Guozhong Cao. Nanostructures & Nanomaterials: Synthesis, Properties & Applications
Подождите немного. Документ загружается.

176
Nanostructures and Nanomaterials
structure as that of the substrate. Homoepitaxy is a simple extension of the
substrate, and thus virtually there
is
no interface between the substrate and
the depositing film and no nucleation process. Although the deposit has a
chemical composition different from that of the substrate, the growth
species prefers to bind to the substrate rather than to each other. Because
of the difference in chemical composition, the lattice constants of the
deposit will most likely differ from those of the substrate. Such a differ-
ence commonly leads to the development of stress in the deposit; stress is
one of the common reasons for the island-layer growth.
Island-layer growth is a little more complicated and involves
in
situ
developed stress. Initially the deposition would proceed following the
mode of layer growth. When the deposit is elastically strained due to, for
example, lattice mismatch between the deposit and the substrate, strain
energy would be developed.
As
each layer of deposit is added, more stress
is developed and
so
is
the strain energy. Such strain energy is proportional
to the volume
of
the deposit, assuming there is no plastic relaxation.
Therefore, the change of volume of Gibbs free energy should include the
strain energy and
Eq.
(5.2)
is modified accordingly:
(5.5)
16~~~.
2
-
3~0s
8
+
COS~
8
4
AG*=( 3(AGv
+
o)*
)(
where
o
is the strain energy per unit volume generated by the stress in the
deposit. Because the sign of AGv is negative, and the sign of
o
is positive,
the overall energy barrier to nucleation increases. When the stress exceeds
a critical point and cannot be released, the strain energy per unit area of
deposit is large with respect to
yvf,
permitting nuclei to form above the
initial layered deposit. In this case, the surface energy
of
the substrate
exceeds the combination of both surface energy of the deposit and the
interfacial energy between the substrate and the deposit:
(5.6)
Ysv
>
Y/S
+
Yvf
If should be noted that there are other situations when the overall volume
of Gibbs free energy may change. For example, initial deposition or nucle-
ation on substrates with cleavage steps and screw dislocations would
result in a stress release and, thus, an increased change of the overall
Gibbs free energy.
As
a result, the energy barrier for the initial nucleation
is reduced and the critical size of nuclei becomes small. Substrate charge
and impurities would affect the
AG*
through the change of surface, elec-
trostatic and chemical energies in a similar manner.
It should be noted that the aforementioned nucleation models and mech-
anisms are applicable to the formation of single crystal, polycrystalline and
amorphous deposit, and of inorganic, organic and hybrid deposit. Whether

Two-Dimensional Nanostructures: Thin Films
177
the deposit is single crystalline, polycrystalline or amorphous, depends on
the growth conditions and the substrate. Deposition temperature and the
impinging rate of growth species are the two most important factors and
are briefly summarized below:
(1)
Growth
of
single crystal films is most difficult and requires: (i) a sin-
gle crystal substrate with a close lattice match, (ii) a clean substrate
surface
so
as to avoid possible secondary nucleation, (iii) a high
growth temperature
so as to ensure sufficient mobility of the growth
species and (iv) low impinging rate of growth species
so
as to ensure
sufficient time for surface diffusion and incorporation
of
growth
species into the crystal structure and for structural relaxation before
the arrival of next growth species.
(2) Deposition of amorphous films typically occurs (i) when a low
growth temperature is applied, there is insufficient surface mobility
of growth species andor (ii) when the influx of growth species onto
the growth surface is very high, growth species does not have enough
time to find the growth sites with the lowest energy.
(3)
The conditions for the growth of polycrystalline crystalline films fall
between the conditions of single crystal growth and amorphous film
deposition. In general, the deposition temperature is moderate ensur-
ing
a
reasonable surface mobility of growth species and the imping-
ing flux of growth species is moderately high.
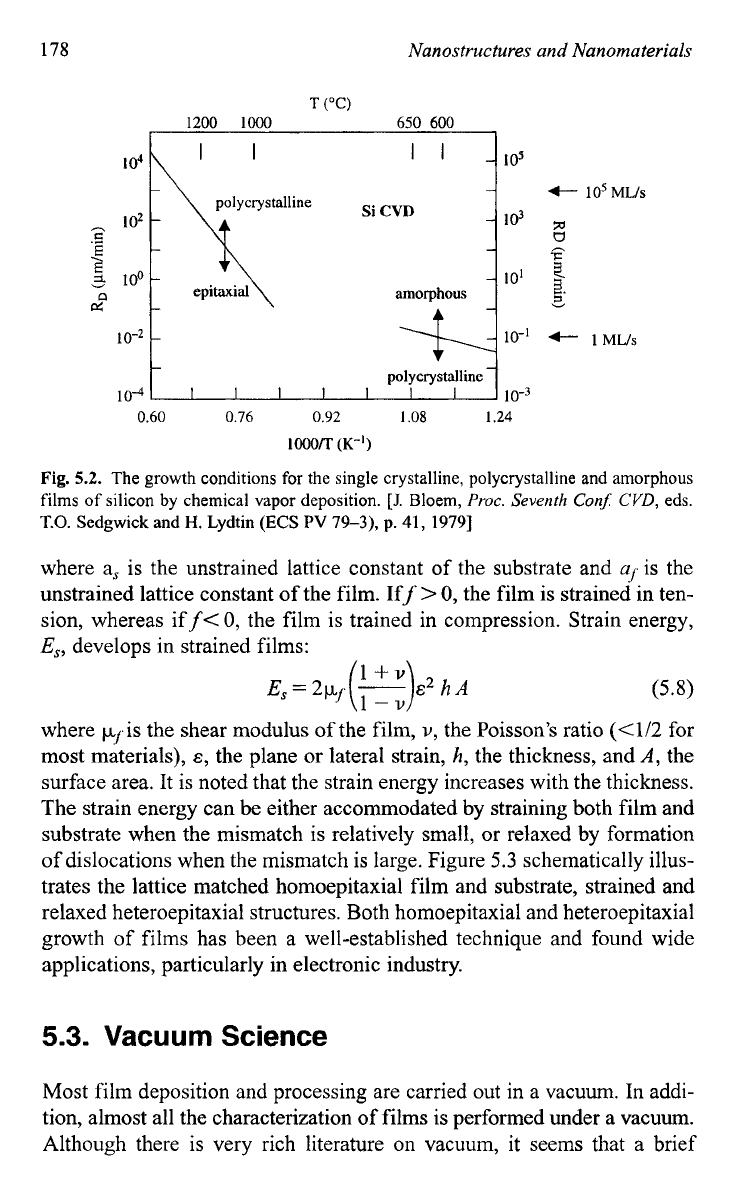
Figure
5.2,
as an example, shows the growth conditions for the single
crystalline, polycrystalline and amorphous films of silicon by chemical
vapor dep~sition.~ The above discussion is applicable to single element
films; however, growth process is complex in the presence of impurities
and additives and in the case of multiple component material systems.
Epitaxy is a very special process, and refers to the formation or growth
of single crystal on top of a single crystal substrate or seed. Epitaxial
growth can be hrther divided into homoepitaxy and heteroepitaxy.
Homoepitaxy is to grow film on the substrate, in which both are the same
material. Homoepitaxial growth is typically used to grow better quality
film or introduce dopants into the grown film. Heteroepitaxy refers to the
case that films and substrates are different materials. One obvious differ-
ence between homoepitaxial films and heteroepitaxial films is the lattice
match between films and substrates. There is no lattice mismatch between
films and substrates by homoepitaxial growth. On the contrary, there will
be a lattice mismatch between films and substrates in heteroepitaxial
growth. The lattice mismatch is also called misfit, given by:
(5.7)
f
-
as
-
ar
af
178
Nanostructures
and
Nanomaterials
T
("C)
1200 lo00
650
600
<
II
Si
CVD
amorphous
A
I
05
103
10'
105
MLI~
lo-'I
,
I
I
,
7:
4-
lMUs
polycrystalline
1
0-4
0.60 0.76
0.92
1.08
1.24
lOOO/T
(K-')
Fig.
5.2.
The growth conditions for the single crystalline, polycrystalline and
amorphous
films
of
silicon
by
chemical vapor deposition.
[J.
Bloem,
Proc.
Seventh
ConJ:
CVD,
eds.
T.O.
Sedgwick and
H.
Lydtin
(ECS
PV
79-3), p.
41,
19791
where a, is the unstrained lattice constant of the substrate and
ar
is the
unstrained lattice constant
of
the film. Iff
>
0,
the film is strained in ten-
sion, whereas iff<
0,
the film is trained in compression. Strain energy,
E,,
develops in strained films:
(5.8)
where kfis the shear modulus of the film,
u,
the Poisson's ratio
(<1/2
for
most materials),
E,
the plane or lateral strain,
h,
the thickness, and
A,
the
surface area. It is noted that the strain energy increases with the thickness.
The strain energy can be either accommodated by straining both film and
substrate when the mismatch is relatively small, or relaxed by formation
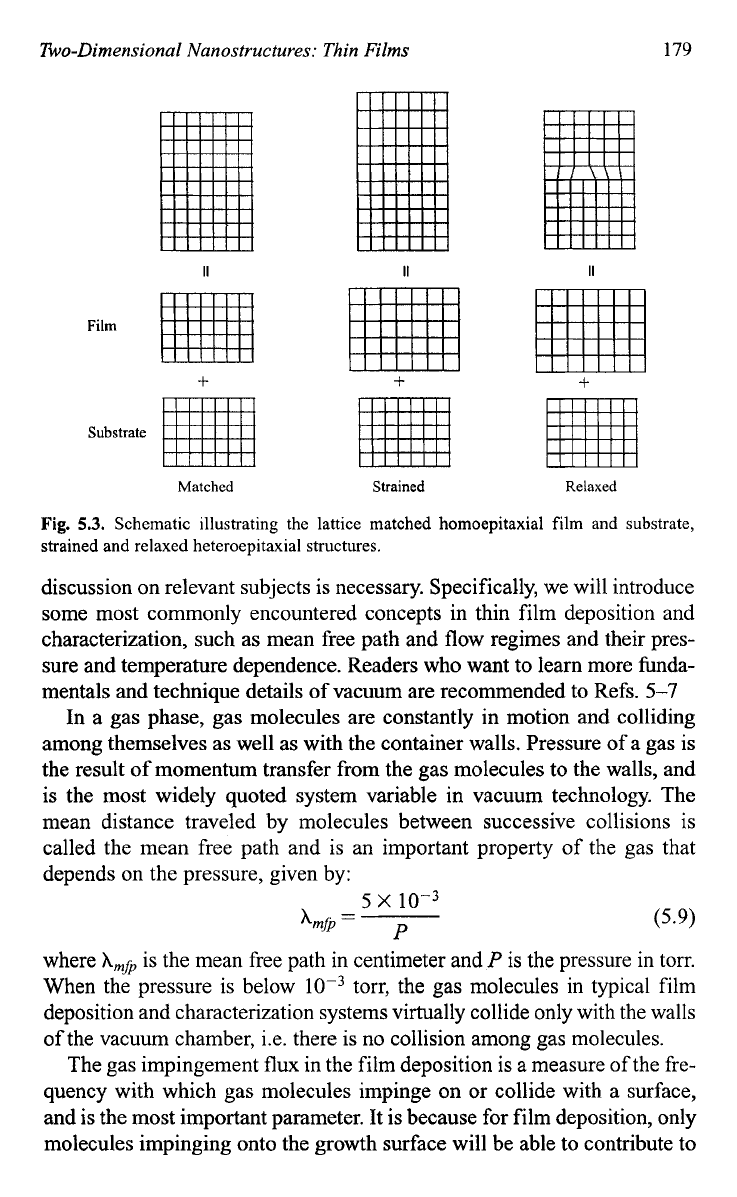
of dislocations when the mismatch is large. Figure
5.3
schematically illus-
trates the lattice matched homoepitaxial film and substrate, strained and
relaxed heteroepitaxial structures. Both homoepitaxial and heteroepitaxial
growth of films has been a well-established technique and found wide
applications, particularly in electronic industry.
5.3.
Vacuum Science
Most film deposition and processing are carried out in a vacuum. In addi-
tion, almost all the characterization of films is performed under a vacuum.
Although there is very rich literature on vacuum,
it
seems that a brief
Two-Dimensional Nanostructures:
Thin
Films
179
II
+
Substrate
Matched
Strained
Relaxed
Fig.
5.3.
Schematic illustrating the lattice matched homoepitaxial film and substrate,
strained and relaxed heteroepitaxial structures.
discussion on relevant subjects is necessary. Specifically, we will introduce
some most commonly encountered concepts in thin film deposition and
characterization, such as mean free path and flow regimes and their pres-
sure and temperature dependence. Readers who want to learn more hda-
mentals and technique details of vacuum are recommended to Refs.
5-7
In a gas phase, gas molecules are constantly in motion and colliding
among themselves as well as with the container walls. Pressure
of
a gas is
the result of momentum transfer from the gas molecules to the walls, and
is the most widely quoted system variable in vacuum technology. The
mean distance traveled by molecules between successive collisions is
called the mean free path and is an important property of the gas that
depends on the pressure, given by:
5
x
10-3
A
.=
mfP
p
(5.9)
where
Amjb
is the mean free path in centimeter and
P
is the pressure
in
torr.
When the pressure is below torr, the gas molecules in typical film
deposition and characterization systems virtually collide only with the walls
of the vacuum chamber, i.e. there is no collision among gas molecules.
The gas impingement flux in the film deposition is a measure of the fre-
quency with which gas molecules impinge on or collide with a surface,
and is the most important parameter. It
is
because for film deposition, only
molecules impinging onto the growth surface will be able
to
contribute to
180
Nanostructures and Nanornaterials
r:
.-
I
E
P
LI
x
0
-
2g
r
'C
-
I
min
-
10min
-
I
hr
:
10hrs
-
1
day
-
1
week
-
1
month
1
year
the growth process. The number of gas molecules that strike a surface per
unit time and area is defined as the gas impingement flux,
CD:
10-4
-
10-2
-
1
0"
-
102
-
104
-
108
-
101"-
P
@=3.513
X
(Mot
-
10-6
-
10-4
-
10-2
-
10"
-
102
-
106
104
(5.10)
where
P
is the pressure in torr,
A4
is the molecular weight and
T
is
temperature.
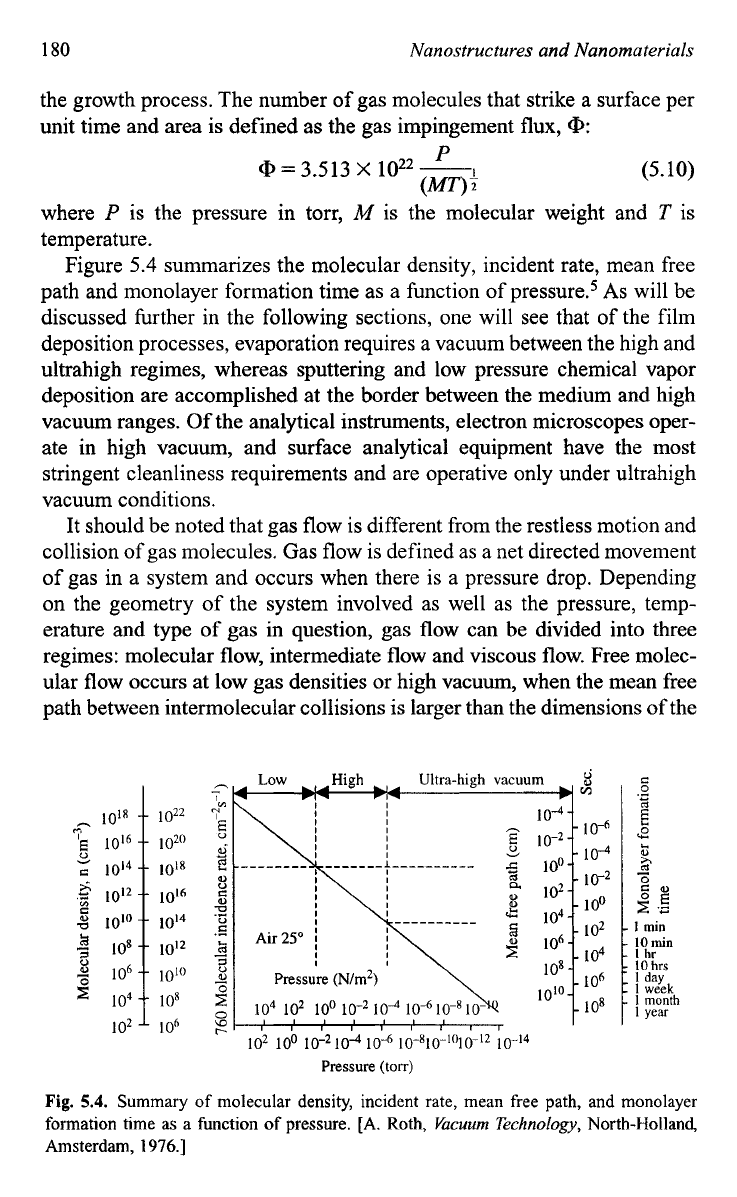
Figure
5.4 summarizes the molecular density, incident rate, mean free
path and monolayer formation time as a function of pre~sure.~
As
will be
discussed hrther in the following sections, one will see that of the film
deposition processes, evaporation requires a vacuum between the high and
ultrahigh regimes, whereas sputtering and low pressure chemical vapor
deposition are accomplished at the border between the medium and high
vacuum ranges.
Of
the analytical instruments, electron microscopes oper-
ate in high vacuum, and surface analytical equipment have the most
stringent cleanliness requirements and are operative only under ultrahigh
vacuum conditions.
It
should be noted that gas flow is different from the restless motion and
collision of gas molecules. Gas flow is defined as a net directed movement
of gas in a system and occurs when there is a pressure drop. Depending
on the geometry of the system involved as well as the pressure, temp-
erature and type of gas in question, gas flow can be divided into three
regimes: molecular flow, intermediate flow and viscous flow. Free molec-
ular flow occurs at low gas densities or high vacuum, when the mean free
path between intermolecular collisions is larger than the dimensions of the
Ultra-high vacuum
FI
r
I-
Low
High
h
v
g
s
8
B
a
t
$4
I
IIIIlIlll
102
100
10-2
10-4
IO-~
IO-~IO-~~)IO-~~
10-l4
Pressure
(torr)
Fig.
5.4.
Summary of molecular density, incident rate, mean free path, and monolayer
formation time as a function
of
pressure. [A. Roth,
Vacuum
Technology,
North-Holland,
Amsterdam,
1976.1
Two-Dimensional Nanostructures: Thin Films
181
system and the molecules collide with the walls of the system only. At
high pressure, intermolecular collisions become predominant since the
mean free path is reduced and the gas flow is referred to as in the viscous
flow regime. Between free molecular flow and viscous flow, there is a
transition regime: intermediate flow. The above
gas
flow can be defined
by the magnitude of the Knudsen number,
K,,
given by:
D
K
=-
(5.1
1)
where
D
is the characteristic dimension
of
the system, e.g. the diameter of
a pipe, and
Amfb
is
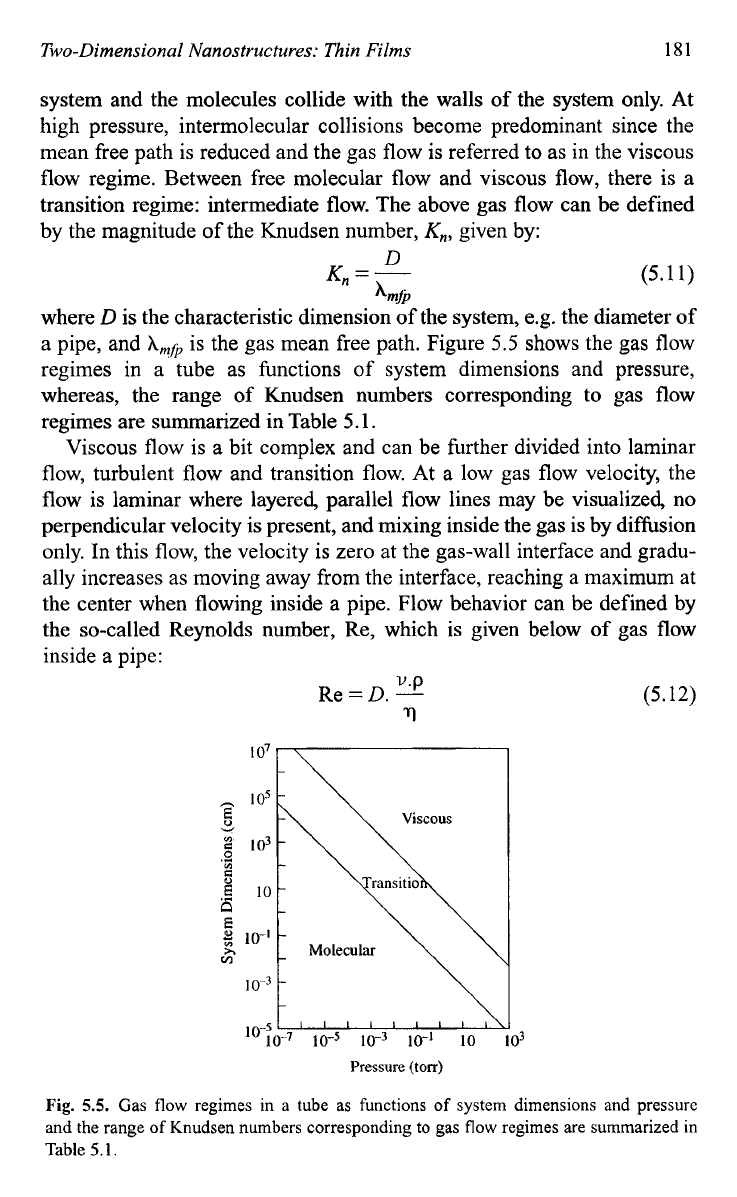
the gas mean free path. Figure
5.5
shows the gas flow
regimes in a tube as functions of system dimensions and pressure,
whereas, the range of Knudsen numbers corresponding to gas flow
regimes are summarized in Table
5.1.
Viscous flow is a bit complex and can be further divided into laminar
flow, turbulent flow and transition flow. At a low gas flow velocity, the
flow is laminar where layered, parallel flow lines may be visualized, no
perpendicular velocity is present, and mixing inside the
gas
is by diffusion
only. In this flow, the velocity is zero at the gas-wall interface and gradu-
ally increases as moving away from the interface, reaching a maximum at
the center when flowing inside a pipe. Flow behavior can be defined by
the so-called Reynolds number, Re, which is given below of gas flow
inside a pipe:
ti
XmfP
V.P
Re=D.-
rl
(5.12)
11111111,1\
1
~-i~-~
10-5
10-I
10
103
Pressure
(tom)
Fig.
5.5.
Gas
flow
regimes in a tube as functions
of
system dimensions and pressure
and the range
of
Knudsen numbers corresponding to gas
flow
regimes are summarized in
Table
5.1.

182
Nanostructures and Nanomaterials
Table
5.1.
Summary
of
gas
flow
regions.
Gasflow
regimes
Knudsen
number
D.P
Molecular
flow
K,,<
1
D.P
<
5
X
cm.torr
Intermediate
flow
1
<K,<
110
5X
10-3<D.P.<5X
IO-'cm.torr
Viscous
flow
Kn>l10
D.P
>
5
X
10-Icm.torr
D
is
the characteristic dimension
of
the system and
P
is the pressure.
where
D
is the diameter
of
the pipe,
v,
the velocity,
p,
the density, and
q,
the viscosity of the gas. Laminar
flow
corresponds to a small Re
<
2
100.
At a high gas velocity, the flow is turbulent, where the gas is constantly
under intermixing, where Re
>
4000.
At
2100
<
Re
<
4000,
a transition
from laminar to turbulent flow occurs and is referred to as transition flow.
There is always a laminar
flow
near to the solid surface in both turbulent
and transition flows, since the friction viscous forces a deceleration
of
the
gas at the surface.
DiEusion is one of the mass transfer mechanisms in gases, which also
occurs in liquids and solids. Difision is the motion of atoms or molecules
from regions of higher to lower concentration, thus increasing the entropy
of the system. Another mechanism is convection, a bulk gas flow process.
Convection arises from the response to gravitational, centrifugal, electric
and magnetic forces. Convection can play an important role in high-pressure
film deposition. For example, a hotter and less dense gas above a hot sub-
strate would rise, whereas a cooler and denser gas would replace the gap.
Such a situation is often encountered in cold wall CVD reactors.
5.4.
Physical Vapor Deposition (PVD)
PVD is a process of transferring growth species from a source or target and
deposit them on a substrate to form a film. The process proceeds atomisti-
cally and mostly involves no chemical reactions. Various methods have
been developed for the removal of growth species from the source or tar-
get. The thickness of the deposits can vary from angstroms to millimeters.
In general, those methods can be divided into two groups: evaporation and
sputtering. In evaporation, the growth species are removed from the source
by
thermal means. In sputtering, atoms or molecules are dislodged from
solid target through impact of gaseous ions (plasma). Each group can be
hrther divided into a number of methods, depending on specific tech-
niques applied to activate the source or target atoms or molecules and the
deposition conditions applied.
Two-Dimensional
Nanostructures:
Thin
Films
183
5.4.1.
Evaporation
Evaporation is arguably the simplest deposition method, and has been
proven particularly useful for the deposition
of
elemental films. Although
formation
of
thin films by evaporation was known about
150
years ago,8
it acquired a wide range of applications over
50
years when the industrial
scale vacuum techniques were de~eloped.~ Many excellent books and
review articles have been published on evaporated films.I0
A
typical evap-
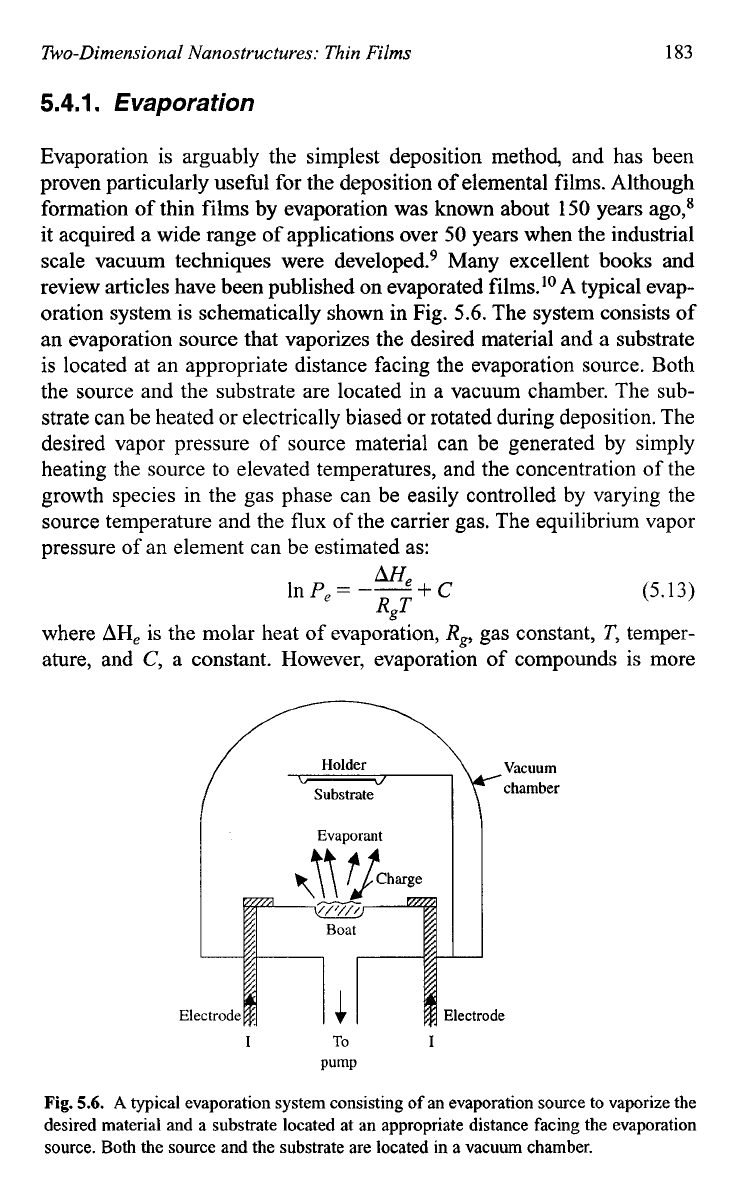
oration system is schematically shown in Fig.
5.6.
The system consists of
an evaporation source that vaporizes the desired material and a substrate
is located at an appropriate distance facing the evaporation source. Both
the source and the substrate are located in a vacuum chamber. The sub-
strate can be heated or electrically biased or rotated during deposition. The
desired vapor pressure of source material can be generated by simply
heating the source to elevated temperatures, and the concentration of the
growth species in the gas phase can be easily controlled by varying the
source temperature and the
flux
of the carrier gas. The equilibrium vapor
pressure
of
an element can be estimated as:
(5.13)
where
AHe
is the molar heat of evaporation,
R,,
gas constant,
T,
temper-
ature, and
C,
a constant. However, evaporation of compounds is more
I
To
I
Pump
Fig.
5.6.
A
typical evaporation system consisting
of
an evaporation source to vaporize the
desired material and a substrate located at an appropriate distance facing the evaporation
source. Both the source and the substrate are located in a vacuum chamber.

184
Nanostructures
and
Nanomaterials
complicated, since compounds may undergo chemical reactions, such as
pyrolysis, decomposition and dissociation, and the resulting vapor com-
position often differs from the source composition during evaporation at
elevated temperatures.
The rate of evaporation
is
dependent on the material in question:
a,
=
a,
NA(P,
-
PJ42
,mRgT)’’2
(5.14)
where
a,
is the evaporation rate,
a,,
the coefficient
of
evaporation
varying between
0
and
1,
NA,
Avogadro’s constant,
P,,
the vapor pressure,
Ph,
the hydrostatic pressure acting on the source,
m,
the molar weight,
Rg,
the gas constant and
T,
the temperature. When a mixture of elements or
compounds is used as a source for the growth of a complex film, the
chemical composition of the vapor phase
is
most likely to be different
from that in the source. Adjusting the composition or molar ratio of the
constituents in the source may help. However, the composition of the
source would change as the evaporation proceeds, since one element may
evaporate much faster than another resulting in the depletion of the first
element. As a result, the composition in the vapor phase will change. For
a multicomponent system, the chemical composition of evaporated film
is likely to be different from that of the source and varies with thickness.
Therefore it is in general difficult to deposit complex films using evapo-
ration method.
Deposition of thin films by evaporation is carried out in a low pressure
(
torr); atoms and molecules in the vapor phase do not collide
with each other prior to arrival at the growth surface, since the mean free
path is very large as compared to the source-to-substrate distance. The
transport of atoms or molecules from the source to the growth substrate is
straightforward along the line of sight, and therefore the conformal cover-
age is relatively poor and a uniform film over a large area is difficult to
obtain. Some special arrangements have been developed to overcome such
a shortfall; these include
(i)
using multiple sources instead of single point
source, (ii) rotating the substrates, (iii) loading both source and substrate
on
the surface
of
a sphere, and (iv) combination
of
all the above.
In addition to evaporation of source by resistance heat, other techniques
have been developed and have attracted increasing attention and gained
more popularity. For example, laser beams have been used to evaporate the
material. Absorption characteristics of the material to be evaporated deter-
mine the laser wavelength to be used. In order to obtain the high power
density required in many cases, pulsed laser beams are generally employed.
Such a deposition process is often referred to as laser ablation. Laser abla-
tion has proven to be an effective technique for the deposition of complex

Two-Dimensional
Nanostructures:
Thin Films
185
films including complex metal oxides such as high T, superconductor
films. One of the great advantages that laser ablation offers is the control
of the vapor composition. In principle, the composition of the vapor phase
can be controlled as that in the source. The disadvantages of laser ablation
include the complex system design, not always possible to find desired
laser wavelength for evaporation, and the low energy conversion efficiency.
Electron beam evaporation is another technique, but it is limited to the case
that the source is electrically conductive. The advantages of electron beam
evaporation include a wide range of controlled evaporation rate due to a
high power density and low contamination. Arc evaporation is another
method commonly used for evaporation of conductive source.
5.4.2.
Molecular beam epitaxy
(MBE)
MBE can be considered as a special case of evaporation for single crystal
film growth, with highly controlled evaporation of a variety of sources in
ultrahigh-vacuum of typically
-lo-'*
torr."-13 Besides the ultrahigh vac-
uum system, MBE mostly consists of realtime structural and chemical
characterization capability, including reflection high energy electron dif-
fraction (RHEED), X-ray photoelectric spectroscopy
(XPS),
Auger elec-
tron spectroscopy (AES). Other analytic instruments may also been
attached to the deposition chamber or to a separate analytic chamber, from
which the grown films can be transferred to and from the growth cham-
ber without exposing to the ambient. Both ultrahigh vacuum and various
structural and chemical characterization facilities are responsible for the
fact that the typical MBE reactor can be easily over $1M.
In MBE, the evaporated atoms or molecules from one or more sources
do not interact with each other in the vapor phase under such a low pres-
sure. Although some gaseous sources are used in MBE, most molecular
beams are generated by heating solid materials placed in source cells,
which are referred to as effusion cells or Knudsen cells.
A
number of effu-
sion cells are radiatically aligned with the substrates as shown in Fig.
5.7.
The source materials are most commonly raised to the desired tempera-
tures by resistive heating. The mean free path of atoms or molecules
(-100m) far exceeds the distance between the source and the substrate
(typically
-30
cm) inside the deposition chamber. The atoms or molecules
striking on the single crystal substrate results in the formation of the
desired epitaxial film. The extremely clean environment, the slow growth
rate, and independent control of the evaporation of individual sources
enable the precise fabrication of nanostructures and nanomaterials at a
