Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

570 Charged Particle and Photon Interactions with Matter
(2) The yields of base lesions and clustered damage sites including base lesions have been revealed
using enzymatic probes of BER proteins for low-LET irradiation. (3) Higher-LET irradiation, how-
ever, induces more complicated damage, which cannot be recognized by BER enzymes. (4) Thus,
some of the complex damage sites, which consist of two or more base lesions, are more poorly
repaired by BER, and, ultimately, induce mutagenesis in living cells. (5) Oxygen K-ionization in
DNA (and, presumably, water hydrated to DNA) plays an important role in inducing base lesions.
(6) Finally, a new water beam technique combined with synchrotron radiation has been developed
to
explore the electric properties of DNA in water.
reFerenCes
Agrawala, P. K., Eschenbrenner, A., Hervè Du Penhoat, M. A., Boissière, A., Eot-Houllier, G., Abel, F., Politis,
M. F., Touati, A., Sage, E., and Chetioui, A. 2008. Induction and repairability of DNA damage caused by
ultrasoft
X-rays: Role of core events. Int. J. Radiat. Biol. 84: 1093–1103.
Ahnstrom,
G. and Bryant, P. E. 1982. DNA double-strand breaks generated by the repair of X-ray damage in
Chinese
hamster cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 41: 671–676.
Akamatsu,
K., Fujii, K., andYokoya, A. 2004a. Low-energy Auger- and photo-electron effects on the degrada-
tion
of thymine by ultrasoft X-irradiation. Int. J. Radiat. Biol. 80: 849–853.
Akamatsu,
K., Fujii, K., and Yokoya, A. 2004b. Qualitative and quantitative analyses of the decomposition
products that arise from the exposure of thymine to monochromatic ultrasoft X rays and 60Co gamma
rays
in the solid state. Radiat. Res. 162: 469–473.
Becker,
D. and Sevilla, M. D. 1993. The chemical consequences of radiation damage to DNA. Adv. Radiat.
Biol.
17: 121–180.
Becker,
D., La Vere, T., and Sevilla, M. D. 1994. ESR detection at 77K of the hydroxyl radical in the hydration
layer
of gamma-irradiated DNA. Radiat. Res. 140: 123–129.
Becker,
D., Bryant-Friedrich, A., Trzasko, C., and Sevilla, M. D. 2003, Electron spin resonance study of DNA
irradiated with an argon-ion beam: evidence for formation of sugar phosphate backbone radicals. Radiat.
Res.
160: 174–185.
Bellon,
S., Shikazono, N., Cunniffe, S., Lomax, M., and O’Neill, P. 2009. Processing of thymine glycol in a
clustered
DNA damage site: Mutagenic or cytotoxic. Nucl. Acids Res. 37: 4430–4440.
Berkowitz,
J. 2002. Atomic Molecular Photoabsorption: Absolute Total Cross Sections, J. Berkowitz (ed.)
(Argonne
National Laboratory). San Diego, CA:
Academic
Press.
Bernhard,
W. A. and Close, D. M. 2004. DNA damage dictates the biological consequence of ionizing radiation:
The chemical pathways. In Charged Particle Interactions with Matter: Chemical, Physicochemical,
and Biological Consequences with Applications. A. Mozumder and Y. Hatano (eds.), pp. 471–489.
NewYork: Marcel Dekker.
Blaisdell, J. O. and Wallace, S. S. 2001. Abortive base-excision repair of radiation-induced clustered DNA
lesions
in Escherichia coli. Proc. Natl. Acad. Sci. USA 98: 7426–7430.
Boiteux,
S., Gajewski, E., Laval, J., and Dizdaroglu, M. 1992. Substrate specicity of the Escherichia coli Fpg
protein (formamidopyrimidine-DNA glycosylase): Excision of purine lesions in DNA produced by ionizing
radiation
or photosensitization. Biochemistry 31: 106–110.
Boudaïffa,
B., Cloutier, P., Hunting, D., Huels, M. A., and Sanche, L. 2000. Resonant formation of DNA strand
breaks
by low-energy (3 to 20
eV)
electrons. Science 287: 1658–1660.
Bowman,
M. K., Becker, D., Sevilla, M. D., and Zimbrick, J. D. 2005. Track structure in DNA irradiated with
heavy
ions. Radiat. Res. 163: 447–454.
Breimer,
L. H. and Lindahl, T. 1984. DNA glycosylase activities for thymine residues damaged by ring satu-
ration, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J. Biol.
Chem.
259: 5543–5548.
Chang,
P. W., Zhang, Q. M., Takatori, K., Tachibana, A., and Yonei, S. 2005. Increased sensitivity to sparsely
ionizing radiation due to excessive base excision in clustered DNA damage sites in Escherichia coli. Int.
J. Radiat. Biol.
81: 115–123.
Chetsanga,
C. J. and Lindahl, T. 1979. Release of 7-methylguanine residues whose imidazole rings have
been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucl. Acids Res. 6:
3673–3684.
Cunniffe, S. M., Lomax, M. E., and O’Neill, P. 2007. An AP site can protect against the mutagenic potential
of
8-oxoG’ when present within a tandem clustered site in E. coli. DNA Repair (Amst.) 6: 1839–1849.
Spectroscopic Study of Radiation-Induced DNA Lesions 571
de Lara, C. M., Jenner, T. J., Townsend, K. M., Marsden, S. J., and O’Neill, P. 1995. The effect of dimethyl
sulfoxide on the induction of DNA double-strand breaks in V79-4 mammalian cells by alpha particles.
Radiat. Res.
144: 43–49.
de
Lara, C. M., Hill, M. A., Jenner, T. J., Papworth, D., and O’Neill, P., 2001. Dependence of the yield of DNA
double-strand breaks in Chinese hamster V79-4 cells on the photon energy of ultrasoft X-rays. Radiat.
Res.
155: 440–448.
Demple, B. and Linn, S. 1980. DNA N-glycosylases and UV repair. Nature 287: 203–208.
Dianov,
G. L., Timchenko, T. V., Sinitsina, O. I., Kuzminov, A. V., Medvedev, O. A., and Salganik, R. I. 1991.
Repair of uracil residues closely spaced on the opposite strands of plasmid DNA results in double-strand
break
and deletion formation. Mol. Gen. Genet. 225: 448–452.
Dizdaroglu,
M., Laval, J., and Boiteux, S. 1993. Substrate specicity of the Escherichia coli endonuclease III:
Excision of thymine- and cytosine-derived lesions in DNA produced by radiation-generated free radicals.
Biochemistry
32: 12105–12111.
D’Souza,
D. I. and Harrison, L. 2003. Repair of clustered uracil DNA damages in Escherichia coli. Nucl. Acids
Res.
31: 4573–4581.
Dugle,
D. L., Gillespie, C. J., and Chapman, J. D. 1976. DNA strand breaks, repair, and survival in x-irradiated
mammalian
cells. Proc. Natl. Acad. Sci. USA 73: 809–812.
Eschenbrenner,
A., Hervè Du Penhoat, M. A., Boissière,A., Eot-Houllier, G., Abel, F., Politis, M. F., Touati, A.,
Sage, E., and Chetioui, A. 2007. Strand breaks induced in plasmid DNA by ultrasoft X-rays: Inuence of
hydration
and packing. Int. J. Radiat. Biol. 83: 687–697.
Faubel,
M. and Kisters, T. 1989. Non-equilibrium molecular evaporation of carboxylic acid dimmers. Nature
339:
527–529.
Faubel,
M. and Steiner B. 1992. Strong bipolar electrokinetic charging of thin liquid jets emerging from 10μm
PtIr
nozzles. Ber. Bunsenges. Phys. Chem. 96: 1167–1172.
Faubel,
M., Shlemmer, S., and Toennies, J. P. 1988. A molecular beam study of the evaporation of water from
a
liquid jet. Z. Phys. D 10: 269–277.
Faubel,
M., Steiner, B., and Toeneies, J. P. 1997. Photoelectron spectroscopy of liquid water, some alcohols,
and
pure nonane in free micro jet. J. Chem. Phys. 106: 9013–9031.
Faubel,
M., Steiner, B., and Toeneies J. P. 1999. Measurements of He I photoelectron spectra of liquid water, for-
mamide and ethylene glycol in fast-owing microjets. J. Electron Spectrosc. Rel. Phenom. 95: 159–169.
Fayard, B., Touati, A., Abel, F., Herve du Penhoat, M. A., Despiney-Bailly, I., Gobert, F., Ricoul, M., L’hoir, A.,
Polities, M. F., Hill, M. A., Stevens, D. L., Sabatier, L., Sage, E., Goodhead, D. T., and Chetioui, A. 2002.
Cell inactivation and double-strand breaks: The role of core ionization, as probed by ultrasoft X rays.
Radiat. Res.
157: 128–140.
Fuchs, H. and Legge, H. 1979. Flow of a water jet into vacuum. Acta Astronaut. 6: 1213–1226.
Fujii,
K., Akamatsu, K., and Yokoya, A. 2004a. Ion desorption from DNA components irradiated with 0.5keV
ultrasoft
X-ray photons. Radiat Res. 161: 435–441.
Fujii,
K., Akamatsu, K., and Yokoya, A. 2004b. Near-edge x-ray absorption ne structure of DNA nucleobases
thin
lm in the nitrogen and oxygen K-edge region. J. Phys. Chem. B 108: 8031–8035.
Fujii,
K., Shikazono, N., and Yokoya, A. 2009. Nucleobase lesions and strand-breaks in dry DNA thin lm
selectively
induced by monochromatic soft X-rays. J. Phys. Chem. 113: 16007–16015.
Fulford,
J. 2000. Quantication of complex DNA damage by ionising radiation: An experimental and theoreti-
cal
approach. PhD thesis, University of Brunel, London, U.K.
Fulford,
J., Nikjoo, H., Goodhead, D. T., and O’Neill, P. 2001. Yields of SSB and DSB induced in DNA by
AlK X-rays and α-particles: Comparison of experimental and simulated yields. Int. J. Radiat. Biol. 77:
1053–1066.
Georgakilas, A. G., Bennett, P. V., Wilson III D. M., and Sutherland, B. M. 2004. Processing of bistra-
nded abasic DNA clusters in gamma-irradiated human hematopoietic cells. Nucl. Acids Res. 32:
5609–5620.
Goodhead, D. T. 1994a. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA.
Int. J. Radiat. Biol.
65: 7–17.
Goodhead,
D. T. 1994b. In Synchrotron Radiation in the Biosciences, B. Chance, J. Deisenhofer, S. Ebashi, D.
T. Goodhead, J. R. Helliwell, H. E. Huxley, T. Iizuka etal. (eds.), pp. 683–705. Oxford, U.K.: Clarendon
Press.
Goodhead, D. T., Thacker, J., and Cox, R. 1981. Is selective absorption of ultrasoft x-rays biologically impor-
tant
in mammalian cells? Phys. Med. Biol. 26: 1115–1127.
Gulston,
M., de Lara, C., Jenner, T., Davis, E., and O’Neill, P. 2004. Processing of clustered DNA damage gener-
ates additional double-strand breaks in mammalian cells post-irradiation. Nucl. Acids Res. 32: 1602–1609.
572 Charged Particle and Photon Interactions with Matter
Hada, M. and Sutherland, B. M. 2006. Spectrum of complex DNA damages depends on the incident radiation.
Radiat Res.
165: 223–230.
Hanai,
R., Yazu, M., and Hieda, K. 1998. On the experimental distinction between SSB and DSB in circular
DNA.
Int. J. Radiat. Biol. 73: 457–479.
Harrison,
L., Brame, K. L., Geltz, L. E., and Landry, A. M. 2006. Closely opposed apurinic/apyrimidinic sites
are converted to double strand breaks in Escherichia coli even in the absence of exonuclease III, endo-
nuclease
IV, nucleotide excision repair and
AP
lyase cleavage. DNA Repair (Amst.) 5: 324–335.
Hatahet,
Z., Kow, Y. W., Purmal, A. A., Cunningham, R. P., and Wallace, S. S. 1994. New substrates for old
enzymes. 5-Hydroxy-2’-deoxycytidine and 5-hydroxy-2’-deoxyuridine are substrates for Escherichia
coli endonuclease III and formamidopyrimidine DNA N-glycosylase, while 5-hydroxy-2’-deoxyuridine
is
a substrate for uracil DNA N-glycosylase. J. Biol. Chem. 269: 18814–18820.
Hieda,
K. and Ito, T. 1991. Radiobiological experiments in the X-ray region with synchrotron radiation. In:
Handbook on Synchrotron Radiation, Vol. 4, S. Ebashi, M. Koch, and E. Rubenstein (eds.), p. 431.
Amsterdam,
the Netherlands: North-Holland.
International
Commission on Radiation Units and Measurements. 1970. Linear Energy Transfer. ICRU Report
16. Washington,
DC.
Ito,
T. and Saito, M. 1988. Degradation of oligonucleotides by vacuum-UV radiation in solid: role of the phosphate
group and bases. Photochem. Photobiol. 48: 567–572.
Kashtanov, S., Augustsson, A., Luo, Y., Guo, J. H., Såthe, C., Rubensson, J. E., Siegbahn, H., Nordgren, J., and
Ågren, H. 2004. Local structure of liquid water studied by x-ray emission spectroscopy. Phys. Rev. B 69:
024201-1–024201-8.
Kozmin, S. G., Sedletska, Y., Reynaud-Angelin, A., Gasparutto, D., and Sage, E. 2009. The formation of dou-
ble-strand breaks at multiply damaged sites is driven by the kinetics of excision/incision at base damage
in
eukaryotic cells. Nucl. Acids Res. 37: 1767–1777.
Krisch,
R. E., Flick, M. B., and
Trumbore,
C. N. 1991. Radiation chemical mechanism of single- and double-
strand
break formation in irradiated SV40 DNA. Radiat. Res. 126: 251–259.
La
Vere, T., Becker, D., and Sevilla, M. D. 1996.Yields of OH in gamma-irradiated DNA as a function of DNA
hydration:
Hole transfer in competition with OH formation. Radiat. Res. 145: 673–680.
Malyarchuk,
S., Youngblood, R., Landry, A. M., Quillin, E., and Harrison, L. 2003. The mutation frequency
of 8-oxo-7,8-dihydroguanine (8-oxodG) situated in a multiply damaged site: comparison of a single and
two
closely opposed 8-oxodG in Escherichia coli. DNA Repair (Amst.) 2: 695–705.
Malyarchuk,
S., Brame, K. L., Youngblood, R., Shi, R., and Harrison, L. 2004. Two clustered 8-oxo-7,8-
dihydroguanine (8-oxodG) lesions increase the point mutation frequency of 8-oxodG, but do not result in
double
strand breaks or deletions in Escherichia coli. Nucl. Acids Res. 32: 5721–5731.
Malyarchuk,
S., Castore, R., and Harrison, L. 2008. DNA repair of clustered lesions in mammalian cells:
Involvement
of non-homologous end-joining. Nucl. Acids Res. 36: 4872–4882.
Melvin,
T., Cunniffe, S. M., O’Neill, P., Parker, A. W., and Roldan-Arjona, T. 1998. Guanine is the target for
direct
ionisation damage in DNA, as detected using excision enzymes. Nucl. Acid Res. 26: 4935–4942.
Milligan,
J. R.,Aguilera, J.A., Nguyen, T. T., Paglinawan, R. A., and Ward, J. F. 2000. DNA strand-break yields
after post-irradiation incubation with base excision repair endonucleases implicate hydroxyl radical pairs
in
double-strand break formation. Int. J. Radiat. Biol. 76: 1475–1483.
Morgner,
H. 1998. Electron spectroscopy for the determination of concentration depth profoles and for the
investigation
of local electric elds. Surf. Investig. 13: 463–474.
Nikjoo,
H., Charlton, D. E., and Goodhead, D. T. 1994. Monte Carlo track structure studies of energy deposi-
tion
and calculation of initial DSB and RBE. Adv. Space Res. 14: (10)161–(10)180.
Nikjoo,
H., O’Neill, P., Goodhead, D.
T.,
and
Terrissol,
M. 1997. Computational modelling of low-energy
electron-induced DNA damage by early physical and chemical events. Int. J. Radiat. Biol. 71:
467–483.
Nikjoo, H., Uehara, S., Wilson, W. E., Hoshi, M., and Goodhead, D. T. 1998. Track structure in radiation biol-
ogy: Theory
and applications. Int. J. Radiat. Biol. 73: 355–364.
Nikjoo,
H., O’Neill, P., Terrissol, M., and Goodhead, D. T. 1999. Quantitative modelling of DNA damage using
Monte
Carlo track structure method. Radiat. Environ. Biophys. 38: 31–38.
Nikjoo,
H., O’Neill, P., Wilson, W. E., and Goodhead, D. T. 2001. Computational approach for determining the
spectrum
of DNA damage induced by ionizing radiation. Radiat. Res. 156: 577–583.
Nikjoo,
H., Bolton, C. E., Watanabe, R., Terrisol, M., O’Neill, P., and Goodhead, D. T. 2002. Modelling of
DNA
damage induced by energetic electrons (100
eV
to 100
keV).
Radiat. Prot. Dosim. 99: 77–80.
O’Neill,
P. 2001. Radiation-induced damage in DNA. In Radiation Chemistry, pp. 585–622. Dordrecht,
the Netherlands: Elsevier Science.
Spectroscopic Study of Radiation-Induced DNA Lesions 573
O’Neill,
P. and Fielden, E. M. 1993. Primary free radical processes in DNA. Adv. Radiat. Biol. 17: 53–120.
Ormerod,
M. G. 1965. Free-radical formation in irradiated deoxyribonucleic acid. Int. J. Radiat. Biol. Relat.
Stud. Phys. Chem. Med.
9: 291–300.
Pearson,
C. G., Shikazono, N.,
Thacker,
J., and O’Neill, P. 2004. Enhanced mutagenic potential of 8-oxo-7,8-
dihydroguanine
when present within a clustered DNA damage site. Nucl. Acids Res. 32: 263–270.
Prise,
K. M., Pullar, C. H., and Michael, B. D. 1999. A study of endonuclease III-sensitive sites in irradiated
DNA:
Detection of alpha-particle-induced oxidative damage. Carcinogenesis 20: 905–909.
Purkayastha,
S., Milligan, J. R., and Bernhard, W. A. 2006. The role of hydration in the distribution of free
radical
trapping in directly ionized DNA. Radiat. Res. 166: 1–8.
Saitoh,
Y., Nakatani, T., Matsushita, T., Agui, A., Teraoka, Y., and Yokoya, A. 2001. First results from the
actinide
science beamline BL23SU at SPring-8. Nucl. Instrum. Methods A 474: 253–258.
Shikazono,
N. and O’Neil, P. 2009. Biological consequences of potential repair intermediates of clustered base
damage
site in Escherichia coli. Mutat. Res. 669: 162–168.
Shikazono,
N., Pearson, C., O’Neill, P., and Thacker, J. 2006. The roles of specic glycosylases in determining
the
mutagenic consequences of clustered DNA base damage. Nucl. Acids Res. 34: 3722–3730.
Siegbahn, K. 1974. Electron spectroscopy —
An
outlook. J. Electron Spectrosc. Relat. Phenom. 5: 3–97.
Sutherland,
B. M., Bennett, P.V., Sidorkina, O., and Laval, J. 2000. Clustered DNA damages induced in isolated
DNA
and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 97: 103–108.
Tao,
N. J., Lindsay, S. M., and Rupprecht, A. 1989. Structure of DNA hydration shells studied by Raman spec-
troscopy.
Biopolymers 28: 1019–1030.
Taucher-Scholz,
G. and Kraft, G. 1999. Inuence of radiation quality on the yield of DNA strand breaks in
SV40
DNA irradiated in solution. Radiat. Res. 151: 595–604.
Tchou,
J., Kasai, H., Shibutani, S., Chung, M. H., Laval, J., Grollman, A. P., and Nishimura, S. 1991.
8-Oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specicity. Proc. Natl. Acad. Sci.
USA
88: 4690–4694.
Ukai,
M., Yokoya, A., Fujii, K., and Saitoh, Y. 2008. X-ray absorption spectrum for guanosine-5’-monophos-
phate in water solution in the vicinity of the nitrogen K-edge observed in free liquid jet in vacuum.
Radiat. Phys. Chem.
77: 1265–1269.
Ukai,
M., Yokoya, A., Nonaka, Y., Fujii, K., and Saitoh, Y. 2009. Synchrotron radiation photoelectron studies
for primary radiation effects using a liquid water jet in vacuum: total and partial photoelectron yields for
liquid
water near the oxygen K-edge. Radiat. Phys. Chem. 78: 1202–1206.
Urushibara,
A., Shikazono, N., O’Neill, P., Fujii, K., Wada, S., and Yokoya, A. 2008. LET dependence of the
yield of single-, double-strand breaks and base lesions in fully hydrated plasmid DNA lms by
4
He
2+
ion
irradiation.
Int. J. Radiat. Biol. 84: 23–33.
von
Sonntag, C. 1987. The Chemical Basis of Radiation Biology. London, U.K.:
Taylor
& Francis.
Ward,
J. F. 1988. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of
formation,
and reparability. Prog. Nucl. Acid Res. Mol. Biol. 35: 95–125.
Watanabe,
R., Yokoya, A., Fujii, K., Saito, K. 2004. DNA strand breaks by direct energy deposition by Auger
and photo-electrons ejected from DNA constituent atoms following K-shell photoabsorption. Int. J.
Radiat. Biol.
80: 823–832.
Wernet,
Ph., Nordlund, D., Bergmann, U., Cavalleri, M., Odelius, M., Ogasawara, H., and Näslund, L. Å. 2004.
The
structure of the rst coordination shell in liquid water. Science 304: 995–999.
Wilson,
K. R., Rude, B. S., Smith, J., Cappa, C., Co, D. T., Schaller, R. D., Larsson, M., Catalano, T., and
Saykally, R. J. 2004. Investigation of volatile liquid surfaces by synchrotron x-ray spectroscopy of liquid
microjet.
Rev. Sci. Instrum. 75: 725–736.
Yamashita,
S., Katsumura,Y., Lin, M., Muroya,Y., Miyazaki, T., Murakami, T., Meesungnoen, J., and Jay-Gerin,
J. P. 2008. Water radiolysis with heavy ions of energies up to 28GeV. 3. Measurement of G(MV
*+
) in
deaerated methyl viologen solutions containing various concentrations of sodium formate and Monte
Carlo
simulation. Radiat. Res. 170: 521–533.
Yang,
N., Galick, H., and Wallace, S. S. 2004. Attempted base excision repair of ionizing radiation damage in
human lymphoblastoid cells produces lethal and mutagenic double strand breaks. DNA Repair (Amst.)
3:
1323–1334.
Yang,
N., Chaudhry, M. A., and Wallace, S. S. 2006. Base excision repair by hNTH1 and hOGG1: A two edged
sword in the processing of DNA damage in gamma-irradiated human cells. DNA Repair (Amst.) 5: 43–51.
Yeh, J. J. and Lindau, I. 1985. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤
Z
≤ 103. At. Data Nucl. Data Tables 32: 1–155.
Yokoya,
A., Watanabe, R., and Hara, T. 1999. Single- and double-strand breaks in solid pBR322 DNA induced
by
ultrasoft x-rays at photon energies at 388, 435 and 573
eV.
J. Radiat. Res. 40: 145–158.
574 Charged Particle and Photon Interactions with Matter
Yokoya, A., Cunniffe, C. M. T., and O’Neill, P. 2002. Effect of hydration on the induction of strand breaks
and
nucleobase lesions in plasmid DNA lms by gamma-radiation. J. Am. Chem. Soc. 124: 8859–8866.
Yokoya,
A., Cunniffe, C. M. T., Stevens, D. L., and O’Neill, P. 2003. Effect of hydration on the induction of
strand breaks, nucleobase lesions and clustered damage in DNA lms by α-radiation. J. Phys. Chem. B
124:
832–837.
Yokoya,
A., Fujii, K., Ushigome, T., Shikazono, N., Urushibara, A., and Watanabe, R. 2006. Yields of soft
X-ray
induced strand breaks and nucleobase lesions in plasmid DNA. Radiat. Prot. Dosim. 122: 86–88.
Yokoya,
A., Fuji, K., Shikazono, N., Akamatsu, K., Urushibara, A., and Watanabe, R. 2008a. Studies of soft
X-ray-induced Auger
effect on the induction of DNA damage. Int. J. Radiat. Biol. 84: 1069–1081.
Yokoya,
A., Shikazono, N., Fujii, K., Urushibara, A., Akamatsu, K., and Watanabe, R. 2008b. DNA damage
induced
by the direct effect of radiation, J. Radiat. Phys. Chem. 77: 1280–1285.
Yokoya,
A., Cunniffe, S. M. T., Watanabe, R., Kobayashi, K., and O’Neill, P. 2009a. Induction of DNA strand
breaks, nucleobase lesions, and clustered damage sites in hydrated plasmid DNA lms by ultrasoft x-rays
around
the phosphorus K-edge. Radiat. Res. 172: 296–305.
Yokoya,
A., Fujii, K., Fukuda, Y., and Ukai, M. 2009b. EPR study of radiation damage to DNA irradiated with
synchrotron
Soft X-rays around nitrogen and oxygen K-edge. Radiat. Phys. Chem. 78: 1211–1215.

575
21
Application of Microbeams
to
the Study of the Biological
Effects
of Low Dose Irradiation
Kevin M. Prise
Queen’s University Belfast
Belfast,
United Kingdom
Giuseppe Schettino
Queen’s University Belfast
Belfast,
United Kingdom
21.1 introduCtion
Humans are continually exposed to ionizing radiation from a range of sources including environ-
mental and occupational sources. The doses from these various sources are very low and generally
of low dose rates. At the level of individual cells within the human body, this can equate to only
Contents
21.1 Introduction .......................................................................................................................... 575
21.2 Low
Dose Radiation Effects and Radiation Risk................................................................. 576
21.3
Introduction
to Microbeams ................................................................................................. 576
21.4
History,
Rationale, and Key Aspects.................................................................................... 577
21.5
Charged-Particle
Microbeams..............................................................................................580
21.5.1
Microbeam Production.............................................................................................580
21.5.2 Beam
Detection ........................................................................................................ 581
21.5.3
Beam
Orientation and Sample Holder...................................................................... 582
21.5.4
Optics
and Sample Preparation ................................................................................ 583
21.5.5
Positioning
Stage ......................................................................................................584
21.6
X-Ray Microbeams...............................................................................................................584
21.6.1 X-Ray
Source............................................................................................................ 585
21.6.2
X-Ray
Optics ............................................................................................................ 587
21.6.3
X-Ray
Detection and Dosimetry ..............................................................................588
21.7
Electron
Microbeams ...........................................................................................................588
21.8
Future Developments............................................................................................................ 589
21.8.1 Beam
Spot Scanning ................................................................................................ 589
21.8.2
Non-Staining
Imaging ..............................................................................................590
21.8.3
Hard
X-Ray Optics ...................................................................................................590
21.8.4
Biological
Advances ................................................................................................. 591
21.9
Summary .............................................................................................................................. 591
Acknowledgments..........................................................................................................................592
References...................................................................................................................................... 592
576 Charged Particle and Photon Interactions with Matter
single tracks of radiation crossing a cell over periods of weeks or years. Our understanding of
the effects of these low doses requires technological approaches that can deliver highly localized
radiation beams into biological models. This chapter reviews the use of novel microbeam technologies
that
allow relevant doses to be tested in biological systems. We outline some of the key technologi-
cal
approaches used to produce microbeams using different types of ionizing radiations and give
examples of some of the biological results that have been obtained with them.
21.2 low dose radiation eFFeCts and radiation risk
Humans are exposed to multiple physical, chemical, and biological agents during their lifetime.
Of these, ionizing radiation(s) has long been known to be deleterious after high dose exposure
(>100mSv), predominantly due to cancer induction; although very high dose exposures cause tissue
damage and ultimately death (see Hall, (2000) for a general textbook). Ionizing radiations are widely
used in society, play a key role in the treatment of cancer, and are an important diagnostic tool.
Despite a century of study, risk estimates for cancer induction in humans to be used for radiation
protection purposes are extrapolated from the Japanese atomic bomb survivors, who were exposed
to both relatively high doses and dose rates. Around 120,000 survivors have been followed up to the
present date; 20,000 of these had received doses of greater than 5 mSv. By 1990, around 6000 deaths
resulted from cancer, of which ∼400 were considered to be deaths due to excess radiation. Other
exposed populations have been studied, including those treated with radiation for various medical
conditions such as ankylosing spondylitis, conditions related to leukemia risk, and other tumors.
Several studies of radiation workers have been undertaken as these populations were exposed to
protracted low-dose exposures (Cardis etal., 1995; Muirhead etal., 1999). From these epidemio-
logical data, a simple extrapolation of risk has been made to low doses generally found in envi-
ronmental and most occupational exposures. This has been called the linear non-threshold model
(LNT), which assumes a linear dose–response relationship between dose and risk. Currently, with
the exception of radiotherapy, the doses that members of the population can be typically exposed to
are lower than the doses received by the bomb survivors and are therefore areas where epidemio-
logical data is scarce. Against a typical background dose of around 3 mSv/year, examples of routine
medical exposures include 3mSv for a breast mammogram and 0.7mSv for a dental x-ray (Brenner
etal., 2003). From this background, a range of experimental approaches have been taken to study
the effects of low dose and low dose rates of radiation exposure and to test the assumptions of the
LNT model. Ionizing radiations are typically compared on the basis of their linear energy transfer
(LET). Sparsely ionizing radiations such as γ-rays and x-rays are classied as low LET and densely
ionizing radiations such as alpha-particles and neutrons are classied as high LET. For low-LET
radiations, a single radiation track will deposit around 1mGy in a human cell. A novel approach has
been the use of microbeams that allow radiation to be precisely targeted to a particular biological
target of interest. They have contributed enormously to our understanding of the effects of low dose
radiation
exposure and this will be the subject of the rest of this chapter.
21.3 introduCtion to miCrobeams
Charged-particle microbeams have been used since the 1960s for quantitative elemental analysis
of geological, historical, and biological samples (Watt and Grime, 1987) where two-dimensional
elemental maps can be obtained by scanning a small ion beam across a sample and monitoring
the x-rays produced by the sample elements (Watt etal., 1982). However, it was only toward the
end of the 1990s that microbeams have attracted the interest of the radiobiology community and
been developed into specic tools to investigate the effect of ionizing radiation on living samples.
Modern “radiobiological microbeams” are instruments capable of delivering accurate predeter-
mined doses of ionizing radiation to individual cells (or parts of cells) and which can assess the
damage induced on a cell-by-cell basis. The advantages of a deterministic irradiation achieved by
Application of Microbeams to the Study of the Biological Effects of Low Dose Irradiation 577
targeting and analyzing cells individually have been recognized since the beginning of radiobio-
logical studies. Using basic setups, Zirkle and colleagues (Zirkle and Bloom, 1953) tried to correlate
radiation-induced cell damage to the type and energy of radiation, the number of ions per cell, and
even the subcellular compartment irradiated. Despite the limited control and precision offered by
their polonium-tipped syringe needle, important observations were made regarding the nuclear and
cytoplasmic sensitivity, strengthening the hypothesis that considerable benet could be achieved
with a deterministic irradiation approach. However, it was only in the last decade of the twenti-
eth century that improvements in radiation production, detection and delivery, image processing,
and micropositioning have the required precision and speed been achieved to successfully develop
radiobiological
microbeam facilities.
21.4 history, rationale, and key aspeCts
The rst microbeam experiment has to be attributed to Zirkle and Bloom (Zirkle and Bloom, 1953)
who in 1953 used a 2MV Van de Graaff accelerator and microcollimators to study the process
of cell division following proton exposure. Their collimator consisted of two metal plates with a
groove etched on one, clamped together to achieve a 2.5μm beam or adjustable cross slits to form
a nominal proton beam of any size from a few microns to a few millimeters. Later a 25μm micro-
beam was developed using the cyclotron facility at the Brookhaven National Laboratories using a
similar collimator approach and 11MeV/amu proton or 22MeV/amu deuterons (Baker etal., 1961),
to investigate radiation damage to mouse-brain cells (Zeman etal., 1959). These rst microbeams
were limited to relatively high doses and impossibility to control and/or determine the number of
particles reaching the samples. As a solution, the GSI Darmstadt microbeam (Kraske etal., 1990)
based on a UNILAC accelerator to collimate ions from carbon to uranium (1.4MeV/amu) used
plastic track detectors to monitor the particle traversals and, a posteriori, determine the traversals
experienced by each sample. Such an approach, however, was extremely time consuming and only
20
cells could be irradiated with a single ion during 10
h
of beam time.
The
incentive to develop modern microbeams came mainly from the necessity to evaluate the
biological effects of exactly one particle traversal in order to evaluate environmental and occupational
radiation risks, where only a few cells in the human body experience isolated traversals by charged
particles (Brenner etal., 1995) separated by intervals of months or years (see Figure 21.1). Due to the
random Poisson distribution of tracks, such scenarios cannot be simulated in vitro using conventional
broad eld techniques, and current excess cancer risks associated with exposure to very low doses of
ionizing radiation are estimated by extrapolating high dose data. These data obtained from in vitro
experiments or from epidemiological data from the atomic bomb survivors, however, suffer from
limited statistical power and are unable to resolve uncertainties from confounding factors, forcing the
adoption of the precautionary LNT model. Moreover, there is experimental evidence that non-linear
effects may play a considerable role at low doses. Genomic instability (Kadhim etal., 1995), hypersen-
sitivity (Marples and Joiner, 1993), and bystander effect (Prise etal., 2005; Morgan and Sowa, 2007),
for example, may increase the initial radiation risk, while the adaptive response (Boreham etal.,
2000) may act as a protective mechanism reducing the overall risks at low doses. Charged-particle
microbeams therefore represent a unique tool. They allow targeting of single cells and analysis of
the induced damage on a cell-by-cell basis, which is critical to assess the shape of the dose–response
curve in the low-dose region. A second driving question was related to the radiation-sensitivity of
the whole cell and the cell nucleus in particular. Traditional radiobiological theories were based on
the assumption that the DNA was the only critical target and that any biological effect observed
was entirely the result of DNA alteration. Following some observation of nonuniformity of cellular
response (Datta etal., 1976), the investigation of radiosensitivity across the cell nucleus was consid-
ered to be of great interest in improving radiobiology models. Modern microbeam facilities were
developed almost in parallel at the Pacic Northwest Laboratories (United States) (Braby and Reece,
1990), Columbia University (United States) (Michael etal., 1995), and the Gray Laboratory(UK)
578 Charged Particle and Photon Interactions with Matter
(Folkard etal., 1997a,b). They all adopted, at least initially, a collimating approach using either adjust-
able knife-edges, sets of microapertures, etched grooves, or microcapillaries to produce micron size
beams of proton and helium ions. The main difference between these microbeams and the previ-
ous facilities were the ability to a priori determine the number of particles to be delivered to each
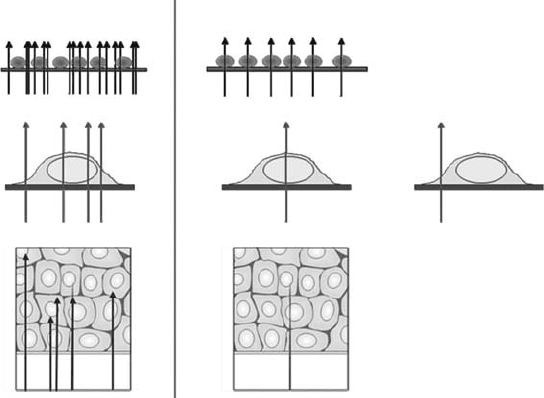
sample, and the individual cell or part of it to be irradiated (see Figure 21.1). This has been achieved
using custom-designed radiation detectors (see below for details) coupled to fast electrostatic beam
defectors able to shut down the beam when the desired number of particles have been delivered. The
availability of accurate micropositioning stages and computer resources for automatically driving the
experiments were also essential elements.
The initial success of these rst microbeam facilities was related to the ability to measure radia-
tion effects at very low doses with great accuracy. The Columbia charged-particle microbeam was
used to measure the oncogenic transforming efciency of the nuclear traversal of exactly one α
particle (Miller etal., 1999). It was found to be signicantly lower than that predicted by a Poisson-
distribution delivery by an average of one α particle. Such ndings suggested that multiple tra-
versals dominate the biological response, and extrapolation from multiple particle traversals may
overestimate the single traversal risk. However, similar experiments (Hei etal., 1997) highlighted
how even a single α-particle traversal has considerable toxic (∼20%) and mutagenic probability
(average 110 mutants per 10
5
survivors). Similarly, using 3.2MeV protons, Prise and coworkers at
the Gray Laboratory (Prise etal., 2000) used the micronucleus assay as a measure of predomi-
nantly lethal chromosome damage showing a linear dose response in the range 1–30 protons per
nucleus with a single proton responsible for micronuclei formation in less than 2% of exposed
cells. A single-particle traversal was also shown to induce a signicant increase in the proportion
of aberrant human T-lymphocyte cells, 12–13 population doublings after exposure (Kadhim etal.,
2001). The unstable phenotype indicated by the high level of chromatid-type aberrations suggested
that a single α particle through the cell nucleus can also induce genomic instability. Moreover, by
targeting the cytoplasm, microbeams have shown that intracellular signaling between the cyto-
plasm and the nucleus can also cause DNA damage, undermining therefore the fundamental para-
digm of radiobiology that considers direct DNA exposure a prerequisite for the manifestation of
Conventional Microbeam
Nuclear
(A)
(B)
(C)
Non-nuclear
Figure 21.1 Schematic showing differences between conventional radiation exposure experiments and
those using a microbeam. (A) depicts the ability to deliver uniform numbers of charged-particle tracks to indi-
vidual cells, including that of a single track. (B) represents the ability to target radiation to different subcellular
locations.
(C) represents the ability to deliver localized radiation to dened depths within tissue.
Application of Microbeams to the Study of the Biological Effects of Low Dose Irradiation 579
the radiation effects. This is well summarized in the work of Wu and colleagues (Wu etal., 1999),
where cytoplasmic irradiation by α particles was found to induce mutations in human hamster
hybrid (A
L
) cells, indicating that the cytoplasm is also a critical target. By comparing the mutant frac-
tion induced by nuclear and cytoplasmic α-particle traversals for an equitoxic dose, cytoplasmic
irradiation was found to be as much as seven times more mutagenic (due to the low cell killing)
than nuclear exposure and therefore potentially more harmful. Moreover, it was noticed that the
spectrum of aberrations induced by the cytoplasm irradiation was quite different from that pro-
duced by nuclear irradiation, suggesting that different mutagenic mechanisms may be involved.
The importance of cytoplasmic irradiation has also been highlighted by other microbeam experi-
ments directed at investigating the bystander phenomenon. Using both α particles (Shao etal.,
2004) and soft x-rays, increased micronuclei formation and decreased clonogenic survival have
been measured following the irradiation of one or a few cells through their cytoplasm. This nding
showed that direct DNA damage is not required for switching on of important intra cell-signaling
mechanisms. Another advantage offered by the microbeam approach is the possibility of assessing
radiation damage on a cell-by-cell basis, thus avoiding the statistical uncertainty that affects some
conventional assays. For conventional assays, accurate measurements of the radiation effect may
be limited by both the uncertainty in the number of samples exposed and the dose delivered. This
is a particular problem in the low-dose region, where only small effects are expected. However,
using the Gray Laboratory charged-particle microbeam, it has been possible to precisely measure
the survival of V79 cells exposed to 3.2MeV protons at doses below 1Gy (Schettino etal., 2001).
As a result of the precise particle delivery system and knowing the number of cells exposed, it was
possible to detect small variations in the initial slope of the survival curve indicating the presence
of a hypersensitivity region that had been shown previously using x-rays (Marples and Joiner, 1993)
and proving that microbeams are ideally suited to investigate cell survival at very low doses.
Since then, microbeams have established themselves as a powerful, often unique, radiobiological
tool facilitating our understanding of a variety of biological responses. For example, the micro-
beam approach is considered particularly appropriate to investigate the so-called bystander effect
(Morgan and Sowa, 2007), where radiation damage is being detected in unexposed samples follow-
ing the irradiation of neighboring cells. Even though this type of study could be undertaken using
basic shielding arrangements, comprehensive investigations could only be done using the precise
pre-selected dose delivery and the individual cell system assay offered by the microbeam. Using
the Gray Laboratory microbeam (Prise etal., 1998), it was possible to demonstrate that the level
of bystander damage is independent of the number of cells targeted (up to 25%) and of the number
of particle traversals leading to a binary all-or-nothing interpretation of the bystander response
(Schettino etal., 2005). Bystander studies have now been extended to tissues and 3D cell models in
order to understand the role of cell-to-cell communication and the implication of tissue architecture
on radiation response. It is anticipated that the new generation of microbeams, employing longer-
range radiations and deep-tissue imaging techniques, may offer a unique contribution. Moreover,
increasing scientic interest toward subcellular targets and signaling pathways that regulate the
various radiobiological responses greatly benets from the use of microbeam facilities (see Figure
21.1). Only by using facilities that are able to localize the radiation dose to specic areas of interest,
is it possible to address where the triggering effects for specic biological responses (Lyng etal.,
2006) (such as apoptosis or the bystander effect) are initiated and whether the nonuniformity of the
damage
induction may be responsible for the differential damage expression.
Following
the success of the rst microbeams at the end of the twentieth century, considerable inter-
est has been generated in the scientic communities regarding microbeam technologies, resulting in a
sharp increase in the number of facilities worldwide (Gerardi etal., 2005). Many different experimental
setups have been exploited in order to assess the damage induced following irradiation by a micrometer
(or submicrometer in some cases) beam that targets individual cells or part of cells. Some of the most
popular approaches are reviewed and discussed below. In general, however, there are a few basic require-
ments that a microbeam facility has to fulll in order to perform accurate radiobiological experiments.
