; muscle, including the
heart; liver; kidney; adipose tissue, etc.) and in central
tissues (central nervous system, including pituitary
gland) is explained by the divergent distribution of
selenium-containing iodothyronine deiodinases. Type
1 is present mainly in peripheral tissues, while type 2
is present mainly in the central nervous system. Type
2 deiodinase has a much lower affinity constant for
T
4
than type 1 deiodinase (1 nmol l
1
versus 23 mmol
l
1
). This explains why, within the central nervous
system, in normal physiological conditions, 80% of
tbl0002Table 2 Kinetic properties of iodothyronines in humans
Property T
4
T
3
ReverseT
3
Total serum concentration
(mgdl
1
)
8.1 0.11 0.012
Free serum concentration
(ng dl
1
)
1.2 0.29 0.04
Distribution volume (l) 10 35 90
Metabolic clearance rate
(per day 70 kg s
1
)
1.2 25 111
Serum half-life (days) 7 1 0.2
Production
(mg day
1
70 kg s
1
)
100 31 39
Relative metabolic potency 0.3 1.0 0.0
Nuclear T
3
receptor
binding in vitro
10
9
10
10
No data
T
4
, tetraiodothyronine; T
3
, triiodothyronine.
tbl0001 Table 1 Properties of the major human thyroid hormone-
binding proteins
Property Thyroxine-
binding
globulin
Tr a n s t h y r e t i n A lb u m i n
Molecular weight (kDa) 54 55 66
Peptide structure Glycoprotein Peptide
tetramer
Single
chain
Concentration (mol l
1
)310
7
2 10
6
6 10
4
Half-life (days) 5 2 15
% of tetraiodothyronine
carried
75 10–15 10–15
HORMONES/Thyroid Hormones 3143
intracellular T
3
derives from local deiodination of
T
4
, while, in peripheral tissues, this percentage is less
than 50%. Moreover, in case of incipient biochem-
ical hypothyroidism, when serum T
4
is already de-
creased but serum T
3
is still normal, there is
increased type 2 deiodinase activity and increased
thyroid type 1 deiodinase activity, while there is
decreased type 1 deiodinase activity in other periph-
eral tissues (liver, mucle, heart, kidney). These mech-
anisms involve central hypothyroidism reflected by
increased serum TSH, while peripheral tissues are
still euthyroid (there is no evidence of hypothyroid-
ism in peripheral tissues). The advantage of such a
complex feedback mechanism is to stimulate max-
imally the thyroid by TSH in case of incipient bio-
chemical hypothyroidism.
0026 At the nuclear level (Figure 5), thyroid hormone
acts through a T
3
response element (TRE). Most thy-
roid hormone activity described up to now is medi-
ated via these high-affinity nuclear receptors – even if
some extranuclear actions of thyroid hormones are
also described. The principal differentiation effects of
thyroid hormones (brain development, bone matur-
ation) and the metabolic effects of thyroid hormones
(uncoupling protein, fatty acid metabolism, malic
enzyme activity) are mediated via these receptors.
The binding of thyroid hormone T
3
to its receptor
involves an induction (positively regulated genes) or a
repression (negatively regulated genes) of specific
messenger RNAs and the translation of these
mRNAs in specific proteins. Examples of positively
regulated genes include b-adrenergic receptor, fatty
acid synthetase, malic enzyme, growth hormone, un-
coupling protein, and type 1 iodothyronine deiodi-
nase (except in thyroid). Examples of negatively
regulated genes are TSH, prolactin, type 2 iodothyr-
onine deiodinase, and type 1 iodothyronine deiodi-
nase in thyroid.
0027The translated protein may also act indirectly, for
example, thyroid hormones influence growth via
insulin-like growth factor 1 (IGF-1). Nevertheless,
this action is not present in hypophysectomized rats.
So, the thyroid hormone action on liver IGF-1 is
indirect:
T
3
*!Pituitary and circulating growth hormone *
! Liver and circulating IGF-1 *
0028The TRE belongs to the family of erb-related recep-
tors (including receptors for estrogen, progesterone,
glucocorticoid, mineralocorticoid, androgen, vitamin
D, retinoic acid). The receptor binds to the DNA via
zinc-fingers. It forms a dimer which is stabilized when
bound to T
3
(homodimer) or to 9-cis-retinoic acid
(heterodimer).
0029A congenital mutation in TRE is a – rare – cause of
resistance to thyroid hormones (200 cases have been
Cytoplasm
Circulating T
3
Circulating T
4
T
3
T
3
TRE
Nucleus
Gene
Se-Diase 1
mRNA
fig0005Figure 5 Simplified model of thyroid hormone action on
nuclear thyroid hormone receptor. T
4
, tetraiiodothyronine; T
3
,
triiodothyronine; Se-Diase 1; selenium-dependent iodothyronine
deiodinase 1; TRE, T
3
response element.
TSH
Pituitary
Thyroid
Liver
Cellular level
TG
TG-T
3
Circulating T
3
Circulating T
3
Circulating T
4
T
4
T
3
TG-T
4
TG-MIT
TG-DIT
Se-Diase 1
Se-Diase 1 or 2
Nuclear T
3
receptor
+
I
−
I
0
−
T
3
T
4
fig0004 Figure 4 Some metabolic steps in iodine metabolism. Pituitary
thyrotropin (TSH) stimulates thyroid I
uptake, and enzymatic
organification of iodine (I
0
) by thyroperoxidase (TPO) in a mono-
and diiodotyrosine (MIT and DIT), which are further coupled by
the same enzyme thyroperoxidase (TPO) in tri- and tetraiodo-
thyronine (T
3
and T
4
). Part of T
4
is further deiodinated to T
3
in
the liver by selenium-containing enzymes selenium-dependent
iodothyronine deiodinases 1 and 2 (Se-Diase 1 and 2). At the
cellular level, the active hormone is T
3
, which binds to a specific
nuclear T
3
receptor. T
3
comes directly from circulating serum T
3
or from the intracellular conversion of T
4
to T
3
.
3144 HORMONES/Thyroid Hormones

described in the world) characterized by elevated
serum free T
4
and elevated serum TSH. Individuals
with this syndrome do not in general exhibit signs of
hypothyroidism, presumably because the resistant
state is compensated for by increased levels of thyroid
hormones. In some cases, the syndrome may include
attention-deficit hyperactivity disorder, reduced intel-
lectual development, delayed skeletal maturation,
tachycardia, and deafness.
Calcitonin
0030 Thyroid also synthesizes calcitonin. The parafollicu-
lar or C cells are distributed among follicular cells.
These cells derive from the neural crest (by opposi-
tion to the endodermal origin of follicular cells). In
human adults, these cells represent less than 1% of
the follicular cells. Parafollicular cells are also secret-
ing other hormones like gastrin and somatostatin.
During ontogeny, around embryonic day 24 in
humans, C cells of neural crest origin colonize a ves-
tigial organ – the ultimobranchal organ, derived from
the fourth pharyngeal pouch. This ultimobranchial
organ, with its embryonic C cells, migrates toward
the thyroid, where its cells diffuse among the folli-
cules.
0031 Calcitonin is derived from a 136 amino acid pre-
cursor peptide which, after cleavage, leaves an active
protein of 32 amino acids.
0032 Calcitonin inhibits bone resorption, peculiarly
during growth. For example, it decreases serum cal-
cium after administration of pharmacologic doses. In
adults, however, the effect of calcitonin on calcium
homeostasis is marginal. Moreover, children born
without thyroid (thyroid aplasia) and adequately
treated with substitutive T
4
hormonetherapy do not
present alterations of calcium homeostasis nor osteo-
porosis. So, if there is no doubt that calcitonin exerts
an effect at pharmacological doses, its role in normal
human physiology, even during growth, remains
largely unknown.
See also: Iodine: Properties and Determination;
Physiology
Further Reading
Caillou B, Dupuy C, Lacroix L et al. (2001) Expression of
reduced nicotinamide adenine dinucleotide phosphate
oxidase (ThoX, LNOX, Duox) genes and proteins in
human thyroid tissues. Journal of Clinical and Endo-
crinological Metabolism 86: 3351–3358.
Gut Hormones
L M Morgan, University of Surrey, Guildford, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001The gut is the body’s largest endocrine organ, both in
terms of the number of endocrine cells and number of
hormones. The very word ‘hormone’ was first used by
Bayliss and Starling in 1902 to describe the action of
the gastrointestinal (GI) hormone secretin. However,
the firm establishment of the gut’s endocrine status
was not achieved until 1970 when the primary struc-
ture of the three classical endocrine gut hormones
secretin, gastrin, and cholecystokinin (CCK) was
finally determined. Since the 1970s there has been a
rapid explosion in our knowledge of the variety and
complexity of bioactive peptides found in the gut,
made possible by new investigative technologies, es-
pecially those of molecular biology. The gut has been
shown to contain not only endocrine hormones but a
huge variety of neuropeptides, and many gut peptides
which were formerly believed to be classical hormones
have since been shown to be widespread neurotrans-
mitters. This has led to a much broader understand-
ing of the diversity of physiological roles for gut
peptides. These roles extend far beyond the absorp-
tion and digestion of food in the gut itself to the
coordination and regulation of many bodily functions
outside the gut. This article reviews the major hor-
mones of the GI tract, their characteristics, physio-
logical effects, and the regulation of their secretion by
nutrients and other stimuli. It focuses on those aspects
of gut hormone physiology that are concerned with
the digestion and metabolism of nutrients and the role
of gut hormones in the regulation of appetite and
food intake itself.
Distribution and Synthesis
0002GI hormones were first identified within gut endo-
crine cells, which are scattered throughout the
mucosal epithelium lining the gastric glands, intes-
tinal crypts, and villi. Typically these cells are pyr-
amidal and lie on the basement membrane. Some
cells extend to the lumen of the gut by means of
tufts of microvilli and some connect to one another
via cytoplasmic processes. In addition, the neuronal
cells of the gut which form the submucosal and
myenteric plexi also produce a wide variety of gut
peptides. The peptide-secreting cells share many fea-
tures, and they were probably derived from a
common precursor cell that differentiated to
HORMONES/Gut Hormones 3145

produce each endocrine or neurocrine cell line, a
hypothesis which accounts for the widespread distri-
bution of gut peptides outside the gut itself, in
neural tissue and the central nervous system. With
the exception of secretin, gastric inhibitory polypep-
tide (GIP), and peptide YY (PYY), most of the gut-
regulatory peptides are indeed also present in brain
tissue. Many of the gut peptides thus act as para-
crine or neurocrine transmitters rather than as endo-
crine hormones. This enables many functions of the
digestive tract, e.g., secretory or motor functions, to
be regulated by interaction between the endocrine
and nervous systems. In addition, gut neuropeptides
are involved in many centrally mediated functions,
such as the perception of appetite and regulation of
food intake.
0003The GI hormones are low-molecular-weight single-
chain polypeptides, of chain length generally fewer
than 50 amino acids. Most are synthesized by a pro-
hormone mechanism in a manner similar to insulin.
However, unlike insulin, GI hormone genes often ex-
press several different bioactive peptides. After the
initial translation productof thegene is formed, a series
of modifications, collectively called posttranslational
processing, can result in a number of peptide products
of varying chain lengths and biological activities.
Characterization
0004The major gut hormones of known structure are
listed in Table 1, together with the primary attributes
by which they have been characterized. Each year
tbl0001 Table 1 Distribution and biological actions of the major gastrointestinal (GI) hormones
Hormone/peptide Site of distributionin GI tract Major biological effects
Secretin family
Secretin Endocrine cells, duodenum, and jejunum Stimulation of pancreatic exocrine secretion
Gastric-inhibitory polypeptide Endocrine cells, duodenum, and jejunum Stimulation of insulin secretion
Glucagon-like peptides (GLP-1, GLP-2,
glicentin, oxyntomodulin)
Endocrine cells throughout GI tract. Highest
concentrations in terminal ileum and
colon
Stimulation of insulin secretion and satiety.
Inhibition of gastric acid secretion. Trophic
effect on gut mucosa
Vasoactive intestinal peptide Nerve fibers throughout GI tract Modulation of gut motility. Stimulation of
intestinal secretion. Regulation of blood
flow
Gastrin family
Gastrin Endocrine cells, antrum, and duodenum Stimulation of gastric acid secretion.
Trophic actions on gut mucosa
Cholecystokinin Endocrine cells, duodenum, and jejunum
Nerve fibers, pancreas, and colon
Stimulation of pancreatic secretion,
gallbladder contraction, satiety. Inhibition
of gastric emptying
Pancreatic polypeptide family
Pancreatic polypeptide Endocrine cells, pancreas Inhibition of pancreatic exocrine, gastric
acid secretion
Peptide YY Endocrine cells throughout GI tract.
Highest concentrations in terminal ileum
and colon
Inhibition of gastric acid secretion, gastric
emptying, intestinal motility
Neuropeptide Y Nerve fibers throughout GI tract. Pancreas Inhibition of intestinal and insulin secretion
Tachykinin family
Substance P Nerve fibers throughout GI tract
Smooth-muscle contraction regulation of
blood flowNeurokinin A (neuromedin L) Nerve fibers throughout GI tract
Neurokinin B (neuromedin K) Nerve fibers throughout GI tract
Bombesin-like peptides
Gastrin-releasing peptide Nerve fibers throughout GI tract
8
<
:
Smooth muscle contraction. Trophic
actions on gut mucosa. Stimulation of
gastrin
Neuromedin B Nerve fibers throughout GI tract
Somatostatin Endocrine cells, nerve fibers throughout GI
tract, especially stomach, colon, pancreas
Inhibition of many gut peptides, especially
gastrin and insulin; gastric, pancreatic,
and biliary secretion
Neurotensin Nerve and endocrine cells throughout GI
tract, especially ileum and jejunum
Inhibition of gastric motility and secretion
Stimulation of pancreatic exocrine secretion
Motilin Endocrine cells throughout small intestine,
especially duodenum, jejunum
Regulation of interdigestive gut motility.
Inhibition of gastric motor activity
Galanin Nerve fibers throughout GI tract, pancreas Inhibition of gastric emptying, gut motility,
insulin, and other gut peptide secretion
3146 HORMONES/Gut Hormones

several new bioactive peptides are added to the grow-
ing list of GI hormones, and currently the number of
regulatory peptides isolated from the GI tract is well
in excess of 50. Many of the GI hormones show a
large degree of structural homology, and can be
divided up into distinct families on the basis of simi-
larities in their amino acid sequence. There is often
considerable overlap in the biological activities of
hormones in the same group, for example most
members of the secretion family have the ability to
potentiate insulin secretion. Gut peptides which do
not fit into family groups are termed ‘orphan’ pep-
tides. The ability of GI hormone genes to express
multiple phenotypes has led to great molecular het-
erogeneity and complexity of their hormone prod-
ucts. For example, secretin was originally believed
only to exist as a 27-amino-acid peptide. However,
by 1990, three additional secretins with full bioactiv-
ity had been identified, two of similar size to secretin-
27 and formed by variable trimming of secretin-27’s
carboxyl terminal, and one large bioactive molecule,
secretin-71, produced by trimming out the mid-
sequence of the prohormone, preprosecretin. Pro-
hormones can also contain more than one active
sequence. Within the secretin family, a group of closely
related glucagon-like peptides is derived from a single
preproglucagon gene. Posttranslational processing is
cell-specific; thus in the pancreas glucagon is the hor-
monal gene product, whilst in the gut, glicentin and
oxyntomodulin (extended versions of glucagon) are
produced, together with two additional bioactive
peptides, glucagon-like peptides 1 and 2.
0005 Some of the neurally distributed gut peptides were
first isolated from brain tissue. Somatostatin was
originally isolated and characterized from the hypo-
thalamus before its discovery in gastric mucosal cells
and the pancreas. The enkephalins, members of the
tachykinin family, were first isolated from brain
tissue, where they are often detectable in higher
concentrations than in tissues within the GI tract.
0006 The gut hormones occurred very early on in evolu-
tionary terms and various members of the gut hor-
mone families are extensively distributed throughout
the animal kingdom. Some of the mammalian gut
hormones have amphibian counterparts. The bombe-
sin-like peptides, of which gastrin-releasing peptide
(GRP) is the chief mammalian example, were origin-
ally isolated in amphibian skin. In addition, neuro-
tensin, CCK and the tachykinin, substance P, have
their amphibian skin counterparts – xenopsin, caeru-
lein, and physalaemin respectively. Substance P and
CCK-like peptides have been found in invertebrate
neurons and a CCK-like peptide has even been isol-
ated in hydra, one of the simplest present-day multi-
cellular organisms.
Physiological Effects
0007Gut peptides can be separated into three functional
categories: endocrine, paracrine, and neurocrine,
reflecting their cellular location and mode of delivery
to target cells (Table 2). Endocrine peptides reach
their targets via the general circulation, paracrine by
diffusion or via the local circulation, and neural pep-
tides by synaptic transmission.
0008Essential first steps in identifying the physiological
function of a gut peptide are to determine its cellular
location, pharmacological properties, and the regula-
tion of its secretion. This is hindered by two features of
the neurendocrine system of the gut. The first
is the wide dispersion and comingling of peptide-
containing cells which make it difficult to excise
one endocrine or neural system without interfering
with another. The second feature, characteristic of
neurons, is the existence of different peptides within
the cell along with nonpeptide neurotransmitters,
leading to the possibilities of very complex inter-
actions. Nevertheless, the physiological roles of gut
hormones in several major functions of the GI tract
and the interaction between endocrine and nervous
systems have been elucidated and are described below.
Control of Gastric, Pancreatic, and Biliary
Secretion
0009There are three major endogenous stimulators of gas-
tric acid secretion: gastrin, acetylcholine, and hista-
mine; it is probable that all three are interdependent
under physiological conditions in humans. The major
action of gastrin is to release acid from parietal cells
in the fundus and body of the stomach, although
it also exerts indirect effects on acid secretion via
the stimulation of histamine release from ECL cells.
Regulation of gastrin secretion is under the control
of both humoral and local factors and also the
tbl0002Table 2 Functional categories of gut hormones
Hormone Endocrine Paracrine Neurocrine
Secretin þ
Gastric-inhibitory peptide þ
Glucagon-like peptides þ
Vascular-inhibitory peptide þ
Gastrin þ
Cholecystokinin þþ
Pancreatic polypeptide family þþ
Tachykinin family þ
Bombesin-like peptides þ
Somatostatin þþþ
Neurotensin þ
Motilin þþ
Galanin þ
HORMONES/Gut Hormones 3147

autonomic nervous system, illustrating the complex
interaction between endocrine, neurocrine, and para-
crine systems. Gastrin release is stimulated directly by
the ingestion of food, especially protein, and by the
neuropeptide GRP, which is itself released by choli-
nergic stimulation. Gastrin secretion is inhibited by
acid, and by somatostatin, acting in a paracrine
manner. The ingestion of fat, once the fat has passed
though the stomach and into the small intestine, in-
hibits gastric acid secretion via the stimulation of GI
hormones GIP, glucagon-like polypeptide-1 (GLP-1),
and CCK. It seems likely that their modes of action
vary and include both direct inhibitory effects on
parietal cells, as in the case of CCK, and indirect
effects on acid secretion via gastrin and somatostatin.
0010 Pancreatic exocrine secretion contains two major
components. The first is an aqueous solution contain-
ing a high concentration of bicarbonate whose
function is to neutralize acid entering the duodenum
from the stomach. The second is a solution of
enzymes which aid the absorption and digestion of
carbohydrate, fat, and protein. Secretin and cholecys-
tokinin are traditionally thought to be responsible for
pancreatic exocrine secretion, but other gut peptides,
notably vasoactive intestinal peptide (VIP), neuroten-
sin, and pancreatic polypeptide, may also influence
secretion. Secretin is responsible for most of the water
and bicarbonate secretion. CCK acts synergistically
with secretin to augment pancreatic bicarbonate
output, and interactions between neurotensin and
secretin also appear to be important. During diges-
tion, pancreatic exocrine secretion is partly controlled
by efferent vagal fibers, mediated by VIP. Pancreatic
polypeptide inhibits pancreatic secretion and may
be involved with the damping-down of pancreatic
exocrine secretion following a meal.
0011 CCK has been considered a major hormone con-
trolling gallbladder emptying, and release of pancre-
atic enzymes. However, the lack of reliable assays
to measure circulating levels of CCK has caused dif-
ficulty in ascribing a physiological, as opposed to
pharmacological, role for the hormone. Recent stud-
ies using CCK-A (the predominant CCK receptor
found in the pancreas and gallbladder) receptor
antagonists have confirmed the role of CCK as
the primary stimulator of gallbladder contraction,
although the effects of CCK receptor antagonists on
pancreatic secretion are less clear, probably reflecting
multiple pathways of control for pancreatic exocrine
secretion.
Gastric Emptying and Gut Motility
0012 Between the striated muscles of the esophagus and the
external anal sphincter, which are dependent upon
efferent nerves for proper functioning, lie the smooth
muscles which form the bulk of the GI tract. They are
capable of executing their function without extrinsic
innervation, and sympathetic and parasympathetic
nerves act only in a modulatory capacity. The intrin-
sic nerves of the gut form two major networks or
plexi: the myenteric plexus which lies between the
longditudinal and circular muscles of the gut, and the
submucous plexus, which lies between the mucosa
and circular muscle layer. Control of gut motility
involves many neuronally located gut peptides. Re-
ceptors for many peptides have been located on gut
smooth muscle cells, including motilin, the opioid
peptides, the tachykinins, VIP, and neuropeptide Y.
0013Regulation of the rate of gastric emptying depends
on many factors, including the physical state and size
of the meal and its macronutrient content. Three
endocrine gut hormones have been implicated in the
control of gastric emptying. Secretion of CCK is
responsible for the delay in gastric emptying when
fat is added to a meal; the presence of fat in the
duodenum is a powerful stimulus for CCK release.
GLP-1, secreted in response to the presence of all
three macronutrients in the upper small intestine,
also delays gastric emptying and regulates the influx
of nutrients into the upper part of the gut. Peptide YY,
which is colocalized and often cosecreted with GLP-
1, inhibits gastric emptying and also small intestinal
motility. It has been termed the ‘ileal brake’ and
its actions following a meal are thought to enable
sufficient time to occur for the meal’s absorption.
Following the ingestion of food, waves of alternate
contraction and relaxation descend from the gut, fa-
cilitating the passage of food and aiding its absorp-
tion. VIP causes smooth muscle relaxation and its
distribution is consistent with the role of a transmitter
in the descending inhibitory nerves. Opioid peptides
increase the contractile activity of the gut and their
net effect is to slow the transit of food. The tachyki-
nins also have potent exitatory and spasmogenic
effects on smooth muscle and appear to be involved
in regulation of the peristaltic reflex.
0014When fasting is prolonged, interdigestive myoelec-
tric complexes occur. These are waves of contraction
beginning in the stomach which move slowly down
the small intestine, whose function is to clear out the
intestine between feeds and keep the intraluminal
bacterial population low. Motilin is primarily in-
volved in the regulation of this interdigestive activity.
Potentiation of Insulin Secretion and other
Metabolic Effects
0015One of the more important metabolic actions of gut
hormones is their ability to modulate insulin secre-
tion. Orally administered glucose leads to far higher
circulating insulin levels than the same amount of
3148 HORMONES/Gut Hormones
