Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

550 Charged Particle and Photon Interactions with Matter
because these radical yields were not fully consistent with the nal induced biological effects. It has
been widely recognized that densely ionizing radiation, such as α-particles, induces cell killing more
efciently than sparsely ionizing radiation, such as γ- or x-rays. The relative biological effectiveness
(RBE) of the lethal effect on cells irradiated with α-particles when compared with γ-irradiation is
about two. Becker etal. (2003) have reported, however, that when hydrated DNA samples at 77K are
irradiated with high-LET argon ions (60 and 100MeV/nucleon), nucleobase ion radicals were induced
at a lower yield than that obtained with γ-irradiation, although the neutral deoxyribose radicals, which
are thought to accompany an SSB, were induced with a higher yield than with γ-irradiation.
Few studies have investigated the nucleobase lesions induced by direct energy deposition from
an ion track to a DNA molecule at ambient temperatures. Recent studies using hydrated DNA have
shown that the yield of DSBs induced by α-irradiation (Yokoya etal., 2003; LET = 140 keV/μm)
was twice that induced by γ-irradiation (Yokoya etal., 2002), indicating that dense ionization or
excitation events along the α-particle tracks are more effective at inducing clustered types of strand
breaks. Furthermore, irrespective of the hydration level, α-particle irradiation of DNA induced
more complex types of clusters of DNA damage, including nucleobase lesions, than photon irradia-
tions such as γ- or hard x-rays. Recently, the track size of high-LET argon ions has been experimen-
tally determined using pulsed electron double resonance spectroscopy (PELDOR) techniques by
Bowman etal. (2005). These authors reported that the number of clustered radicals within a 1 nm
length of DNA was 2.6, which is consistent with the distribution of lesions in a cluster damage site,
that
is, 4–10 lesions over several base pairs (Nikjoo etal., 2001).
In
order to focus on the clustered damage sites induced by irradiation with high-LET ion par-
ticles, the yields of nucleobases have been determined by enzymatic probes that convert nucleobase
lesions into readily detectable SSBs (as described previously). In these studies, plasmids or phage
DNA were used as models. The yields of damage were measured as a function of the ionization
density of the radiation using ion particles from accelerator facilities with LET values. The yields of
nucleobase lesions detected by the enzymatic treatments, as well as strand breaks, varied depending
on the experimental conditions. In dilute DNA solutions, the yields of SSBs and DSBs decreased
with increasing LET, although the ratio of DSBs to SSBs increased with increasing LET (Taucher-
Scholz and Kraft, 1999). Similarly, both the nucleobase cluster lesions revealed by Fpg or Nfo pro-
teins and the prompt DSBs induced in T7 DNA decreased with increasing LETs of the ion particles
(Hada and Sutherland, 2006). In the dilute DNA solutions, diffusible OH
•
produced by radiolysis of
water contributed primarily to the induction of DNA damage. It has been well understood that the
yield of OH
•
decreases with increasing LET because the higher density of the radicals in the track
leads to a higher probability of an intratrack recombination process between the radicals than that
for a low-LET radiation track. Recently, Yamashita etal. (2008) discussed the intratrack reactions
along the ion-particle tracks using a model experimental system for the formation of methyl violo-
gen cation radicals. On the other hand, when DNA was irradiated with helium ions under hydrated
conditions, the yield of prompt SSBs did not depend signicantly on the LET of the helium ions,
whereas the yield of DSBs increased with increasing LET. The yields of isolated nucleobase lesions
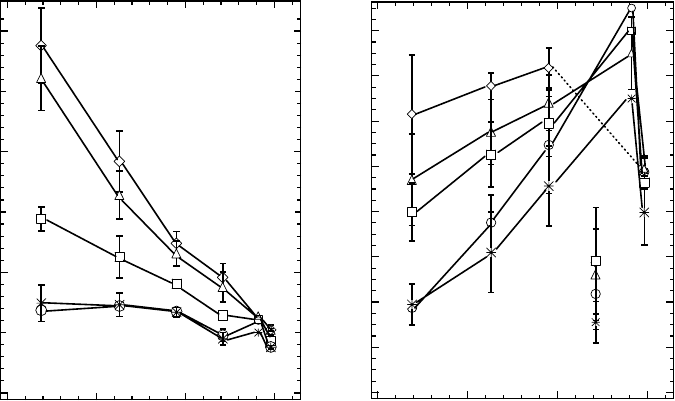
revealed by Nth and Fpg as additional SSBs decreased drastically with increasing LET (Figure
20.5A, Urushibara etal., 2008), and very few enzyme-sensitive sites were induced at 120keV/μm.
These results indicate that a cluster of nucleobase lesions induced at 120keV/μm is less readily
repaired by the BER proteins than that induced at a lower-LET region and, therefore, may show a
high RBE value for cell killing. The sum of the yields of DSBs and additional DSBs revealed by
Nth and Fpg increased with increasing LET. These studies concluded that the yields of clustered
damage, revealed as DSB and non-DSB clustered damage sites but not isolated lesions (i.e., SSBs),
increased with increasing ionization density of the He ions under 140keV/μm (Figure 20.5B).
Chang etal. (2005) observed no enhancement of cell killing after exposing an Fpg-overexpressing
E. coli strain to α-rays. These results may reect the greater complexity of clustered damage sites
generated by high-LET radiation, in which the lesions are processed sequentially to avoid the DSB
formation
as described in Section 20.4.
Spectroscopic Study of Radiation-Induced DNA Lesions 551
20.4 biologiCal relevanCe oF Clustered damage
Individual lesions, whether they are clustered or isolated, are thought to be recognized and processed
mainly by base-excision repair (BER). In the initial phase of BER, DNA glycosylases recognize the
damaged base and excise the N-glycosidic bond to generate an AP site. A variety of nucleobase
lesions are recognized by several DNA glycosylases, each of which shows broad substrate speci-
cities. The DNA backbone at the AP site is excised by the accompanying AP lyase activity of the
glycosylase or by the AP endonucleases (step 1) to generate an SSB. Blocks at the 3′-end of the SSB
generated by the AP lyase activity are also removed by AP endonucleases. One or more nucleotides
are inserted at the SSB (step 2), followed by displacement of the strand (step 3), cleavage of the
displaced nucleotide(s), and ligation of the break (step 4). Depending on the number of nucleotides
inserted, BER proceeds through either of the two subpathways: short-patch repair or long-patch
repair. The major enzymes from different species involved in the process are listed in Table 20.4.
Although a considerable amount of clustered DNA damage appears to be induced in cells, how
and to what extent clustered DNA damage is processed in vivo and, perhaps more importantly, is
related to biological consequences have long remained unknown. Apart from the difculties in
interpreting the results of clustered damage revealed as DSBs by glycosylases or AP endonucleases,
this is probably because (1) radiation-induced clustered DNA lesions are difcult to fully detect
experimentally and (2)they have random congurations in terms of the types, numbers, and relative
positions of the lesions. To overcome the difculty posed by the random nature of radiation-induced
lesions, many research groups have used synthetic clusters, in which the type, the number, and the
relative position of the lesions are specied, to examine how the clusters are processed. Whether
a clustered DNA damage site is indeed relevant to biological end points has mostly been investi-
gated using plasmid-based assays with model synthetic lesions. A plasmid-based assay, in principle,
30
20
10
0
15010050
(A) (B)
0
LET(keV/μm) LET(keV/μm)
DSB×10
–12
(Gy/Da)
SSB× 10
–11
(Gy/Da)
16
14
12
10
8
6
4
2
0
150100500
Figure 20.5 Dependence of the yield of SSBs (A) and DSBs (B) on LET for fully hydrated DNA irradi-
ated with
4
He
2+
ions at 279K (⚪) or after a postirradiation incubation for 30 min at 310K in the absence (
m
)
or presence of either Nth (▵), Fpg (◽), or both Nth and Fpg (♢). The vertical error bars are ±SD for the SSB
values determined from the slope of the dose-response curves from three independent experiments. The data
at 141 keV/μm taken from the previous report (Yokoya etal., 2003) for α-irradiation are shown for comparison,
assuming that the LET of Pu-α particles in the sample lm comprising hydrated plasmid DNA and TE buffer
solutes (ρ = 1.2) is estimated at 141keV/μm. (Data from Urushibara, A. et al., Int. J. Radiat. Biol., 84, 23, 2008.)

552 Charged Particle and Photon Interactions with Matter
transforms cells with model clusters that are ligated into the plasmids. Transformation efciencies
as well as mutation frequencies are monitored to evaluate the ability of the plasmids with clusters
to replicate and generate mutations in cells. Various types of synthetic nucleobase lesions, such as
8-oxoG, thymine glycol (Tg), DHT, dihydrouracil (DHU), 5-hydroxyuracil (5-OHU), as well as AP
sites,
have been subjected to this analysis.
Several
studies indicated that the mutagenic potential of an 8-oxoG is enhanced in E. coli cells
by the presence of another nucleobase lesion or an AP site within close proximity on the oppo-
site strand (Malyarchuk etal., 2003, 2004; Pearson etal., 2004; Shikazono etal., 2006; Bellon
etal., 2009). An 8-oxoG is a mutagenic lesion, and E. coli is known to avoid mutagenesis by this
lesion with at least two DNA glycosylases: Fpg, which excises 8-oxoG residues from DNA, and
MutY, which excises adenine residues incorporated opposite 8-oxoG after DNA synthesis or rep-
lication. With clusters containing 8-oxoG, a lack of MutY led to a marked increase in mutation
frequency compared with that in wild type. The importance of MutY in mutagenesis suggests
that 8-oxoG:adenine base pairs can arise from the presence of 8-oxoG at replication because of
retardation or absence of excision in the cluster; however, they are readily repairable, because,
after replication, they are no longer part of a cluster. The frequencies of mutation are very similar
for the DHT/8-oxoG cluster and the 8-oxoG/8-oxoG cluster in the same bacterial strains, sug-
gesting that the same key intermediate (AP/8-oxoG or SSB/8-oxoG) is involved. In the studies
with clusters containing 8-oxoG, the majority of the mutations found were G-to-T transversions
at the 8-oxoG sites, with few deletions. In addition, the transformation efciency of bacteria by
table 20.4
enzymes
a
ssociated
with ber and ssbr
enzymes species Functions
Glycosylase Fpg E. coli Removal
of damaged purines
yOgg1 S. cerevisiae
hOGG1 human
Glycosylase Nth E. coli Removal
of damaged
pyrimidines
Ntg1 S. cerevisiae
Ntg2 S. cerevisiae
yNTH1 Human
Glycosylase Nei E. coli Removal
of damaged
pyrimidines
hNEIL1 Human
hNEIL2 Human
AP
endonuclease Xth E. coli Incision at
AP
sites
(including
oxidized
AP)
Nfo E. coli
APE1 S. cerevisiae
Human
Polymerase PolI E. coli Insertion
of nucleotides
Polβ
Human
Polδ
Human
dRPase/FLAP endonuclease PolI E. coli Removal of
deoxyribophosphate/FLAP
Polβ
Human
Polδ
Human
FEN1 Human
Ligase Ligase E. coli Sealing
of breaks
LigaseI Human
LigaseIIIα
Human
BER,
base-excision repair; SSBR, single-strand break repair; AP, abasic sites.
Spectroscopic Study of Radiation-Induced DNA Lesions 553
plasmids carrying these clusters was comparable to that by undamaged DNA, which indicated
that most of these clusters were not processed into DSBs during repair. The in vivo results from
these model bistranded damage sites indicate that (1) the repair of an 8-oxoG is retarded so that
some of the clusters remain partly unrepaired by the time of replication, (2) lesions are repaired
sequentially and the formation of DSBs is minimized, and (3) there appears to be a preferential
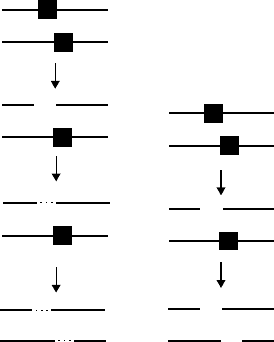
order for excising different lesions (Figure 20.6). These suggestions have recently been rein-
forced by the results of a study by Kozmin etal., in which they used up to ve different lesions
in yeast (Kozmin et al., 2009). The existence of a hierarchy points toward the biological impor-
tance of the complex interplay between various repair proteins to avoid the generation of DSBs
that
could be deleterious to the cells. In contrast, the mutagenic potential of an 8-oxoG placed in
tandem with an AP site remained similar to that of a single 8-oxoG in wild-type cells of E. coli,
and even decreased in fpgmutY cells (Cunniffe etal., 2007). This implies that the position of the
base damage greatly inuenced the mutagenic consequences of a cluster. A model that explains
this difference in mutagenesis is shown as follows. In a bistranded cluster, the strand opposite
8-oxoG could undergo a long-patch repair or its rate of amplication could be reduced, so that
the 8-oxoG-carrying strand serves as the major template for DNA synthesis (Figure 20.6A).
This might lead to an enhanced mutagenic potential of 8-oxoG. It is important to note that with
a long-patch repair, as long as the repair patch size is larger than the distance between lesions,
a nucleotide will always be inserted opposite 8-oxoG by repair synthesis. In the case of tandem
clusters with an adjacent lesion of 8-oxoG positioned on the same strand, a similar scheme results
in the induction of fewer or no mutations because an undamaged strand is the more preferred
template for DNA synthesis.
With bistranded uracils, which are thought to be quickly converted into bistranded AP sites
in vivo, the induction of DSBs through the processing of clustered DNA damage in E. coli has
been inferred from the formation of deletions (Dianov etal., 1991). In addition, a reduction of
transformation efciency, implying the formation of DSBs, was also observed when the two ura-
cils (Us) or AP sites were positioned in close proximity on both strands in E. coli (separated
by less than 7–8 bp) (D’Souza and Harrison, 2003; Shikazono and O’Neil, 2009), as well as in
Bistranded clusters
DSB
(A) (B)
Sequential
repair
replication
Mutation
Figure 20.6 Pathways of the processing of bistranded clusters in vivo. Black squares indicate DNA
lesions. (A) Base lesions are processed and repaired sequentially by BER to avoid the formation of a DSB.
The unrepaired base damage may serve as a template for repair synthesis or replication, resulting in muta-
tions. (B) AP sites are processed simultaneously before completion of repair, resulting in the formation
of a DSB.
554 Charged Particle and Photon Interactions with Matter
yeast (Kozmin etal., 2009). Interestingly, in E. coli, a reduced transformation efciency was still
found in the absence of the enzymatic activities, such as those of AP endonucleases (Xth, Nfo,
and endoV), AP lyases (Nth, Fpg, and Nei), and nucleotide excision repair (NER) (UvrA), needed
for the incision of an AP site (Harrison etal., 2006). A similar situation with low transformation
efciency of bistranded Us or AP sites in mutants decient for AP endonuclease (apn1apn2 double
mutant) was also found in yeast (Kozmin etal., 2009). These results suggest that bistranded clus-
ters comprising lesions that block replication strongly diminish the transformation efciency. It
has recently been shown in mouse cells that two opposed tetrahydrofurans could be cleaved into a
DSB by AP endonuclease(s), and a fraction of the lesions could be inaccurately repaired by NHEJ
repair resulting in deletions (Malyarchuk etal., 2008). Tetrahydrofuran is a stable AP-site analog.
This formation of DSBs in mammalian cells is consistent with the results from in vitro process-
ing of bistranded AP sites in cell extracts. The bistranded clusters comprising an AP site and a
one-nucleotide gap (GAP) have also been shown to have very low transformation efciencies,
similar to those of bistranded Us or AP sites (Shikazono and O’Neil, 2009). Taking these results
together, it was inferred that bistranded nucleobase lesions, that is, single-base lesions on each
strand but not clusters containing only AP sites and strand breaks, are repaired in a coordinated
manner; thus, the formation of DSBs is avoided. In other words, when either nucleobase lesion is
initially excised from a bistranded damage site, the remaining nucleobase lesion will only rarely
be converted into an AP site or an SSB in vivo.
All of the above information suggests that the presence of one or more vicinal lesions affects the
rate, delity, and pathways of DNA repair and determines the outcome of processing. It is worth
noting that in most, if not all, cases, the repair of clustered lesions was retarded compared with the
repair of isolated lesions. The less effective repair of lesions in a cluster should not simply be con-
sidered deleterious or harmful to cells, as it may often protect against the formation of lethal DSBs.
A major consequence of retarded processing of clustered DNA damage is the extended lifetime of
lesions within the cluster, which allows the cluster to be present at replication.
20.5 intermediate speCies and degradation proCesses
o
F dna revealed by s
ynChrotron
s
o
F
t
x
-rays
as p
robes
In order to overcome the difculty posed by the random nature of the radiation damage to DNA,
atom-selective ionization is another powerful tools that induces spatially regulated energy deposi-
tion in DNA. In general, as discussed above, it is difcult to induce specic lesions in DNA using
conventional radiation sources such as γ- or hard x-rays, which nonspecically ionize the DNA’s
constituent
atoms and cause damage with random congurations. In order to achieve atom-specic
ionization, many studies have used soft x-ray photons, particularly those with energies below 10 keV.
Soft x-ray photons mainly interact with matter in a living cell through a photoelectric process: as
a consequence, a photoelectron and Auger electrons are ejected from the atom that absorbs the
soft x-ray photon. The biological effects induced by soft x-irradiation are thought to arise from the
formation of DNA damage through both the ionization of DNA and the impact of the secondary
electrons, which ionize or excite nearby molecules through inelastic scattering. The main products
resulting
from such electron loss are oxidized nucleobases, such as 8-oxoG.
Synchrotron
radiation has been used as an intense soft x-ray source. Monochromatic photons
from a high-resolution monochromator can be used to induce inner-shell photoionization at a
particular atom in order to explore the details of the photoelectric effect in biological samples
(Hieda and Ito, 1991). The atom-selective irradiation of a biological system provides potentially
new insights into the role of photoabsorption events for each constituent element of the DNA in
the induction of the nal biological effects (Figure 10.7). K-shell ionizations of carbon, nitrogen,
and oxygen atoms in DNA have been explored using monochromatic soft x-ray photons around
the K-edge energies: carbon, 284 eV; nitrogen, 410eV; and oxygen, 543 eV. Early studies, how-
ever, reported that the yields of DNA strand breaks were almost constant or slightly enhanced at
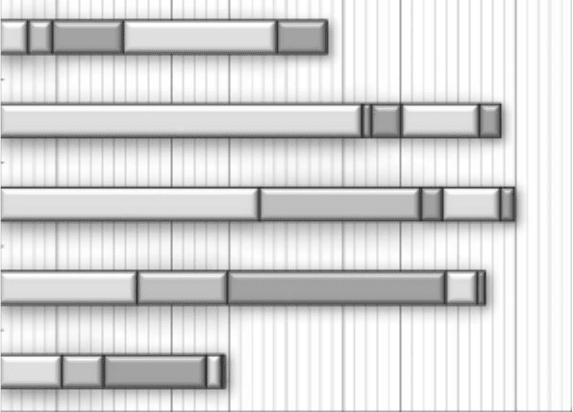
Spectroscopic Study of Radiation-Induced DNA Lesions 555
the oxygen K-edge energy (Yokoya etal., 1999; Fayard etal., 2002). Recent studies also showed
a similar enhancement of the yield of DSBs above the oxygen K-edge region by a factor of 1.4–2
(Eschenbrenner et al., 2007; Agrawala et al., 2008). However, these energy dependencies of
the yields of DNA strand breaks are not larger than those initially expected. As indicated by
Goodhead, how inner-shell ionization can cause specic biological and biochemical effects has
been a long-standing question (Goodhead, 1994b and Goodhead etal., 1981). In order to answer
this question and understand the mechanism by which soft x-irradiation produces such a high
efciency of biological effects, not only DNA strand breaks but also oxidized nucleobases as
discussed above should be examined.
Knowledge about the yield of nucleobase lesions has been, however, very scarce in the studies of
DNA damage by soft x-irradiation. Only the yield of nucleobase lesions detected by Fpg treatment
has been reported in a DNA lm irradiated with monochromatic soft x-rays (250, 380, and 760eV)
(Agrawala etal., 2008). We still lack clear evidence to determine whether the photoelectric effect on the
DNA’s constituent atoms induces particular types of nucleobase lesions as well as DNA strand breaks.
Recently, Fujii and coworkers determined the yields of pyrimidine and purine nucleobase lesions
as well as SSBs and DSBs induced in dry DNA lms by soft x-irradiation using monochromatic
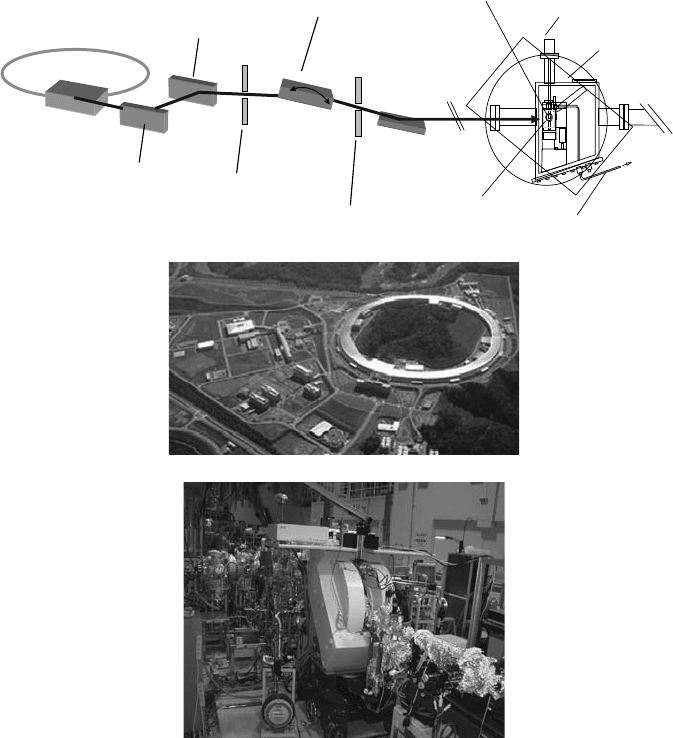
synchrotron radiation in the carbon, nitrogen, and oxygen K-edge regions (280–760 eV) (Fujii etal.,
2009) using a soft x-ray beamline at SPring-8 (Figure 20.8). The role of the photoelectric effect on
the DNA’s constituent atoms was discussed in terms of the yield of nucleobase lesions relative to
strand breaks. They also showed the potential utility of the selective induction of a specic lesion
achieved by irradiation with highly monochromatic synchrotron radiation in the research eld of
DNA damage and its enzymatic repair processes. The dependence of the damage yield on photon
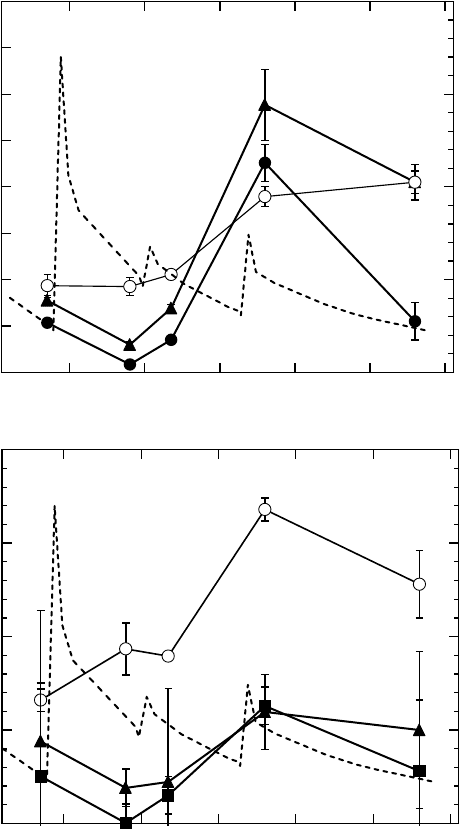
energy is shown in Figure 20.9. The main ndings from their study on the direct ionization of DNA’s
constituent atoms are as follows: (1) The yields of both oxidative pyrimidine and purine nucleobase
lesions, revealed as Nth- and Fpg-sensitive sites, respectively, were strikingly enhanced by oxygen
270 eV
380 eV
435 eV
560 eV
760 eV
0.0 1.0 2.0 3.0
Photoabsorption cross section (× 106 cm
2
/g)
4.0 5.0 6.0 7.0 8.0 9.0 10.0
C
C
N
N
O
O
P
P
Na
Na
C N O P Na
C N O P Na
C N O PNa
Figure 20.7 Photoabsorption cross section of pUC18 plasmid DNA at the energies above phosphorus
L-edge (below carbon K-edge, 270eV), above carbon K-edge (380eV), above nitrogen K-edge (435eV), above
oxygen K-edge (560 eV) and below phosphorus K-edge (760eV). The partial cross sections corresponding to
each
atom are shown by hatched areas.
556 Charged Particle and Photon Interactions with Matter
K-ionization at 560eV and were much lower at the photon energy just below the nitrogen K-edge
(380eV) than those observed at the other photon energies tested. (2) The yield of prompt SSBs was
also enhanced by oxygen K-ionization, but not as distinctly when compared with the yield of nucleo-
base lesions. (3) The yield of the purine lesions (Fpg-sensitive sites) induced by 760eV photons was
signicantly lower than that of pyrimidine lesions (Nth-sensitive sites). (4) Finally, clustered lesions
detected as DSBs after treatment with Nth or Fpg were similarly enhanced by oxygen K-ionization
(560eV), and also showed large yields from 270eV photons, although the yield of prompt DSBs was
only enhanced by oxygen K-ionization. Recently, Agrawala etal. (2008) also reported similar values
for Fpg-sensitive sites from three soft x-ray energies (1.05, 0.49, and 0.92 × 10
−11
Gy
−1
/Da for 250,
380,
and 760
eV
photons, respectively).
Ultrasoft x-ray
beamline (BL-23SU)
X-band EPR apparatus
SPring-8
electron
storage ring
Variably
polarizing
undulator
Grating
monochromator
Post-
focusing
mirror
Vacuum chamber
He cryogenic system
Microwave cavity
(X-band, 9 GHz)
Microwave guide
Pellet sample
Vertical
focusing
mirror
Horizontal
focusing
mirror
Entrance slit
Exit slit
(A)
(B)
Figure 20.8 Schematic layout of the ultrasoft x-ray beamline (BL-23SU) at SPring-8 and the biophysics
end-station (A) and the EPR apparatus installed in the end-station (B). A variably polarizing undulator (APPLE
II type) was used as an intense photon source of ultrasoft x-rays. High-energy resolution (E/ΔE ∼ 10,000) of the
ultrasoft x-ray energy selected by rotating the valid-line-spacing grating (Saitoh etal., 2001) was achieved.
Spectroscopic Study of Radiation-Induced DNA Lesions 557
The irradiation of nucleobases with ionizing radiation is known to produce a variety of unpaired
electron species observed as free radicals by EPR or electron and nuclear double resonance (ENDOR)
methods (see review by Bernhard and Close, 2003). Purkayastha etal. (2006) have studied the G-values
of free radicals trapped in hydrated DNA that were directly ionized by x-irradiation (70kV, tungsten
target) using a low-temperature (4K) EPR technique. They suggested that the observed free radicals
were mainly localized at the nucleobases (80%–90%). These trapped radicals were divided into two
groups: purine radical cations resulting from one-electron oxidations or holes, and pyrimidine radi-
cal anions resulting from one-electron attachments (Bernhard and Close, 2003). The pathways that
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
800700600500400300
Photon energy(eV)
SSB and ESS×10
–11
(Gy/Da)
DSB and enzymatically induced
DSB×10
–12
(Gy/Da)
Photoabsorption cross section(relative)Photoabsorption cross section(relative)
800700600500400300
Photon energy(eV)(B)
(A)
2.0
1.5
1.0
0.5
0.0
Figure 20.9 Dependence of the yield of Nth- and Fpg-sensitive sites and prompt SSBs (A) and prompt
and enzymatically induced DSBs (B) induced by irradiation of pUC18 plasmid DNA on the photon energy
at 277 K under a vacuum. The dotted lines are photoabsorption cross sections of DNA. (Reproduced from
Fujii, K. etal., J. Phys. Chem., 113, 16007, 2009. With permission.)
558 Charged Particle and Photon Interactions with Matter
produce the chemically stable nucleobase lesions initiated by the production of oxidative or reductive
radicals are reviewed in the previous chapter (see Schemes 19.1, 19.2, and 19.4).
One of the major nucleobase radicals is the cytosine anion radical formed by trapping one elec-
tron at low temperature (4–10K). At higher temperature (>180K), it is primarily thymine that traps
a single electron to produce the thymine anion radical. This radical easily converts to 5,6-dihydro-
thymine-5-yl- radical (5-thymyl radical) and shows a specic eight-line EPR spectrum (Ormerod,
1965). The 5-thymyl radical is likely to be a precursor of DHT, which is one of the primary sub-
strates of the Nth protein. When a K-shell electron in the constituent atoms of thymine is ionized by
soft x-irradiation, one or two holes are left in the thymine as a consequence of the Auger process,
and an electron adduct is not likely to be produced at the thymine. Nevertheless, the 5-thymil radi-
cal was one of the main products induced by oxygen K-shell photoabsorption in a thymine pellet
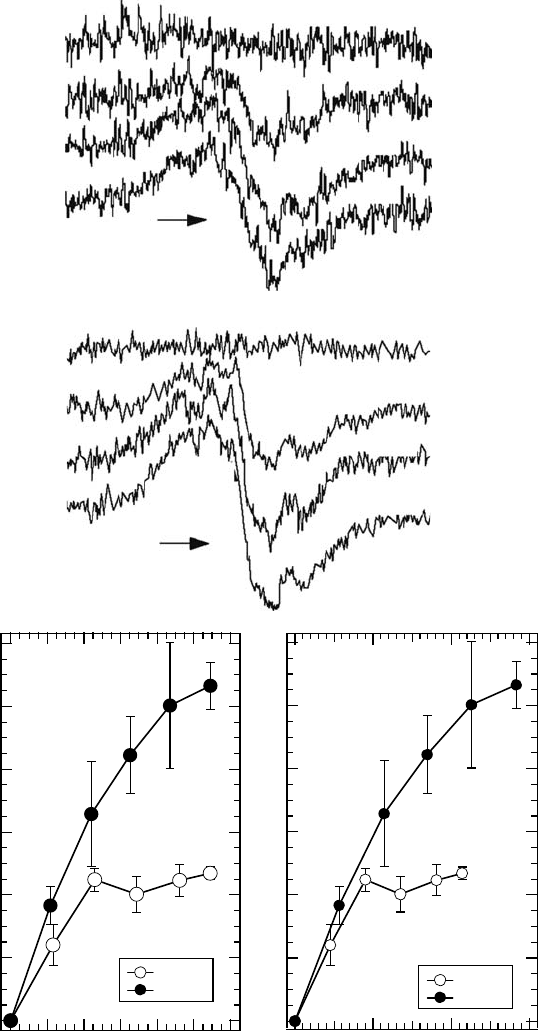
sample that was irradiated with 538eV soft x-rays in a vacuum at 77K (Figure 20.10A; Akamatsu
etal., 2004a). The dose response of the total spin number obtained from the EPR spectrum was
similar to that found using a 407 eV photoirradiation (nitrogen K-shell photoabsorption) in the low
dose range (Figure 20.10B). An high performance liquid chromatography (HPLC) analysis revealed
that the yield of DHT produced in the thymine-pellet sample irradiated in a vacuum with 538eV
soft x-rays was slightly lower than for irradiation with soft x-rays (395 or 407 eV) below the oxygen
K-edge (Figure 20.11; Akamatsu etal., 2004b). These experimental results suggest that the produc-
tion of DHT is not enhanced by oxygen K-ionization when thymine exists alone, and not as a part of
DNA. In other words, either oxygen K-ionization induced in not only thymine but also in the sugar-
phosphate backbone, or hydrating water molecules, which bind tightly to DNA even in vacuum, was
responsible for the strong enhancement of the yield of Nth-sensitive sites. Furthermore, we must
examine the source of hydrogen atoms that are bound to the C6 or both the C5 and C6 positions
in thymine to produce a 5-thymyl radical or DHT. Fujii etal. (2004a) reported that H
+
is the major
ion desorbed by a 538eV soft x-irradiation of a thin lm of thymine as well as 2-deoxy-d-ribose,
thymidine (dThd), and thymidine 5′-monophosphate (dTMP) (Figure 20.12). These results suggest
that H
+
was overwhelmingly produced in the sample by oxygen K-ionization. In addition, the 2.5
hydrating water molecules per nucleotide, which inevitably exist in DNA samples even under high
vacuum (Tao etal., 1989), could be the hydrogen source. Thus, we propose a model of production of
Nth-sensitive
sites enhanced by oxygen K-ionization, as shown in Scheme 20.1.
Similarly,
oxygen K-ionization of the functional groups surrounding guanine or adenine in DNA
could be responsible for the production of Fpg-sensitive sites. Guanine is known to be a major
hole-trapping site in DNA because it has the lowest ionization potential (in the gas phase) among
the nucleobases (see the review by Bernhard and Close, 2003). Not only oxygen K-ionization of the
carbonyl group at the C6 position but also hole transfer from the other ionizing sites to guanine
may produce a guanine cation radical. The production of the oxidative guanine lesions, 8-oxoG
or 2,6-diamino-4-hydroxy-5-formamindopyrimidine (Fapy-Gua), which are substrates of the Fpg
protein, requires the addition of an oxygen atom to an additional carbonyl group at the C8 position
of guanine (or the guanine cation radical). This oxygen atom should come from outside of the par-
ent guanine molecule. The hydrating water molecule proximately located to guanine is probably a
source of the oxygen atom. K-ionization of the oxygen atom in the hydrating water leaves a chemi-
cally reactive oxygen anion (or radical), which could easily react with guanine to produce the oxida-
tive guanine lesions (Scheme 20.2). The yields of enzymatically induced DSBs were enhanced by
not only oxygen K-ionization at 530eV but also irradiation with 270eV (below the carbon K-edge)
photons. On the other hand, the prompt DSBs induced by 270eV photons showed the lowest yield
of the energies tested and were signicantly enhanced by oxygen K-ionization. As presented above,
oxygen K-ionization increases the yield of nucleobase lesions. Consequently, the yields of the clus-
tered nucleobase lesions are thought to increase above the oxygen K-edge energy. The clustering of
nucleobase lesions might be induced through valence electron ionization or phosphorus L-ionization
by 270eV photons (below the carbon K-edge). The ionization of phosphorus L-shell electrons pro-
duces phosphorus LMM Auger electrons (∼120eV) (Watanabe et al., 2004). Yokoya et al., have
Spectroscopic Study of Radiation-Induced DNA Lesions 559
2 mT
2 mT
(A)
10.5 h
10.5 h
7.0 h
7.0 h
3.5 h
3.5 h
Before irradiation
Before irradiation
12
10
8
6
4
2
0
Spin number (nmol)
121086420
Absorbed photon number(nmol)(B)
407 eV
538 eV
12
10
8
6
4
2
0
0.60.40.20
Absorbed energy (J)
407 eV
538 eV
Figure 20.10 (A) EPR signal changes of thymine irradiated with 407 eV (upper) and 538eV (lower) soft
x-rays. The irradiation periods (h) are shown in the gure. (B) Dose-response relationship between the spin
number obtained from EPR spectra of irradiated thymine with 407 (⚪) and 538eV (⚫) photons at 77K in a
vacuum
against absorbed photon number (left) and absorbed energy (right). (Reproduced from Akamatsu, K.
etal.
Int. J. Radiat. Biol., 80, 849, 2004a. With permission.)
