Guozhong Cao. Nanostructures & Nanomaterials: Synthesis, Properties & Applications
Подождите немного. Документ загружается.

206
Nanostructures and Nanomaterials
Fig.
5.18.
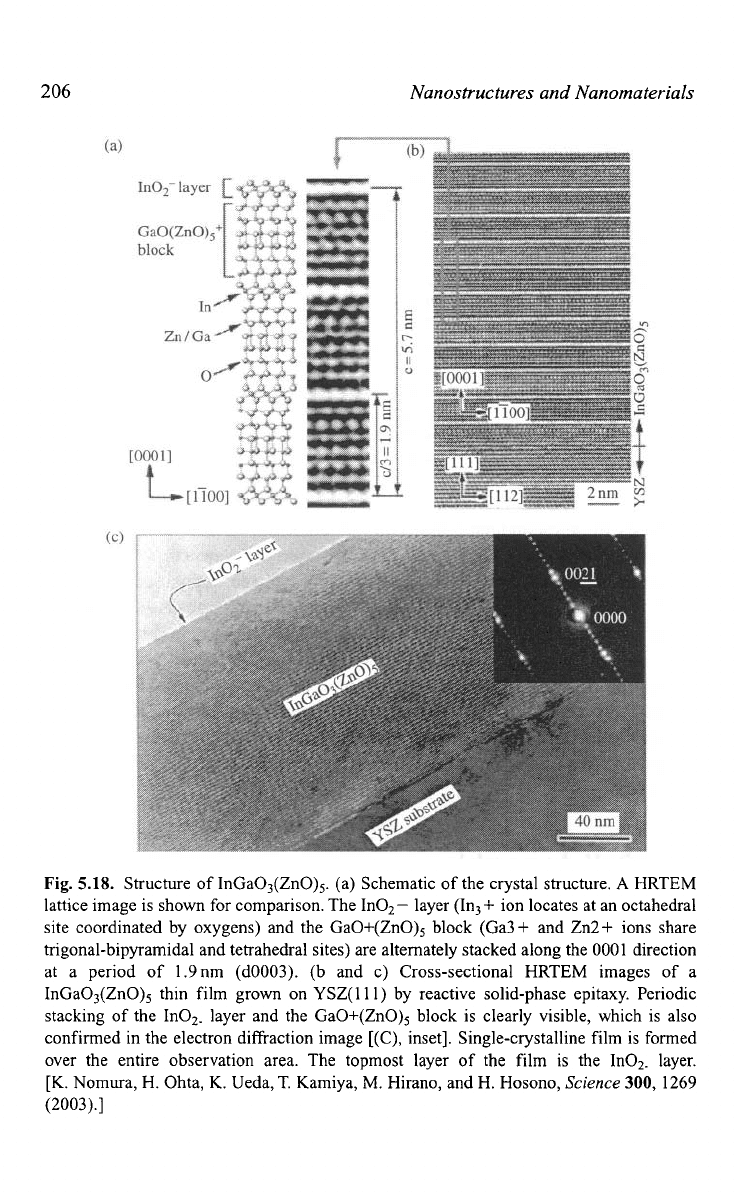
Structure of InGa03(ZnO)5. (a) Schematic of the crystal structure.
A
HRTEM
lattice image is shown for comparison. The InOz- layer (In3+ ion locates at an octahedral
site coordinated by oxygens) and the GaO+(ZnO)5 block (Ga3+ and Zn2+ ions share
trigonal-bipyramidal and tetrahedral sites) are alternately stacked along the
0001
direction
at a period of 1.9nm (d0003). (b and c) Cross-sectional
HRTEM
images of a
InGa03(ZnO)5 thin film grown on YSZ(ll1) by reactive solid-phase epitaxy. Periodic
stacking of the InOz. layer and the GaO+(ZnO)5 block is clearly visible, which is also
confirmed in the electron diffraction image [(C), inset]. Single-crystalline film
is
formed
over the entire observation area. The topmost layer of the film is the In02. layer.
[K.
Nomura, H. Ohta,
K.
Ueda,
T.
Kamiya, M. Hirano, and H. Hosono,
Science
300,
1269
(2003).]

Two-Dimensional
Nanostructures:
Thin Films
207
explored as driving forces for the self-assembly of nanometer subjects as
the fundamental building blocks.
Self-assembled monolayers are molecular assemblies that are formed
spontaneously by the immersion of an appropriate substrate into a solution
of an active surfactant in an organic ~olvent.~~~~~
A
typical self-assembling
surfactant molecule can be divided into three parts as sketched in
Fig.
5.19.
The first part is the head group that provides the most exother-
mic process, i.e. chemisorption
on
the substrate surface. The very strong
molecular-substrate interactions result in an apparent pinning of the head
group to a specific site on the surface through a chemical bond, such as
covalent Si-0 and S-Au bonds, and ionic
-C02-Agf
bond. The second
part is the alkyl chain, and the exothermic energies associated with its
interchain van der Waals interactions are an order of magnitude smaller
than the chemisorption of head groups on substrates. The third molecular
part is the terminal hctionality; these surface hctional groups in SA
monolayers are thermally disordered at room temperature. The most
important process in self-assembly is the chemisorption, and the associ-
ated energy is at the order of tens of kcal/mol (e.g.
-40-45
kcal/mol for
thiolate on g~ld~~,~*).
As
a result of the exothermic head group-substrate
interactions, molecules try to occupy every available binding site on the
surface and adsorbed molecules may diffuse along the surface. In general,
SA
monolayers are considered ordered and closely packed molecular
assemblies that have a two-dimensional crystalline-like structure, though
there exist a lot of defects.
The driving force for the self-assembly includes: electrostatic force,
hydrophobicity and hydrophilicity, capillary force and chemisorption. In
the following discussion, we will focus on the formation of SA monolayers
Surface group
Alkyl,
or
derivatized-
alkyl
group
Interchain van
der
Waals
and
electrostatic interactions
Chemisorption
,+,
at
the
surface
Surface-active headgroup
..
surface
Fig.
5.19.
A
typical self-assembling surfactant molecule consisting of three parts: surface
group, alkyl or derivatized alkyl group, and surface-active headgroup.

208
Nanostructures and Nanomaterials
that chemisorb on the substrates. There are several types of self-assembly
methods for the organic monolayers and these include (i) organosilicon on
hydroxylated surfaces, such as Si02 on Si, A1203 on Al, glass, etc?-l
(ii) alkanethiols on gold, silver and c~pper,~~-~~ (iii) dialkyl sulfides on
(v) alcohols and amines on
platinum,86 and (vi) carboxylic acids on aluminum oxide and ~ilver.
Another way to group the self-assembly methods could be based on the
types of chemical bonds formed between the head groups and substrates.
There are (i) covalent Si-0 bond between organosilicon on hydroxylated
substrates that include metals and oxides, (ii) polar covalent S-Me bond
between alkanethiols, sulfides and noble metals such as gold, silver, plat-
inum and copper, and (iii) ionic bond between carboxylic acids, amines,
alcohols on metal or ionic compound substrates.
One of the important applications of self-assembly that has been exten-
sively studied is the introduction of various desired functionalities and
surface chemistry to the inorganic materials. In the synthesis and fabrica-
tion of nanomaterials and nanostructures, particularly the core-shell struc-
tures, self-assembled organic monolayers are widely used to link different
materials together.
(iv) dialkyl disulfides on
5.8.1.
Monolayers
of
organosilicon or
alkylsilane derivatives
Typical formulas of alkylsilanes are RSiX3, R2SiX2 or R,SiX, where X is
chloride or alkoxy group and
R
is a carbon chain that can bear different
functionalities, such as amine or pyridyl. The chemistry of organosilicon
derivatives has been discussed in a great detail by Pl~eddemann.~~ The
formation of monolayers is simply by reacting alkylsilane derivatives with
hydroxylated surfaces such as Si02, Ti02.
In a typical procedure, a hydroxylated surface is introduced into a
solution (e.g.
-5
X
10-3M) of alkyltrichlorosilane in an organic solvent
(e.g. a mixture of
80/20
Isopar-G/CC14) for a few minutes (e.g.
2-3
min.).
A longer immersion time is required for surfactants with long alkyl chains.
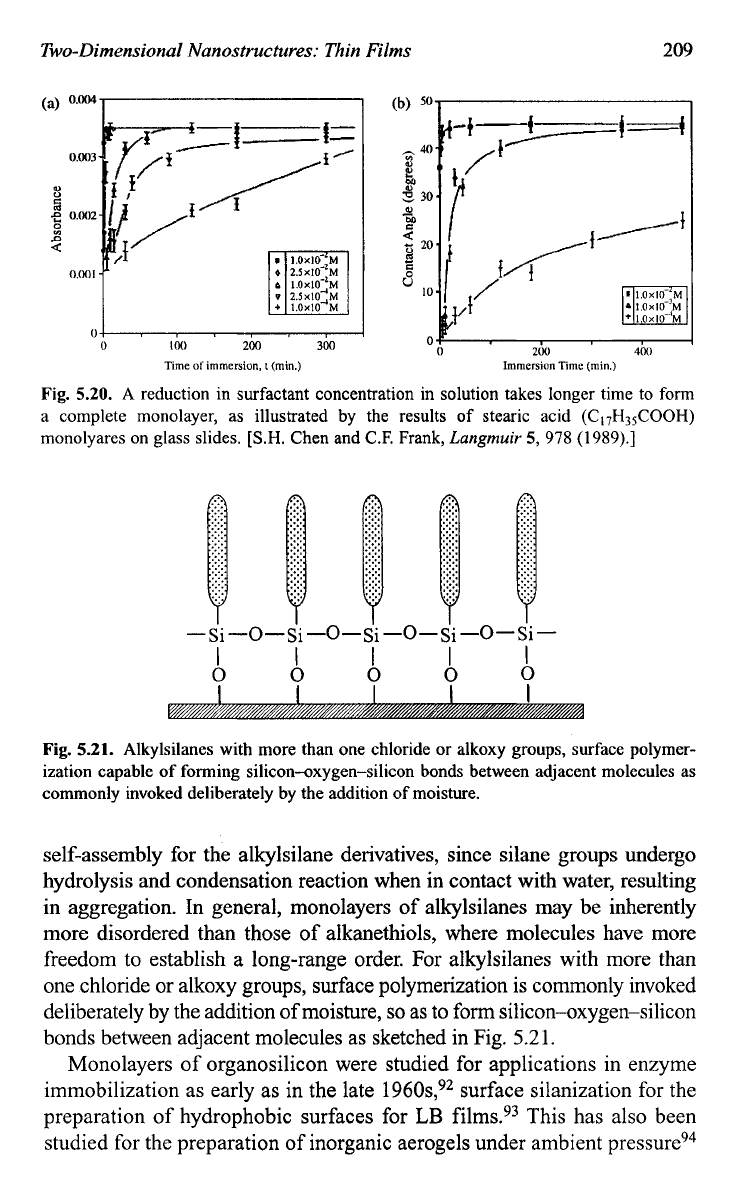
A reduction in surfactant concentration in solution takes longer time to
form a complete monolayer as illustrated in Fig.
5.20,
which presents
the results of stearic acid
(CI7H&OOH)
monolayers on glass
slide^.^'
The ability to form a complete monolayer is obviously dependent on the
substrate, or the interactions between the monolayer molecules and the
substrate surface. After immersion, the substrate is rinsed with methanol,
DI
water and then dried. Organic solvent is in general required for the
Two-Dimensional Nanostructures: Thin Films
209
0.003
0.002
Time
ol
immersion,
I
(min.)
Immersion Time (min.)
Fig.
5.20.
A
reduction in surfactant concentration in solution takes longer time to form
a complete monolayer, as illustrated by the results of stearic acid
(CI7H3,COOH)
monolyares on glass slides.
[S.H.
Chen and C.F. Frank,
Langmuir
5,
978
(1989).]
I
0
I
0
I
0
I
0
I
0
Fig.
5.21.
Alkylsilanes with more than one chloride
or
alkoxy groups, surface polymer-
ization capable of forming silicon-oxygen-silicon bonds between adjacent molecules as
commonly invoked deliberately by the addition of moisture.
self-assembly for the alkylsilane derivatives, since silane groups undergo
hydrolysis and condensation reaction when in contact with water, resulting
in aggregation. In general, monolayers of alkylsilanes may be inherently
more disordered than those of alkanethiols, where molecules have more
freedom to establish a long-range order. For alkylsilanes with more than
one chloride or alkoxy groups, surface polymerization is commonly invoked
deliberately by the addition of moisture,
so
as to form silicon-oxygen-silicon
bonds between adjacent molecules as sketched in Fig.
5.2
1.
Monolayers of organosilicon were studied for applications in enzyme
immobilization as early as in the late
1960~,~~
surface silanization for the
preparation of hydrophobic surfaces for
LB
films.93 This has also been
studied for the preparation of inorganic aerogels under ambient pressure94

210
Nanostructures and Nanomaterials
and in the fabrication of low dielectric constant porous inorganic
rnaterial~.~~
As
will be discussed in the next chapter, the fabrication
of
oxide-metal core-shell nanostructures is heavily relied on the formation of
an organic monolayer linking core and shell materials. For example, in a
typical approach to the fabrication of silica-gold core-shell nanostruc-
tures, organosilicon with amine as a functional group is used to form a
monolayer on the surface of silica nanoparticles by self-assembly. The sur-
face amine groups then attract gold nanoclusters in the solution, which
result in the formation of a gold shell.
One of the ultimate goals of using SA films is the construction of
multilayer films that contain functional groups that possess useful physi-
cal properties in a layer-by-layer fashion. Examples of those functional
groups include electron donor or electron acceptor groups, nonlinear
optical chromophores, moieties with unpaired spins. The construction of
an
SA
multilayer requires that the monolayer surface be modified to be
a hydroxylated surface,
so
that another
SA
monolayer can be formed
through surface condensation. Such hydroxylated surfaces can be pre-
pared by a chemical reaction and the conversion of a nonpolar terminal
group to a hydroxyl group. Examples include a reduction of a surface ester
group, a hydrolysis of a protected surface hydroxy group, and a hydrobo-
ration-oxidation
of
a terminal double bond.96i97 Oxygen plasma etching
followed with immersion in DI-water also effectively makes the surface
hydr~xylated.~~
A
subsequent monolayer is added onto the activated or
hydroxylated monolayer through the same self-assembly procedure and
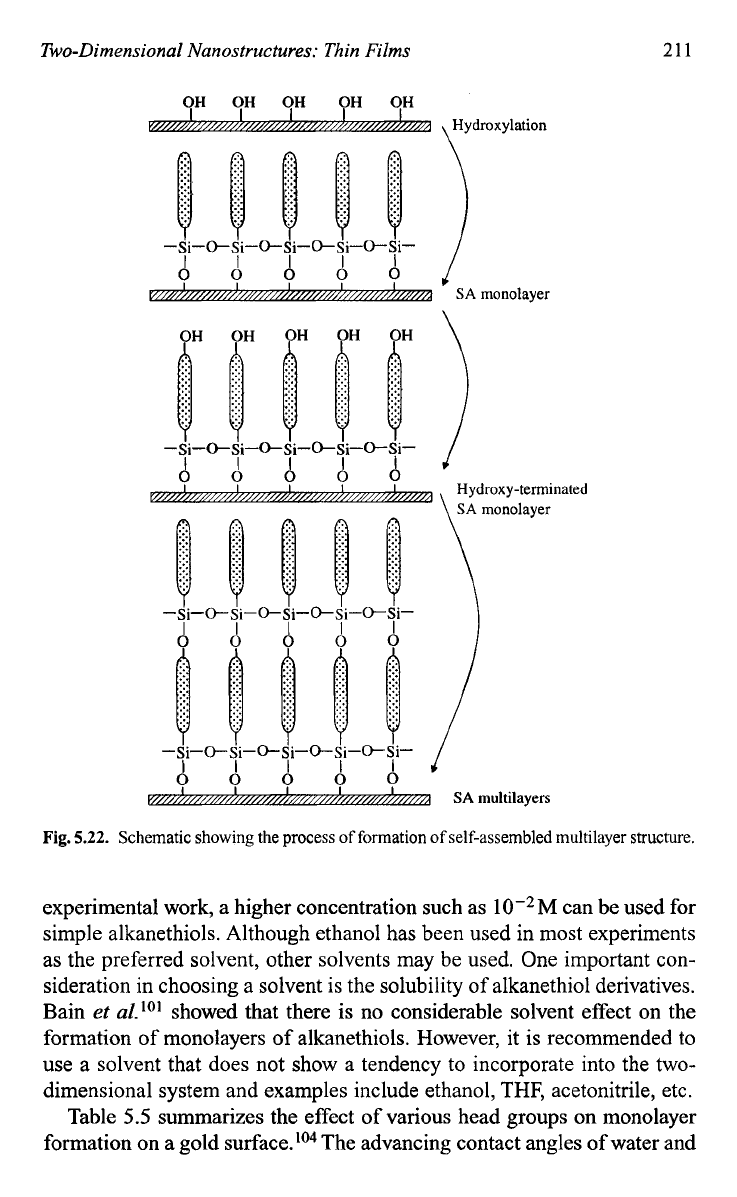
multilayers can be built just by repetition of this process. Figure
5.22
shows such a
SA
multilayer structure. However, it should be noted that in
the construction of multilayers, the quality of monolayers formed by self-
assembly may rapidly degrade as the thickness of the film
increase^.^^.^^
5.8.2.
Monolayers of alkanethiols and sulfides
Monolayers of alkanethiols on gold surfaces are an extensively studied
SA
system since 1983.loo Sulfur compounds can form strong chemical bonds
to go1d,'o'~102 silver,84 copper,
lo3
and platinums6 surfaces. When a fresh,
clean, hydrophilic gold substrate is immersed into a dilute solution (e.g.
10-3M)
of
the organosulfur compound in an organic solvent, a closely
packed and oriented monolayer would form. However, immersion times
vary from a few minutes to a few hours
for
alkanethiols, or as long as sev-
eral days for sulfides and disulfides. For the self-assembly of alkanethiol
monolayers, lov3
M
is a convenient concentration widely used for most
Two-Dimensional Nanostructures: Thin
Films
21 1
OH
OH
OH
OH
OH
-a
,
Hydroxylation
SA
multilayers
Fig.
5.22.
Schematic
showing
the process of
formation of
self-assembled multilayer
structure.
experimental work, a higher concentration such as
lo-*
M
can be used for
simple alkanethiols. Although ethanol has been used in most experiments
as the preferred solvent, other solvents may be used. One important con-
sideration in choosing a solvent is the solubility of alkanethiol derivatives.
Bain
et
dlO1
showed that there is no considerable solvent effect on the
formation of monolayers of alkanethiols. However, it is recommended
to
use a solvent that does not show a tendency
to
incorporate into the two-
dimensional system and examples include ethanol,
THF,
acetonitrile, etc.
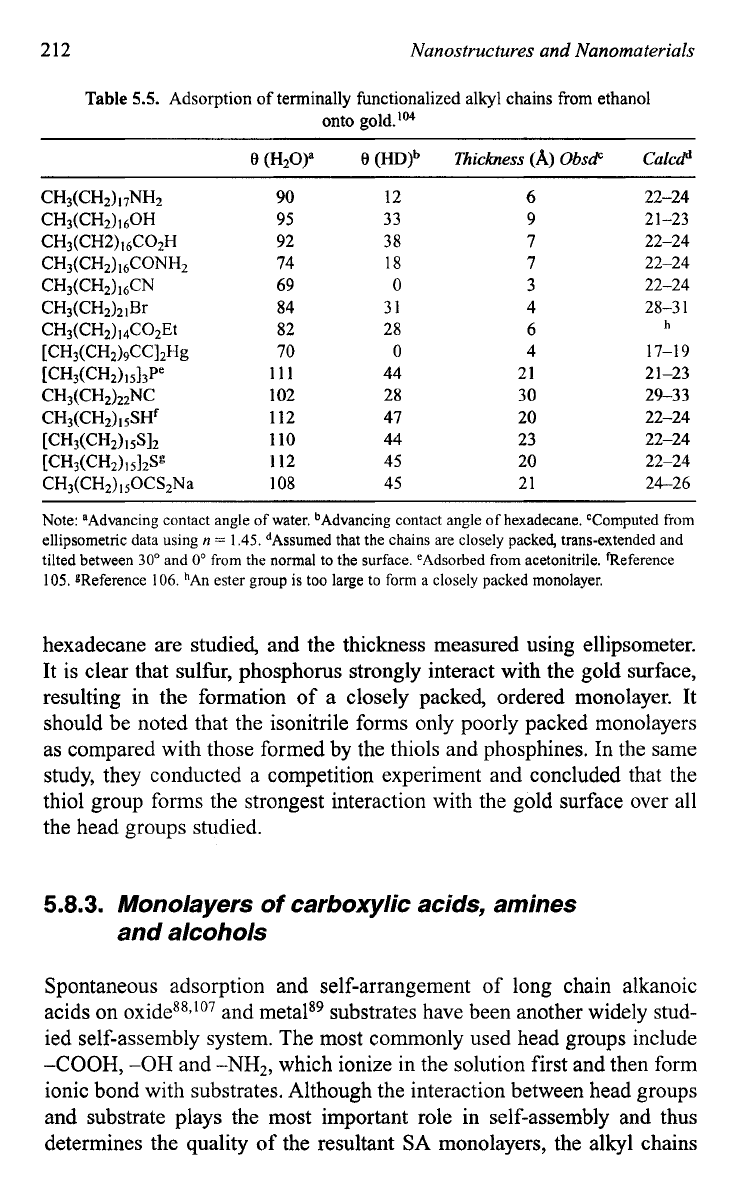
Table
5.5
summarizes the effect of various head groups on monolayer
formation on a gold surface.'"' The advancing contact angles of water and
212
Nanostructures
and
Nanomaterials
Table
5.5.
Adsorption
of
terminally functionalized alkyl
chains
from
ethanol
onto
gold.IW
8
(H20)"
8
(HD)b
Thickness
(A)
Obsdc
Culcdd
CH3(CH2)17NH2
90 12
6
22-24
CH3(CH2)160H
95 33 9 2 1-23
CH3( CH2)
I
6C07.H
92 38
I
22-24
CH3(CH2)16C0NH2
74 18
I
22-24
CH3(CH2)
16CN
69
0
3
22-24
84 31 4 28-3
1
CH3(CH2)2
1
Br
h
CH3(CHZ)14C02Et 82
28
6
[CH3(CH2)9CCl2Hg
70
0
4 17-19
[CH3(CH2)1513Pe
111
44 21 21-23
CH3(CH2)22NC 102 28
30 29-33
CH3(CH2)15SHf
112
47
20 22-24
[CH3(CH2)15Sl2
110
44 23 22-24
[CH3(CH2)
1512s6
112 45 20 22-24
CH3(CH2)150CS2Na
108
45 21 24-26
Note: aAdvancing contact angle of water. bAdvancing contact angle of hexadecane. CComputed from
ellipsometric data using
n
=
1.45.
dAssumed that the chains are closely packed, trans-extended and
tilted between
30"
and
0"
from the normal to the surface. eAdsorbed from acetonitrile. 'Reference
105. gReference 106. hAn ester group
is
too large
to
form a closely packed monolayer.
hexadecane are studied, and the thickness measured using ellipsometer.
It is clear that sulfur, phosphorus strongly interact with the gold surface,
resulting in the formation
of
a closely packed, ordered monolayer. It
should be noted that the isonitrile forms only poorly packed monolayers
as compared with those formed
by
the thiols and phosphines. In the same
study, they conducted a competition experiment and concluded that the
thiol group forms the strongest interaction with the gold surface over all
the head groups studied.
5.8.3.
Monolayers
of
carboxylic acids, amines
and alcohols
Spontaneous adsorption and self-arrangement of long chain alkanoic
acids on and metalg9 substrates have been another widely stud-
ied self-assembly system. The most commonly used head groups include
-COOH,
-OH
and
-NH2,
which ionize in the solution first and then form
ionic bond with substrates. Although the interaction between head groups
and substrate plays the most important role in self-assembly and thus
determines the quality
of
the resultant
SA
monolayers, the alkyl chains

Two-Dimensional Nanostructures: Thin Films
213
also play an important role. In addition to the interchain van der Waals
and electrostatic interactions, alkyl chains may provide space for better
arrangement of head groups resulting in the formation of closely packed
SA monolayers or restrict packing and ordering in the self-assembly,
depending on the molecular structures of alkyl chains.'Os,'10
SA monolayers have been exploited for applications of surface chem-
istry modification, introduction of functional groups on the surface, con-
struction of multilayer structures.
SA
monolayers have also been used
to enhance the adhesion at the
interface^.^^
Various functional groups
can also be incorporated into or partially substitute alkyl chains in surfac-
tant molecules. SA monolayers have also be used in the synthesis and
fabrication of core-shell nanostructures with silane groups linking to
oxides and amines linking to metals.'@
Self-assembly is a wet chemical route to the synthesis of thin films,
mostly organic or inorganic-organic hybrid films. This method is often
used for the surface modification by formation of a single layer of
molecules, which is commonly referred to as self-assembled monolayer
(SAM). This method has also been explored to assemble nanostructured
materials, such as nanoparticles into an ordered macroscale structures,
such as arrays or photonic bandgap crystals. Self-assembly
of
nanoparti-
cles and nanowires is one
of
the topics to be discussed in Chapter
7.
Arguably, all spontaneous growth processes of formation of materials
such as single crystal growth or thin film deposition can be considered as
self-assembly process. In those processes, growth species self-assemble at
low energy sites. Growth species here for self-assembly are commonly
atoms. For more conventional definition
of
self-assembly, the growth
species are commonly molecules. However, nanoparticles or even micron-
sized particles are also used as growth species for self-assembly.
5.9.
Langmuir-Blodgett Films
Langmuir-Blodgett films (LB films) are monolayers and multilayers of
amphiphilic molecules transferred from the liquid-gas interface (commonly
water-air interface) onto a solid substrate and the process is generally
referred to as Langmuir-Blodgett technique (LB technique).'
lo
Langmuir
carried out the first systematic study on monolayers of amphiphilic mole-
cules at the water-air interface and the first study on a deposition of multi-
layers of long-chain carboxylic acid onto a solid substrate was carried out."'
Before discussing in more detail about the
LB
films, let
us
briefly review
what is the amphiphile. The amphiphile is a molecule that is insoluble
in
214
Nanostructures and Nanomaterials
water, with one end that is hydrophilic, and therefore is preferentially
immersed in the water and the other that is hydrophobic and preferentially
resides in the air or in the nonpolar solvent.
A
classical example of an
amphiphile is stearic acid, C17H35C02H. In this molecule, the long hydro-
carbon tail, C17H35
-
is hydrophobic, and the carboxylic acid head group,
-C02H
is
hydrophilic. Since the amphiphiles have one end that is
hydrophilic and the other that is hydrophobic, they like to locate in inter-
faces such as between air and water, or between oil and water. This is the
reason they are also called surfactants. However, it should be noted that the
solubility of an amphiphilic molecule in water depends on the balance
between the alkyl chain length and the strength of its hydrophilic head.
Certain strength of the hydrophilic head is required to form
LB
films. If the
hydrophilicity is too weak, no
LB
film can be formed. However, if the
strength of the hydrophilic head is too strong, the amphiphilic molecule is
too soluble in water to allow the formation
of
a monolayer. Table
5.6
sum-
marizes the properties
of
different head groups.
Il2
The soluble amphiphile
molecules may form micelles in water when their concentration exceeds
their critical micellar concentration, which will be discussed hrther in the
synthesis of ordered mesoporous materials in the next chapter.
The
LB
technique is unique, since monolayers can be transferred to
many different substrates. Most
LB
depositions have involved hydrophilic
substrates where the monolayers are transferred in the retraction mode.113
Glass, quartz and other metal substrates with an oxidized surface are used
as substrate, but silicon wafer with a surface of silicon dioxide is the most
commonly used substrate. Gold is an oxide-free substrate and also com-
monly used to deposit
LB
films. However, gold has a high surface energy
(-1000 mJ/m2) and
is
easily contaminated, which results in an uneven
Table
5.6.
The effect
of
different functional groups
on
LB
film formation
of
C16-compounds.'
l2
hy
weak Weak
Strong
Very
strong
(no
film)
(unstable film)
(stable
LB
film) (soluble)
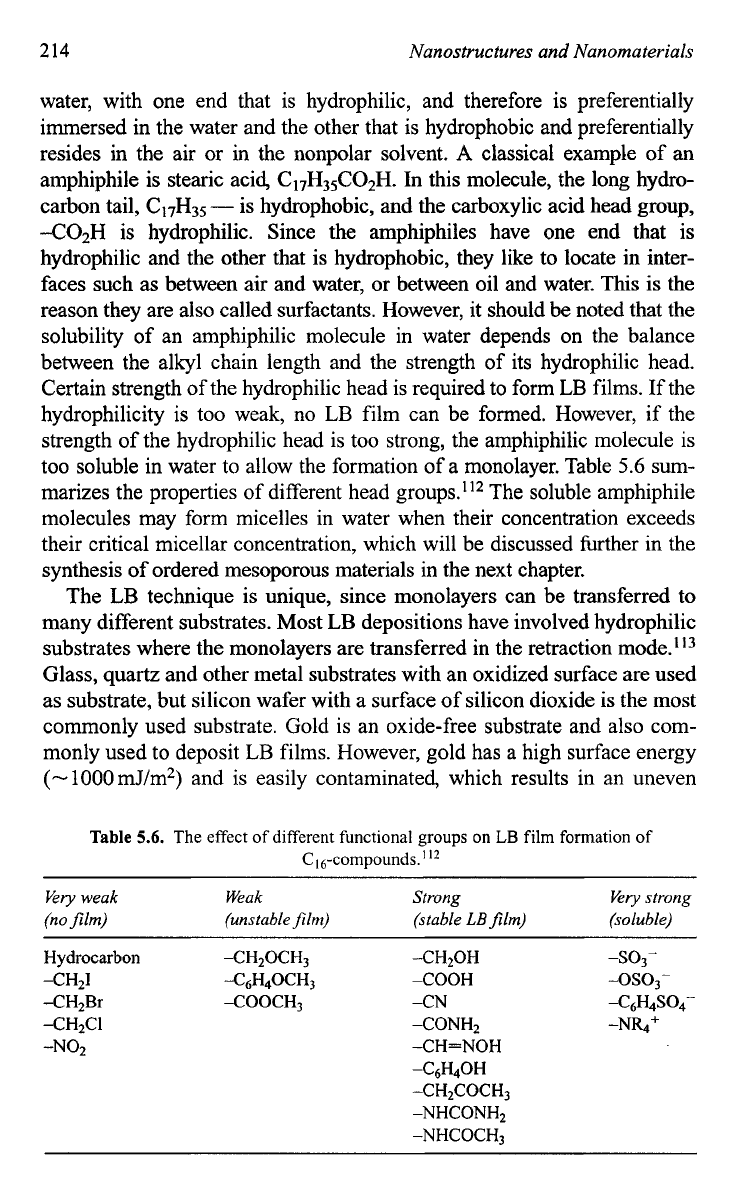
Hydrocarbon XH20CH3 -CH20H
-SO3-
-CH4 -C6H40CH3
-COOH -SO3-
-CH2Br XOOCH3 -CN 46H4s04-
-CH2CI -CONH2 -N%+
-NO2 -CH=NOH
-C&OH
-CH2COCH3
-NHCONHp
-NHCOCH3
Two-Dimensional Nanostructures: Thin Films
215
64
\
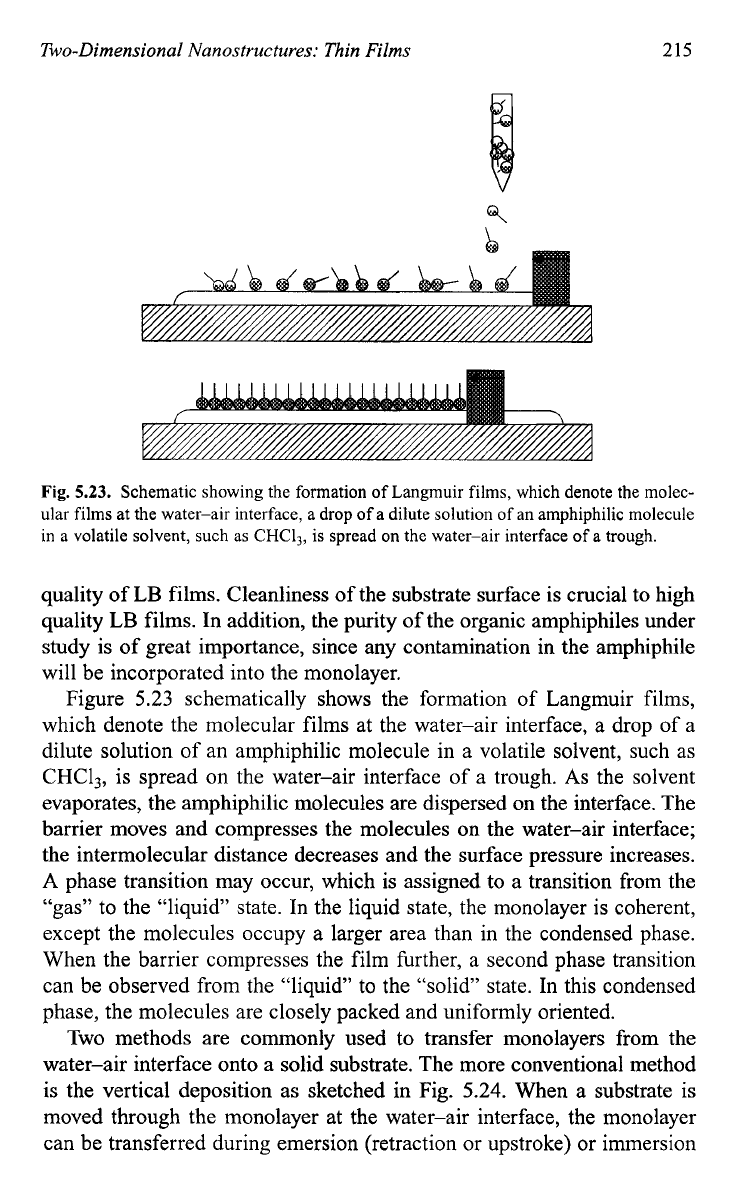
Fig.
5.23.
Schematic showing the formation of Langmuir films, which denote the molec-
ular films at the water-air interface,
a
drop of a dilute solution of an amphiphilic molecule
in
a
volatile solvent, such as
CHC13,
is spread
on
the water-air interface of
a
trough.
quality of LB films. Cleanliness of the substrate surface is crucial to high
quality LB films. In addition, the purity of the organic amphiphiles under
study is of great importance, since any contamination in the amphiphile
will be incorporated into the monolayer.
Figure 5.23 schematically shows the formation of Langmuir films,
which denote the molecular films at the water-air interface, a drop of a
dilute solution of an amphiphilic molecule in a volatile solvent, such as
CHC13, is spread on the water-air interface of a trough.
As
the solvent
evaporates, the amphiphilic molecules are dispersed on the interface. The
barrier moves and compresses the molecules on the water-air interface;
the intermolecular distance decreases and the surface pressure increases.
A phase transition may occur, which
is
assigned to a transition from the
“gas” to the “liquid” state. In the liquid state, the monolayer is coherent,
except the molecules occupy a larger area than in the condensed phase.
When the barrier compresses the film further, a second phase transition
can be observed from the “liquid” to the “solid” state. In this condensed
phase, the molecules are closely packed and uniformly oriented.
Two methods are commonly used to transfer monolayers from the
water-air interface onto a solid substrate. The more conventional method
is the vertical deposition as sketched in Fig. 5.24. When a substrate is
moved through the monolayer at the water-air interface, the monolayer
can be transferred during emersion (retraction or upstroke) or immersion
