Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0005 Oxytocin and vasopressin are synthesized as pre-
cursors in the cell bodies of (different) neurons in the
hypothalamus. They are packaged into granules,
which pass down the axons of these neurons, through
the pituitary stalk, to the swollen axonal endings in
the neurohypophysis, where they are stored. Conver-
sion of precursors to active peptides occurs during
formation and transport of secretory vesicles, cata-
lyzed by prohormone-converting enzymes included
within the vesicles. Also formed by processing of
these precursors are neurophysins, which act as bind-
ing proteins for oxytocin and vasopressin prior to
release. Secretion of the hormones from the nerve
endings occurs by exocytosis in response to nerve
signals coming down the axons of the same cells.
For oxytocin a neural loop links sensory receptors in
the mammary gland (detecting suckling) via a number
of synapses, to the cell bodies of the neurons that
synthesize the hormone. This in turn triggers firing
of these neurons, so that an electrical signal passes
down their axons and causes depolarization of the
plasma membrane of the swollen nerve endings in
the neurohypophysis. The entry of Ca
2þ
which
follows this provides the signal for exocytosis. The
released oxytocin passes via the blood stream to the
mammary gland, where it induces milk ejection
within a few seconds of the initial suckling stimulus.
Similar neural loops regulate secretion of vasopressin,
initiated at receptors in the brain that detect changes
in osmotic pressure, or in the atrium and carotid sinus
that detect changes in blood volume or pressure.
Adenohypophysial Hormones
0006The hormones of the adenohypophysis are all peptide
or protein in nature, and fall into three main families:
(1) corticotropin (ACTH), melanocyte-stimulating
hormone (MSH) and related peptides (the melanocor-
tin family); (2) growth hormone (GH) and prolactin;
(3) thyrotropin or thyroid-stimulating hormone
(TSH), follicle-stimulating hormone (FSH) and lu-
teinizing hormone (LH) – the glycoprotein hormone
family.
The Melanocortin Family
0007ACTH is a peptide of 39 residues concerned mainly
with regulation of the adrenal cortex, including pro-
duction and secretion of corticosteroids. It binds to a
G-protein-linked receptor in the membrane of target
cells in the adrenal cortex, and acts at various points
on the pathway leading to steroid synthesis partly via
action of cyclic AMP as a second messenger. MSHs
tbl0001 Table 1 The hormones of the mammalian pituitary gland
Hormone Abbreviation Chemical nature Main actions
Posterior lobe
Oxytocin Peptide (9 residues) Stimulates milk ejection, uterus contraction
Vasopressin (antidiuretic hormone) VP (ADH) Peptide (9 residues) Antidiuretic, raises blood pressure
Intermediate lobe
a-Melanotropin (a-melanocyte-stimulating
hormone)
a-MSH Peptide (13 residues) Causes skin darkening, especially in
amphibia; functions as neuropeptide in the
brain
Anterior lobe
b-Melanotropin (b-melanocyte-stimulating
hormone)
b-MSH Peptide (18–22 residues) Causes skin darkening, especially in
amphibia
Corticotropin (adrenocorticotropic
hormone)
ACTH Peptide (39 residues) Stimulates corticosteroidogenesis and growth
of adrenal cortex
Lipotropin LPH Peptide/protein
(*91 residues)
Weakly lipotropic (may be primarily a
precursor of b-MSH and b-endorphin)
b-Endorphin Peptide (31 residues) Analgesia?
Growth hormone (somatotropin) GH Protein (*190 residues) Promotes growth, many anabolic actions,
stimulates insulin-like growth factor
1 production
Prolactin PRL Protein (*200 residues) Lactogenic activity (many other actions,
particularly in lower vertebrates)
Thyrotropin (thyroid-stimulating hormone) TSH Glycoprotein (2 subunits) Stimulates thyroid hormone production
Follicle-stimulating hormone FSH Glycoprotein (2 subunits) Stimulates maturation of the Graafian follicle
in female, spermatogenesis in male
Luteinizing hormone (interstitial
cell-stimulating hormone)
LH Glycoprotein (2 subunits) Stimulates maturation and release of ovum
and early development of corpus luteum in
female, steroid production by gonads in
male
Note that some doubt remains as to whether b-MSH, LPH, and b-endorphin are physiologically significant hormones.
HORMONES/Pituitary Hormones 3159

are peptides of 13–22 amino acids, produced charac-
teristically in the intermediate lobe of the pituitary,
which regulate skin color in some species by stimulat-
ing synthesis and altering distribution of pigment in
cells called melanocytes. The actions of MSHs are
particularly marked in amphibia. Although they
occur in the pituitary gland of many mammals their
physiological role there is not clear, although in at
least some species they probably do have a role in
controlling skin pigmentation. MSHs have been de-
scribed in the human pituitary, but there is some
evidence that in the adult at least they are artifacts
of extraction procedures and of little physiological
importance. ACTH and MSHs show similarity in
amino acid sequences, and it is now known that
they are formed from a common biosynthetic precur-
sor, proopiomelanocortin (POMC), together with a
larger peptide b-lipotropin and the opioid peptide
b-endorphin. Despite this, ACTH and a-MSH are
secreted from different cell types (corticotrophs and
melanotrophs), reflecting different processing path-
ways in different cells.
0008 Although MSHs and the other POMC-derived pep-
tides were originally characterized in the pituitary, it
is now clear that they are also expressed in the brain,
and that as neuropeptides they may play an important
role in energy homeostasis and the regulation of
obesity. Recent studies indicate that the peptide hor-
mone leptin, produced by adipose cells, acts at least
partly by stimulating production of a-MSH in hypo-
thalamic neurons. Binding of a-MSH to specific re-
ceptors (MC4 receptors) in hypothalamus and cortex
leads to reduced feed intake and increased energy
expenditure. Animals with defective MC4 receptors
become obese, but the importance of this mechanism
for human obesity has not yet been established.
The Growth Hormone/Prolactin Family
0009 GH and prolactin are both medium-sized proteins
comprising a single polypeptide chain of 190–200
amino acids. They are structurally similar, and are
related to the placental lactogens found in humans,
ruminants, and rodents. GH plays a major role in
regulating somatic growth and is discussed further
below. Prolactin has a number of different actions in
mammals, the most important being concerned with
regulation of lactation and mammary growth. Prolac-
tin acts with other hormones, including insulin, insu-
lin-like growth factors, corticosteroids, thyroid
hormone, and GH (depending on the species) to
stimulate growth of the mammary gland and synthe-
sis and secretion of milk components including pro-
teins (caseins, a-lactalbumin, etc.), sugar, and fat. It
acts partly at the gene level to stimulate production of
specific proteins via a membrane-bound receptor,
Jak kinase and STAT proteins, a mechanism similar
to that of GH (see below). In ruminants milk produc-
tion appears to be stimulated more effectively by GH
than prolactin, and the use of GH to enhance agricul-
tural milk production is being actively pursued.
Whether GH and/or prolactin can influence the nu-
tritional content of milk, as well as total production,
is not clear.
The Glycoprotein Hormone Family
0010The third family of pituitary hormones includes the
glycoprotein hormones: the two gonadotropins, FSH
and LH, and TSH. These are structurally related to
the chorionic gonadotropin (hCG) produced in the
human placenta. They are complex proteins, includ-
ing in each case two subunits (a and b), each of which
is about 100 amino acids long and carries one or more
carbohydrate moieties. The a-subunit is common to
FSH, LH, and TSH (and also hCG), while the b-
subunits are different, and provide the hormonal spe-
cificities. A three-dimensional (3D) structure has been
reported for hCG which shows a number of interest-
ing features, including a ‘disulfide knot’ in each sub-
unit and a very close association between a- and
b-subunits, ‘sealed’ by a peptide strand and disulfide
bridge which act as a kind of ‘seat belt.’ In the female,
FSH and LH control the estrus cycle (menstrual cycle
in primates): FSH stimulates growth of the Graafian
follicle and production of estrogen by granulosa cells,
while LH stimulates maturation of the ovum, ovula-
tion, and development of the corpus luteum. In the
male, LH stimulates the steroid-producing interstitial
cells to produce androgens, while FSH stimulates
spermatogenesis. The actions of both LH and FSH
involve binding to G-protein-linked receptors and are
mediated in part by cyclic AMP. The actions of TSH
are concerned almost exclusively with regulation of
the thyroid gland, including stimulation of growth
of the gland and production of thyroxine and
triiodothyronine.
Regulation of Secretion of Adenohypophysial
Hormones
0011The secretion and synthesis of the hormones of the
pituitary gland are largely under the control of
the hypothalamus. For the neurohypophysis, as dis-
cussed above, such regulation involves nerve signals
operating via the axons of the hypothalamic neurons
within which vasopressin and oxytocin are synthe-
sized and transported to the neurohypophysis.
0012Hypothalamic regulation of synthesis and secre-
tion of adenohypophysial hormones in mammals in-
volves primarily blood-borne neuroendocrine factors
rather than nerve signals. A capillary plexus in the
3160 HORMONES/Pituitary Hormones

hypothalamus allows the blood to pick up neuroen-
docrine secretions; the capillaries then combine to
form the hypothalamic–hypophysial portal vessels
which pass down the pituitary stalk and form a
second capillary network in the adenohypophysis.
This system allows efficient transfer of neuroendo-
crine factors from the hypothalamus to the adenohy-
pophysis (Figure 1). These factors are mostly
peptides, though the catecholamine dopamine is also
an important regulator. Most stimulate secretion of
one or more adenohypophysial hormones, but some
are inhibitors. They are listed in Table 2.
0013 ACTH secretion is stimulated by the hypothalamic
peptide corticotropin-releasing hormone (CRH),
which synergizes with vasopressin and possibly
other factors. a-MSH secretion from the intermediate
lobe may be partly under neural control, but is also
regulated by hypothalamic peptides and inhibited by
dopamine. Secretion of TSH is stimulated by the tri-
peptide TSH-releasing hormone (TRH). Secretion of
FSH and LH is stimulated by the decapeptide LH-
releasing hormone (LHRH: gonadotropin-releasing
hormone (GnRH)); FSH and LH are synthesized in
and secreted from the same cell type (gonadotroph).
Prolactin secretion is largely under inhibitory control,
by hypothalamic dopamine and possibly other
factors. TRH and a number of other peptides can
stimulate prolactin secretion, but their physiological
significance is not clear. GH secretion is stimulated
by the 44-residue peptide GH-releasing hormone
(GHRH), and inhibited by the 14-residue peptide
somatostatin. Both appear to be physiologically im-
portant, though stimulatory control predominates.
Recently another peptide (ghrelin) that stimulates
GH secretion has been characterized (see below).
0014 Many other factors can influence anterior pituitary
secretion, by modulating the actions of the hypothal-
amic regulators and/or in some cases by direct action
at the pituitary. Thus, the secretion of FSH and LH
may be modified by hormones produced by the
gonads, including gonadal steroids and peptide hor-
mones such as inhibin. Such modulation probably
underlies differential release of LH and FSH from a
single cell type. Hormones produced from other
endocrine organs can also affect secretion of the pitu-
itary hormones regulating those organs. Thus thyroid
hormones modulate TSH secretion, and corticoster-
oids affect ACTH secretion. Secretion of GH is modu-
lated by insulin-like growth factors (IGFs).
0015At the physiological level, factors controlling the
release of hypothalamic peptides are obviously im-
portant in the overall control of pituitary hormone
secretion. These factors include neural signals (in-
cluding sensory inputs and signals deriving from en-
dogenous rhythms) and hormonal/metabolic factors,
such as glucose or amino acid levels, steroid hormone
levels, etc. The hypothalamus provides a mechanism
by which the neural and hormonal regulatory systems
can be integrated, providing a combined input to the
pituitary gland which in turn controls many of the
endocrine glands of the body.
Growth Hormone
0016Each of the pituitary hormones has direct or indirect
effects on nutrition. Thus, ACTH and TSH stimulate
production of hormones involved in metabolic regu-
lation. Prolactin, by stimulating lactation, affects
both the nutrition of the offspring and the nutritional
balance of the mother in whom much of the meta-
bolic effort may be diverted towards milk production.
However, the pituitary hormone that is most directly
involved in regulating the nutritional balance of the
organism is GH.
Structure
0017GH is a protein hormone of about 22 kDa molecular
weight. Amino acid sequences of GHs from many
tbl0002 Table 2 The hypothalamic hormones that regulate the adenohypophysis
Hormone Abbreviation Chemical nature Main actions
TSH-releasing hormone TRH Peptide (3 residues) Stimulates release of TSH and possibly prolactin
LH-releasing hormone LHRH Peptide (10 residues) Stimulates release of LH and FSH
Somatostatin
(GH release-inhibiting hormone)
SRIF Peptide (14 residues) Inhibits release of GH (and many other secreted peptides)
Dopamine DA Catecholamine Inhibits release of prolactin and MSH
GH-releasing hormone GHRH Peptide (44 residues) Stimulates release of GH
Corticotropin-releasing hormone CRH Peptide (41 residues) Stimulates release of corticotropin
TSH, Thyroid-stimulating hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; GH, growth hormone; MSH, melanocyte-stimulating
hormone.
Note that these are the hypothalamic factors that have now been well characterized. Other factors have been proposed, but either they have not been fully
characterized or their physiological significance is in doubt. These include oxytocin-related peptides that may be involved in regulating MSH secretion, a
factor that may specifically stimulate FSH secretion, an inhibitor of adenocorticotropic hormone secretion, and factors involved in stimulation of prolactin
release.
HORMONES/Pituitary Hormones 3161

species are available, and the three-dimensional struc-
tures of pig and human GHs have been determined,
using X-ray diffraction. The structure comprises a
four-helix bundle with an unusual (‘up-up-down-
down’) topology. A similar topology has now been
seen in a variety of cytokines and growth factors,
indicating that GH is a member of a large protein
superfamily (the cytokine family). The receptors for
members of this family are also related. There is
considerable sequence variation between GHs from
different mammals. Most notably, human GH is very
different from GHs of nonprimate mammals. Vari-
ation in biological properties is also seen – in particu-
lar, nonprimate GHs are not active in humans, and
human GH, unlike most nonprimate GHs, possesses
lactogenic (prolactin-like) activity. The species speci-
ficity has meant that it has been necessary for thera-
peutic purposes to prepare human GH on a large
scale in order to treat human hypopituitary dwarfism
(see below).
Biological Actions
0018 GH stimulates somatic growth in most vertebrates.
Removal of the pituitary gland leads to marked
slowing of growth in young animals, and the effect
can be reversed by GH treatment. Humans with defi-
cient GH production suffer from retarded growth,
leading to dwarfism, which can often be treated by
exogenous GH. On the other hand, excess GH, due
usually to an adenohypophysial tumor, leads to giant-
ism (if the tumor develops early in life, before the
epiphyses of the long bones fuse) or acromegaly (if
the tumor develops later, when only certain bones,
characteristically those of the face, hands and feet,
can still grow). GH is not the only factor affecting
growth, of course. All animals have a genetic program
that determines their normal adult size range, pre-
sumably optimized by evolutionary pressure to fit a
particular niche. The role of GH is presumably to
regulate growth of the organism within this optimum
range, relative to environmental factors, particularly
nutrition. Thus, if food is in short supply, normal
growth is slowed, and if the short supply is followed
by surplus, growth may be increased to above normal
levels (catch-up growth). The final size that an adult
mammal achieves will be a compromise between the
optimal size that the genetic program determines, and
what can be achieved given the environmental cir-
cumstances, particularly with regard to nutrition.
For an animal to grow in an unregulated fashion
towards a predetermined size (defined mainly in
terms of bone length) could lead to decreased fitness,
due to decreased muscle mass or bone strength. The
link between the effects of nutrition and GH on
growth is emphasized by the observation that
responses to GH in experimental animals may be
influenced markedly by the plane of nutrition.
0019The biological actions of GH are complex, and not
well understood. The overall effect of the hormone is
to promote growth, and it has direct or indirect
effects on many aspects of protein, carbohydrate,
and lipid metabolism, as well as effects on division
and differentiation of some cell types. Whether all of
these effects should be seen as directly related to
stimulation of growth, or whether some of the actions
of the hormone should be considered quite separately
from the growth-promoting effects, is not clear. Some
of the actions of GH are mediated by somatomedins/
IGFs (Figure 2). These are single-chain polypeptides
that show structural similarity to insulin. Two main
IGFs have been characterized – IGF-1 and IGF-2.
IGF-1 is most markedly GH-dependent. IGFs occur
in the circulation largely in association with specific
binding proteins, of which there are at least six types.
These binding proteins extend the half-life and modu-
late the activity of IGFs; at least one of them is itself
GH-dependent.
0020Circulating levels of IGF-1 are low in hypophysec-
tomized rats or GH-deficient animals or people, and
are increased following administration of GH. Unlike
GH, IGF-1 stimulates cartilage growth directly,
which is a prerequisite for growth of bone. For some
time it was considered that circulating IGF-1 (pro-
duced mainly in the liver) was the main mediator of
the actions of GH on cartilage and bone growth, but
it is now clear that GH stimulates IGF-1 production
in many tissues, including cartilage, and that the
growth factor probably acts locally to stimulate tissue
growth, possible synergizing with GH itself.
0021In addition to its actions on cartilage and bone
growth, mediated largely by IGFs, GH also has ana-
bolic effects on various other tissues. For example it
stimulates protein synthesis in muscle and liver, in-
cluding both a general enhancement of the protein
synthetic machinery, and induction of gene expres-
sion for specific proteins, including IGFs and their
binding proteins. GH also has effects on carbohydrate
and lipid metabolism. The effects on carbohydrate
metabolism include short-term insulin-like effects
and longer-term antiinsulin (diabetogenic) effects.
Actions on lipid metabolism include stimulation of
lipid mobilization and uptake, and decrease in lipid
synthesis. Overall the metabolic effect of the hormone
is to promote lipid utilization and decrease carbohy-
drate utilization. The extent to which these metabolic
actions are mediated by IGFs is not clear.
Mechanism of Action of Growth Hormone
0022Understanding of the mechanism by which GH acts
has increased substantially over the past decade. As
3162 HORMONES/Pituitary Hormones
with other polypeptide hormones the interaction
with the target cell is via a membrane-bound receptor.
When the GH receptor was first cloned and charac-
terized in 1987 it proved to be of a type not previously
observed. Since then, receptors of a similar type have
been identified for a number of other polypeptide
signaling molecules (members of the cytokine recep-
tor family), including prolactin, erythropoietin,
and various growth factors and lymphokines. All of
these receptors comprise a single membrane-spanning
domain, with substantial extracellular (ligand-
binding) and intracellular domains. For GH there is
evidence that the hormone binds to two receptor
molecules, effectively dimerizing or cross-linking the
receptor. The most striking demonstration of this
has come from X-ray crystallographic studies,
which revealed the detailed 3D structure of GH
bound to two molecules of the extracellular domain
of the receptor. Cross-linking of two extracellular
domains brings together the corresponding intr-
acellular domains, and it is thought that interaction
between these allows the signal to be transmitted
to the interior of the target cell. Thus far there is
evidence for only one GH receptor gene in any
one mammalian species, but there is generation of
receptor heterogeneity by allelic variation, alternative
splicing during pre-mRNA processing and/or
posttranslational modification. The significance of
such receptor heterogeneity is not yet clear, but a
notable feature of it is the presence of a soluble
form of the extracellular domain in the normal circu-
lation – this acts as a GH-binding protein and may
modulate the activity of the hormone and/or prolong
its half-life.
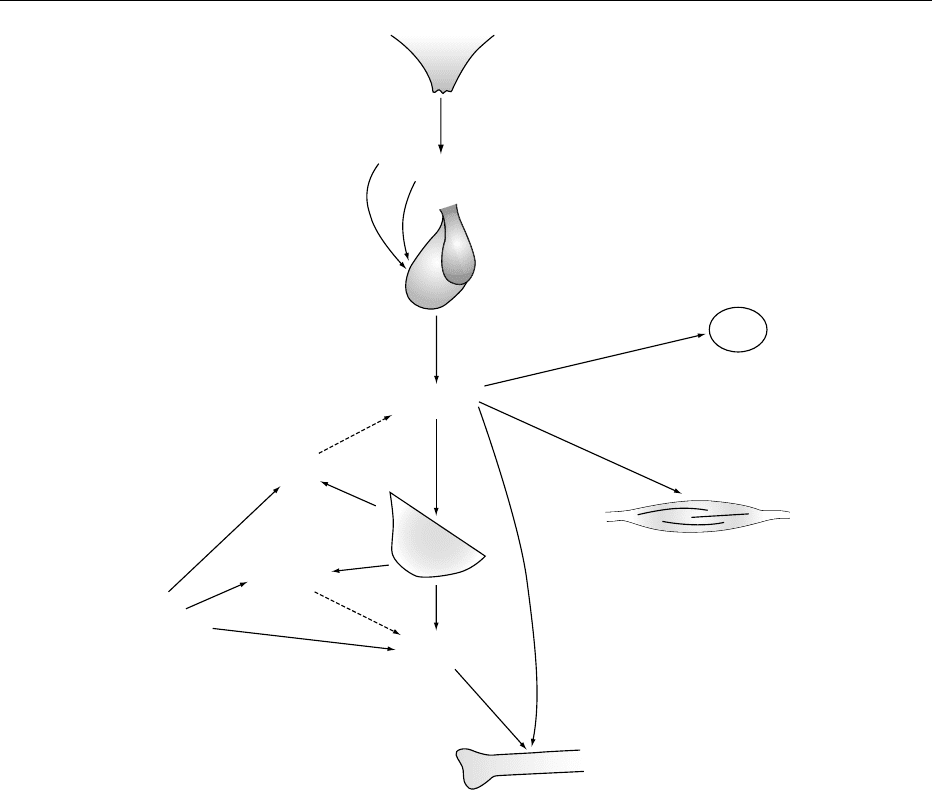
Hypothalamus
Somatostatin
GHRH
Pituitary
Adipose
tissue
Muscle
Skeleton
IGF-1
Other
tissues
GH-BP
Growth
hormone
−+
Liver
IGF-BP
fig0002 Figure 2 The hypothalamic–pituitary–somatic axis. Circulating insulin-like growth factor 1 (IGF-1) derives both from the liver and
other tissues; at least some of the actions of IGF-1 involve local rather than humoral effects. The half-life and actions of growth
hormone (GH) and IGH-1 are modulated by corresponding binding proteins (BP). GHRH, growth hormone-releasing hormone.
Modified from Wallis M (1988) The molecular basis of growth hormone deficiency. MolecularAspects of Medicine 10: 429–509.
HORMONES/Pituitary Hormones 3163

0023 The GH receptor is neither a G-protein-linked re-
ceptor, nor does it include a protein kinase. However,
a major aspect of its mechanism of action is that on
activation, following hormone binding and dimeriza-
tion, it binds and activates a soluble cytosolic protein
kinase Jak2. This leads to phosphorylation of the
receptor itself and of a number of intracellular ef-
fector proteins, including members of the Stat (signal
transduction and activation of transcription) protein
family, particularly Stat5. Phosphorylated Stat5
moves to the nucleus where it acts as a transcription
factor, stimulating the transcription of key GH-de-
pendent genes, including the gene for IGF-1. Acti-
vation of Jak2 also leads to stimulation of various
other intracellular signal transduction mechanisms,
including the MAP kinase pathway.
Regulation of GH Secretion and Synthesis
0024 The secretion of GH is regulated by stimulatory and
inhibitory peptides produced in the hypothalamus,
including GHRH and somatostatin. Stimulatory con-
trol generally predominates. Recently another pep-
tide that stimulates GH release, ghrelin, has been
identified in the stomach. Its physiological role is
not yet clear, but it also stimulates appetite and may
provide a link between GH secretion and food intake.
The actions of these peptides are modulated by
factors acting directly at the pituitary level, including
IGFs, thyroid hormones, and glucocorticoids. Secre-
tion of the hypothalamic peptides is in turn regulated
by neural and metabolic factors. GHRH, and hence
GH secretion, is stimulated by hypoglycemia, high
levels of amino acids such as arginine or leucine
(which may result from a high protein meal), and
lowered free fatty acid levels. GH levels are lowered
in obese subjects, and recent studies suggest that the
‘fat hormone’ leptin may play some role in modulat-
ing GH secretion. GH secretion is normally pulsatile
in animals and humans, which complicates the deter-
mination of ‘normal’ levels.
Growth Hormone Deficiency
0025 In humans GH deficiency gives rise to stunted
growth/dwarfism and can have a number of causes,
some of which are hereditary. Hereditary GH defi-
ciency can be due to: (1) deletion of the gene that
codes for GH; (2) mutations that prevent normal
development of GH-producing cells (somatotrophs)
and GH synthesis; (3) defective GH receptors (so that
although normal levels of the hormone are produced
they fail to produce normal responses in the target
cells); and (4) other reasons, not fully understood.
Most cases of GH deficiency in humans are not her-
editary in origin, however; they often appear to be
due to damage to the link between hypothalamus and
pituitary, so that the normal control of GH secretion
by GHRH is disrupted. Such disruption can be due to
birth trauma, blows to the head in infancy, or other
causes. In such cases the pituitary can often respond
to GHRH administered exogenously, and this is being
explored as a therapeutic approach to treat human
hypopituitary dwarfism.
0026Children suffering from stunted growth due to
clearly established GH-deficiency can be treated
using human GH, and this has become a standard
therapy. Provided GH treatment is started sufficiently
early, growth can be restored to near normal, and
heights within the normal range can be achieved.
For about 20 years the GH used for such therapy
was extracted from human pituitary glands obtained
postmortem, and in many countries extensive
schemes were established for collection of such
material. In the mid-1980s, however, concern arose
about the contamination of some human GH prepar-
ations with infective agents, particularly the
prions causing Creutzfeldt–Jakob disease (CJD),
and use of GH derived from human pituitaries was
largely stopped. Fortunately at that time the possibil-
ity arose of using human GH prepared by genetic
engineering techniques, and this is now widely
employed.
0027The possibility of using GH to treat forms of short
stature other than those due to clear-cut GH defi-
ciency has been considered for some time. The avail-
ability of GH produced by recombinant DNA
methods in potentially unlimited quantities makes
wider usage more feasible, and this is being explored
actively. As well as extending treatment to children
whose short stature is due to primary causes other
than GH deficiency, the use of recombinant DNA-
derived GH to treat adults with GH deficiency is
being considered, as is its use for promoting anabol-
ism in muscle-wasting conditions such as acquired
immunodeficiency syndrome (AIDS), cachexia, and
even old age.
Growth Hormone Excess
0028Oversecretion of GH, usually due to a pituitary
tumor, leads to excessive growth. Where this occurs
before puberty it can lead to giantism, though this is
rare. Where it occurs after puberty (when further
growth of the long bones is not possible) it can lead
to acromegaly, in which those bones which can still
grow in response to GH do so, leading to enlarged
hands and feet and distorted facial features. The
very high circulating levels of GH and IGF-1 seen in
acromegalic patients also lead to thickening of the
skin, enlargement of some soft tissues, and often
3164 HORMONES/Pituitary Hormones

disorders of the cardiovascular, muscular, and endo-
crine systems. Diabetes mellitus is relatively common
in acromegaly, and shows clinical features rather dif-
ferent from normal, presumably because GH itself
has diabetogenic properties.
0029 Acromegaly can be treated by surgical removal
of the pituitary tumor, radiation therapy, administra-
tion of a drug (e.g., bromocriptine or a somatostatin
analog) that inhibits GH secretion and tumor growth
and/or use of a GH receptor antagonist (pegviso-
mant). Very rarely acromegaly is caused by a
GHRH-secreting tumor elsewhere in the body, such
as the pancreas.
Growth Hormone and Malnutrition; Catch-up
Growth
0030 The role of GH in malnutrition and starvation is
complex and poorly understood. In humans, GH
levels increase on fasting, in both normal and obese
subjects, and fall again on refeeding. Paradoxically,
IGF-1 levels fall in response to starvation, despite the
high GH levels. Such a dissociation of GH and IGF-
1 levels is particularly marked in protein-calorie mal-
nutrition (kwashiorkor), where IGF levels may be in
the hypopituitary level despite elevated GH levels, but
it is also seen in conditions such as anorexia. The
mechanism by which the dissociation of GH and
IGF-1 levels arises is poorly understood, as is its
significance for the control of nutritional balance
during malnutrition, but it may effectively allow a
dissociation of the actions of the hormone on growth
promotion (much reduced during periods of malnu-
trition) from those on metabolism (still required since
the hormone promotes utilization of fat). GH re-
sponses to starvation in the rat differ from those
seen in humans – both GH and IGF-1 levels fall;
responses in ruminants and pig are similar to those
in humans.
0031 In children, if nutrition improves after a period of
malnutrition, the phenomenon of catch-up growth
can occur, in which growth increases to an above
normal rate, allowing some or all of the lost ground
to be restored. Involvement of the GH/IGF axis in this
phenomenon is likely, but the mechanism is unclear.
Nutritional Control of IGF Levels
0032 Although GH clearly plays an essential part in the
control of circulating and local IGF-1 levels, it is by
no means the only factor. Insulin and thyroxine are
important, and nutritional level also regulates both
the level and activity of IGF-1. Indeed, IGF-1 levels in
the circulation are remarkably sensitive to nutrients,
and can provide a marker for nutritional status and
effectiveness of treatment. Nutritional influence on
IGF-1 levels may be mediated to some extent by
insulin or thyroid hormones (but not GH, given the
divorce between GH and IGF-1 levels in malnutri-
tion, referred to above), but it is clear that to some
extent they are direct. Actions on GH receptor levels,
which are lowered on fasting, may be involved in
severe dietary restriction, but in less severe protein
restriction postreceptor mechanisms appear to
predominate. Nutritional level also affects the activ-
ity of circulating IGF-1: (1) by altering the balance of
the IGF-binding proteins that modulate the half-life,
tissue availability, and receptor-binding of IGF-1; (2)
by regulating formation of inhibitors of IGF-1; and
(3) by altering type 1 IGF receptor levels. The involve-
ment of so many factors in regulating IGF levels and
activity probably plays an important part in integrat-
ing their various influences on growth.
Growth Hormone and Animal Biotechnology
0033GH can stimulate growth, meat production, and lac-
tation in farm animals and the availability of large
quantities of the hormone produced by recombinant
DNA methodology has stimulated work designed to
assess and apply the hormone in animal agriculture.
Recombinant DNA-derived bovine GH (frequently
referred to as bovine somatotropin, BST) has been
subjected to extensive trials as an agent for promoting
milk production, and is now being used commercially
for this purpose in some countries. The ability of GH
to promote meat production in various farm animals
has been studied extensively. Not only is muscle
growth stimulated, but fat content is much reduced,
so that a potentially healthier product results. Pos-
sible applications of GH in fish farming have also
been studied.
0034The potential for the use of recombinant DNA-
derived GH in agriculture is great, but such usage
remains controversial because of concerns about
food safety, animal welfare, and economic impact.
Indirect ways of modulating GH levels and actions
in farm animals may prove more acceptable than
direct administration of the hormone. Such indirect
approaches include immunological modulation,
modification of endogeneous hypothalamic activity,
and use of the transgenic approach to introduce ad-
ditional genes for GH into animals. The transgenic
approach, coupled with cloning, is particularly
powerful, though again welfare and other consider-
ations may limit its usefulness.
See also: Biotechnology in Food Production; Famine,
Starvation, and Fasting; Growth and Development;
Malnutrition: The Problem of Malnutrition; Renal
Function and Disorders: Kidney: Structure and Function
HORMONES/Pituitary Hormones 3165

Further Reading
Boersma B and Wit JM (1997) Catch-up growth. Endocrine
Reviews 18: 646–661.
Bole-Feysot C, Goffin V, Edery M, Binart N and Kelly PA
(1998) Prolactin (PRL) and its receptor: actions, signal
transduction pathways and phenotypes observed in PRL
receptor knockout mice. Endocrine Reviews 19: 225–
268.
Cone RD (1999) The central melanocortin system and
energy homeostasis. Trends in Endocrinology and Me-
tabolism 10: 211–216.
Drake WM, Parkinson C, Besser GM and Trainer PJ (2001)
Clinical use of a growth hormone receptor antagonist in
the treatment of acromegaly. Trends in Endocrinology
and Metabolism 12: 408–413.
Grossmann M, Weintraub BD and Szkudlinski MW (1997)
Novel insights into the molecular mechanisms of human
thyrotropin action: structural, physiological, and thera-
peutic implications for the glycoprotein hormone family.
Endocrine Reviews 18: 476–501.
Herrington J and Carter-Su C (2001) Signaling pathways
activated by the growth hormone receptor. Trends in
Endocrinology and Metabolism 12: 252–257.
Hwa V, Oh Y and Rosenfeld RG (1999) The insulin-like
growth factor-binding protein (IGFBP) superfamily.
Endocrine Reviews 20: 761–787.
Jordan SA and Jackson IJ (1998) Melanocortin receptors
and antagonists regulate pigmentation and body weight.
Bioessays 20: 603–606.
Kajima M, Hosoda H, Matsuo H and Kangawa K (2001)
Ghrelin: discovery of the natural endogenous ligand for
the growth hormone secretagogue receptor. Trends in
Endocrinology and Metabolism 12: 118–122.
Lapthorn AJ, Harris DC, Littlejohn A et al. (1994) Crystal
structure of human chorionic gonadotropin. Nature
369: 455–461.
Mohr E, Meyerhof W and Richter D (1995) Vasopressin
and oxytocin: molecular biology and evolution of the
peptide hormones and their receptors. Vitamins and
Hormones 51: 235–266.
Norman AW and Litwack GD (1997) Hormones. New
York: Academic Press.
Ohlsson C, Bengtsson BA, Isaksson OGP, Andreassen TT
and Slootweg MC (1998) Growth hormone and bone.
Endocrine Reviews 19: 55–79.
Pell JM and Bates PC (1990) The nutritional regulation of
growth hormone action. Nutrition Research Reviews 3:
163–192.
Thissen J-P, Ketelslegers J-M and Underwood LE (1994)
Nutritional regulation of the insulin-like growth factors.
Endocrine Reviews 15: 80–101.
Wallis M (1988) The molecular basis of growth hormone
deficiency. Molecular Aspects of Medicine 10: 429–509.
Wallis M, Howell SL and Taylor KW (1985) Biochemistry
of the Polypeptide Hormones. Chichester: John Wiley.
Wilson JD, Foster DW, Kronenberg HM and Larsen PR
(eds) (1998) Williams Textbook of Endocrinology, 9th
edn. Philadelphia: Saunders.
Steroid Hormones
A W Norman, University of California-Riverside,
Riverside, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Steroid hormones are well known both physiologic-
ally and clinically as regulators of diverse biological
responses, including profound effects on cellular me-
tabolism, development, and physiology. The steroid
hormones are the estrogens (female sex steroids),
androgens (male sex steroids), progestins, mineralo-
corticoids, glucocorticoids, and vitamin D with its
daughter metabolites.
0002All these different steroid hormones are synthe-
sized from the common precursor, cholesterol, and
structurally differ only in the pattern of chemical
bonds within the rings and modifications on the
side chain. The exquisite specificity of physiological
effects that these steroid hormones evoke is mediated
by high-affinity intracellular receptor proteins that
are exclusively localized in the specific target tissues
for each steroid hormone. Specific interaction of the
hormone–receptor complex with DNA sequences of
the hormone-responsive gene(s) results in the tissue-
specific expression of proteins which either directly or
indirectly generate the biological responses attribut-
able to the steroid hormones. The application of
cellular and molecular biological techniques has
allowed greater understanding of the way in which
such small molecules exert diverse biological effects
with exquisite specificity.
Historical Perspective
0003The first steroid hormone that was discovered,
estrone, was isolated in 1929, before the character-
ization of the ring structure of the steroid
nucleus. Studies by Professor A. Windaus in Go
¨
ttin-
gen, Germany, in the 1930s led to the chemical
characterization and structural determination of
cholesterol, using classical organic chemistry
manipulations. Methodological advances such as
radioisotopes, chromatography, mass spectropho-
tometry, and nuclear magnetic resonance spectros-
copy have all facilitated the elucidation of structures
of the other steroid hormones.
Chemistry
0004In mammalian systems, there are six families of ster-
oid hormones that can be classified on both a struc-
tural and a biological (hormonal) basis. These are
3166 HORMONES/Steroid Hormones

the: (1) mineralocorticoids, which instruct the renal
tubule to retain sodium; (2) glucocorticoids, which
exert manifold effects on carbohydrate metabolism;
(3) progestins, which are essential for reproduction;
(4) estrogens, which induce female secondary sexual
characteristics; (5) androgens, which induce male
secondary sexual characteristics; and (6) the vita-
min-D hormone, 1,25-dihydroxyvitamin D
3
(1,25-
(OH)
2
D
3
), which is important for regulation of
calcium and phosphorus homeostasis, bone growth,
and development. Also, the bile acids are structurally
related to cholesterol and thus constitute a seventh
member of the steroid family. All of these steroids are
biologically derived from cholesterol.
0005 All steroids are derived from a cyclopentano-
perhydrophenanthrene backbone that comprises
three C
6
cyclohexane rings designated as A, B, and
C, fused to a fourth C
5
cyclopentane ring, notated as
the D ring. Within the family of steroid hormones,
each member is distinguished by chemical alterations
such as isomerization, aromatization, dehydrogen-
ation, and fission in the ring structure, and modifica-
tions such as hydroxylations in the side chains of the
cholesterol backbone that are catalyzed by specific
enzymes present in the endocrine glands. The
chemical formulae of these steroid hormones are
depicted in Figure 1.
Biosynthesis of Steroids
0006 Figure 2 summarizes in general terms the metabolic
pathways leading from cholesterol to the six major
steroid hormones (Table 1). The principal tissues
of synthesis of the five classical steroid hormones
(estrogens, androgens, progestins, glucocorticoids,
and mineralocorticoids) are the adrenal cortex, ovar-
ies, and testes. Also, during pregnancy, the fetal–pla-
cental unit can serve as a source of estrogen and some
other hormones. The sites of synthesis of the sixth
class of steroid hormones, the prohormone vitamin
D
3
and its metabolites, are the skin, liver, and kidney.
The bile acids, which are the seventh important struc-
tural class of mammalian steroids, have no known
hormonal activity; they are principally synthesized
in the liver and are secreted from the gallbladder to
participate in digestion and absorption where they
function as a detergent to solubilize fats.
0007 Figure 3 describes the relationship between the
stimulatory peptide hormone and the six classes of
steroid hormones. The biosynthesis of each steroid
hormone by its endocrine gland is stimulated by a
specific cognate peptide hormone. Thus, for example,
the biosynthesis of cortisol in the adrenal cortex is
only stimulated by its tropic (stimulatory) peptide
hormone adrenocorticotropic hormone (ACTH).
ACTH is only secreted by the anterior pituitary gland.
Biosynthesis of Progestins
0008As evident from Figure 2, the common pathway
leading to the production of six classes of steroid
hormones primarily involves conversion of choles-
terol (which possesses 27 carbon atoms) into pregne-
nolone and then progesterone (which possesses 21
carbon atoms). Progesterone is synthesized by the
corpus luteum of the ovary and placenta and consti-
tutes the chief progestational steroid in humans.
Biosynthesis of Glucocorticoids and
Mineralocorticoids
0009The glucocorticoids and mineralocorticoids are
21-carbon corticosteroids produced by the adrenal
cortex (Figure 1). The principal glucocorticoid in
males is cortisol, while aldosterone is the main
mineralocorticoid.
Biosynthesis of Androgens and Estrogens
0010The androgens, which possess 19 carbon atoms, are
produced in the testes in males, and in the ovaries and
placenta in females. Under some circumstances the
adrenal cortex can also produce physiologically sig-
nificant androgens such as androsterone, 4-andro-
stene-3,17-dione, and dehydroepiandrosterone. The
major naturally occurring steroids with androgenic
activity (in decreasing order of relative potency) are
5a-dihydrotestosterone (5a-DHT; 150–200%), tes-
tosterone (100%), androstanediol (65%), androst-
4-ene-3,17-dione (25%), androsterone (10%), and
dehydroepiandrosterone (10%). The hormonally
active form of testosterone in males is 5a-DHT,
which is chiefly produced in the prostate gland,
although skin, testis, and submaxillary glands also
can produce small quantities.
0011The estrogens, which possess 18 carbon atoms, are
produced in the ovarian follicle, corpus luteum,
and fetal–placental unit. In both males and females,
the adrenal cortex can generate small quantities
of estrone from androst-4-ene-3,17-dione. The
major naturally occurring steroids with estrogenic
activity are estra-3,17b-diol, estra-3,16a,17b-triol,
and estrone.
Biosynthesis of Vitamin D Metabolites
0012Chemically, the vitamin D steroids are secosteroids
because of the breakage of the B-ring, leaving the A-,
C-, and D-rings intact (Figure 1). The parent vitamin
D is produced in the skin from 7-dehydrocholesterol
as a consequence of ultraviolet or sunlight action,
which breaks the 9,10 carbon–carbon bond. The
sites of synthesis of the vitamin D metabolites are the
liver and kidney. The hormonally active forms of vita-
min D are 1,25(OH)
2
D
3
and 24,25-dihydroxyvitamin
HORMONES/Steroid Hormones 3167
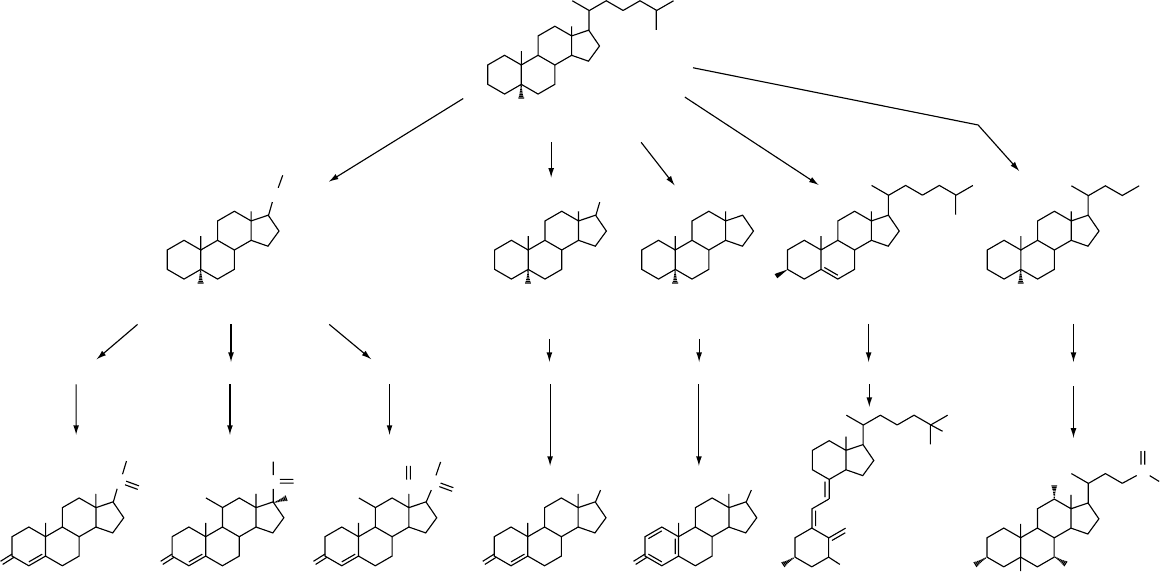
Progesterone Cortisol Aldosterone Testosterone Estradiol 1,25-Dihydroxyvitamin D
3
Cholic acid
1
35
9
H
3
C
H
H
3
C
11
13
19
18
14
16
17
20
21
23
25
26
27
7
H
3
C
H
OH
CH
3
CH
2
CH
3
H
3
C
H
H
CH
3
H
H
CH
3
H
3
C
H
H
3
C
24
C
O
O
CH
3
A
HO
B
CD
Progestins Glucocorticoids
5α-Pregnane (C21)
Mineralocorticoids Androgens Estrogens Vitamin D
Cholesterol (C27)
Cholestane (C27)
5α-Estrane (C18)5α-Androstane (C19) Cholane (C24)
Bile acids
O
HO
C
CH
2
OH
O
O
HO
C
HC
O
CH
2
OH
O
O
OH
HO
OH
OH
OH
HO
OH
OH
H
3
7
12
OH
24
C
O
CH
3
HO
Figure 1 Family tree of the seven principal classes of steroids (bottom row); all of the steroids except cholic acids are steroid hormones. All of the steroids are structurally derived from
cholestane (top row), which is closely related to cholesterol.
fig0001
