Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

560 Charged Particle and Photon Interactions with Matter
recently reported that these LMM Auger electrons were responsible for the clustering of the nucleo-
base lesions induced by phosphorus K-ionization (Yokoya etal., 2009a). However, irradiation with
380eV photons also produced photoelectrons of similar energy (∼100eV) from the carbon K-shell.
Although the detailed mechanism has not yet been claried, not only the core ionizations but also
the valence electron ionization and the resulting hole in the DNA may be involved in causing serious
DNA damage, such as the clustering of nucleobase lesions.
Recently, Yokoya etal. (2008a) have studied short-lived unpaired electron species produced in
DNA lms by soft x-ray irradiation using an EPR spectrometer installed in a synchrotron soft x-ray
beamline (Figure 20.8). They reported that a signicant EPR signal from the short-lived transient
species was observed only during soft x-irradiation (Figure 20.13; Yokoya etal., 2008a). Although
the short-lived EPR spectrum has not yet been assigned to any specic molecular species, one of the
possible origins of the induction of the sharp lines (see Figure 20.13A) is that the low-energy elec-
trons generated as a consequence of inelastic scattering of photo- or Auger electrons in the sample
might be trapped into a specic molecular site that attracts an electron (Yokoya etal., 2009b). The
EPR signal intensity was signicantly enhanced at the oxygen K-edge but not so signicantly at the
nitrogen K-edge (Figure 20.14). They also found the short-lived species arising in evaporated lms of
guanine and adenine (Yokoya etal., 2009b). In this case, the photon energy dependence of the EPR
intensities of the short-lived species coincided with the photoabsorption spectra of the nucleobases,
showing signicant enhancement of the EPR intensity at the nitrogen K-edge region (Figure 20.15A).
0.0
(a) (b) (c) (d)
0.1 0.2 0.3 0.4
HMU
HMU
DHT
DHT
Product 1
Product 2
foU
foU
U
U
Product 3 Product 4
Relative intensityRelative intensity
0.0 0.1 0.2 0.3 0.4 0.0 0.1 0.2 0.3 0.4 0.0 0.1 0.2 0.3 0.4
0.0
(e) Absorbed energy (J)
HN
HN
HN
CH
3
CHO
H
H
H
HN
CH
2
OH
O
O O
O
O
O
O
O
N
H
N
H
N
H
N
H
(f) Absorbed energy (J) (g) Absorbed energy (J) (h) Absorbed energy (J)
0.1 0.2 0.3 0.4 0.0 0.1 0.2 0.3 0.4 0.0 0.1 0.2 0.3 0.4 0.0 0.1 0.2 0.3 0.4
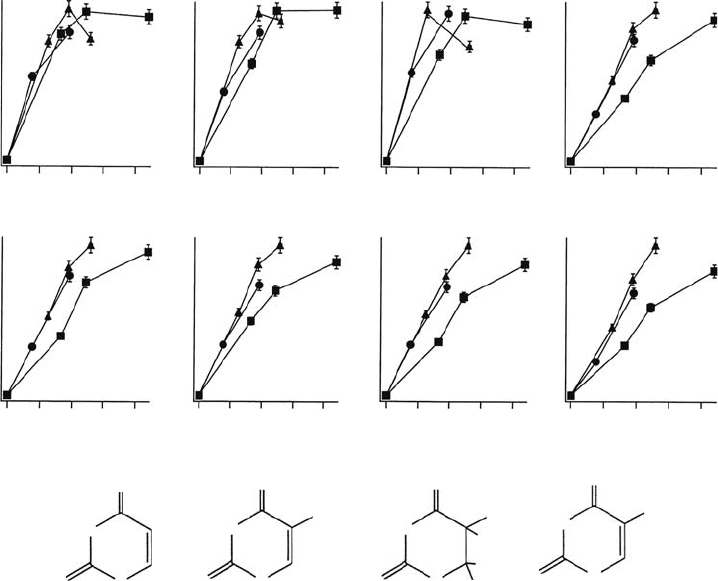
Figure 20.11 Dose-response proles and molecular structures of the thymine decomposition products
analyzed by the HPLC method. The thymine pellet was irradiated with soft x-rays at 395eV (⦁), 407eV (▴),
and 538eV (◾) at 298K under vacuum. (Reproduced from Akamatsu, K. etal., Radiat. Res., 162, 469, 2004b.
With
permission.)
Spectroscopic Study of Radiation-Induced DNA Lesions 561
Interestingly, the enhancement of the EPR intensity of the evaporated adenine lm was reduced
almost completely at the nitrogen K-edge region after a slight exposure to water vapor in the vacuum.
Instead, a signicant oxygen K-edge structure appeared in the spectrum of the photon energy depen-
dence, although adenine does not have any oxygen atoms (Figure 20.15B). These results obtained
from the EPR experiments also support the idea discussed above that the water molecules hydrat-
ing the DNA play an important role in the induction of purine nucleobase lesions. We have reported
80
60
40
20
30
0
25
20
15
10
5
30
0
25
20
15
10
5
0
30
25
20
15
10
5
0
0 20 40
Mass number(m/z)
Intensity(arb. units)
(a)
H
+
H
+
H
+
H
+
CH
2
+
CH
2
+
CH
3
+
CH
3
+
CH
3
+
CH
3
+
C
2
H
2
+
CO
+
CO
+
C
2
H
3
+
CHO
+
C
2
NH
+
C
2
NO
+
CHNO+
C
3
H
3
O
+
C
2
H
3
+
C
2
HN
+
C
2
HO
+
CHNO
+
CHO
+
C
2
H
3
+
C
2
HO
+
C
2
H
2
O
+
C
2
H
3
O
+
2-Deoxy-
D
-ribose
Thymine
dThd
dTMP
CH
3
O
+
CO
+
(b)
(c)
(d)
60 80
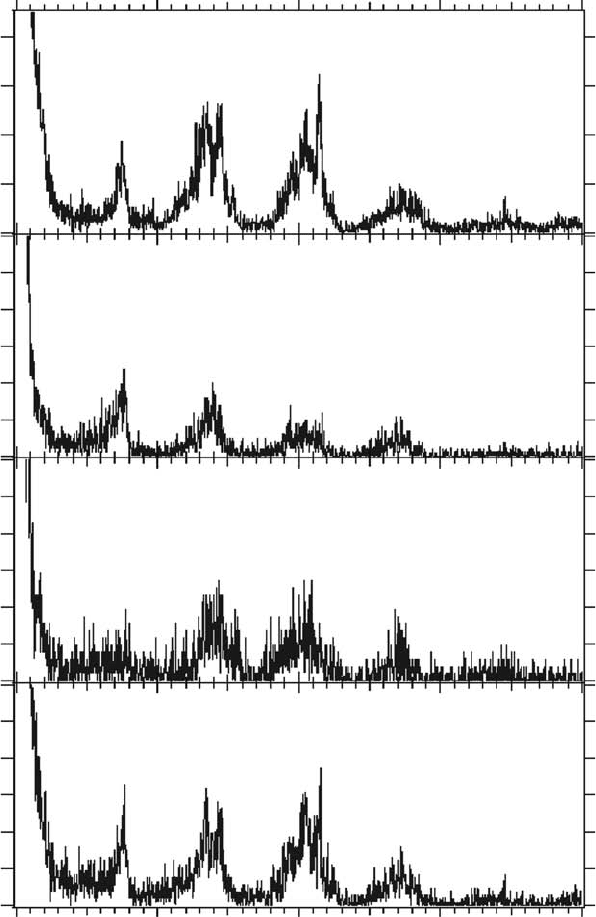
Figure 20.12 Mass spectra of positive ions desorbed from a thin lm of DNA constituent molecules irradi-
ated with soft x-rays (538eV; oxygen K-edge) at 298K under vacuum. (Reproduced from Fujii, K. etal., Radiat
Res.,
161, 435, 2004a. With permission.)
562 Charged Particle and Photon Interactions with Matter
e
SSB
Cytosine
anion
radical
N
O
N
O
H
H
H
H
P
O
O
O
O
+
NH
2
H
H
H
H
NH
ON
O
H
HH
H
H
O
P
O
O
O
O
P
O
O
O
O
H
H
Thymine
anion
radical
O
H
H
+
O
H
H
Hydrated
water
Photo- or
Auger-e
H
+
e
N
O
N
O
H
H
P
O
O
O
NH
2
H
H
H
H
NH
O
N
O
H
HH
H
H
O
P
O
O
O
O
P
O
O
O
O
H
H
Cytosine
e
H
H
+
H
+
e
CH
3
CH
3
Dihydrothymine
Excised by
Nth protein
H
e
O
+
H
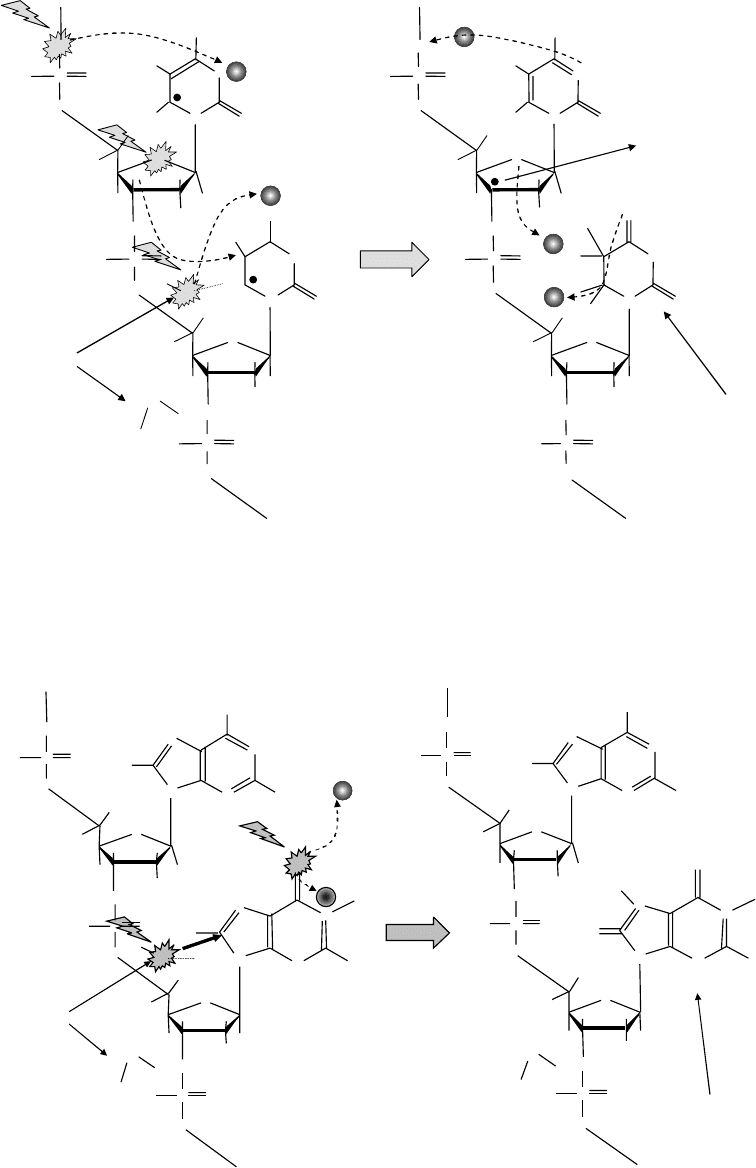
sCheme 20.1 Induction of pyrimidine base damage by oxygen K-ionization.
O
HH
H
H
P
O
O
O
O
H
H
O
H
HH
H
H
P
O
O
O
O
P
O
O
O
O
H
H
O
-
H
H
+
O
H
H
Photo- or
Auger e
–
H
N
N
N
N
H
H
N
H
N
N
N
O
NH
2
H
+
O
H
H
H
H
P
O
O
O
O
H
H
O
H
H
H
H
H
P
O
O
O
O
P
O
O
O
O
H
H
O
O
H
H
H
N
N
N
N
H
H
Hole
N
H
N
N
N
O
NH
2
H
Adenine
Hydrated
water
Guanine
cation
radical
Adenine
8-oxo
Guanine
Excised by
Fpg protein
NH
2
NH
2
e
sCheme 20.2 Induction of purine base damage by oxygen K-ionization.
Spectroscopic Study of Radiation-Induced DNA Lesions 563
that hydrating water molecules surrounding the DNA signicantly increased the yield of nucleobase
lesions, but not that of SSBs when irradiated with γ-radiation (Yokoya etal., 2002).
The yield of the prompt SSBs is also enhanced by oxygen K-ionization, but not as remarkably
when compared with the yield of nucleobase lesions. Ejected electrons in DNA would also cause
molecular damage, including not only the normal ionization of DNA but also the resonantly disso-
ciative attachment process. The latter process preferentially induces DNA strand breaks (Boudaïffa
etal., 2000). The effect of electron impacts would be nonspecically induced regardless of the
atom from which the secondary electrons were ejected. In our previous studies, the prompt SSB
yield for dry pUC18 plasmid DNA was almost constant for a variety of radiations (0.5–1.2 × 10
−10
SSB/Gy/Da), indicatingthatthe DNA strand breaks were mainly induced by random hits of the sec-
ondary electrons. Ito and Saito (1988) reported that the main target for creating SSBs in solid-state
DNA was the pentose ring (deoxyribose). We also concluded that the deoxyribose was a more fragile
site than the nucleobases, as revealed by an ion desorption mass spectroscopy study using soft x-rays
at the oxygen K-edge (Fujii etal., 2004a). The induction of SSBs would not be very sensitive to the
soft x-ray energy.
Thus, irradiation with monochromatic soft x-rays allows us to induce quasi-selective damage in
DNA following the characteristic branching ratios, as shown in Figure 20.16, by tuning the energy
to specic K-edge regions. SSBs are predominantly induced at 380eV, and nucleobase lesions, in
particular Fpg-sensitive sites, are poorly induced. On the other hand, both SSBs and nucleobase
344342340338336334
2 mT
341.0340.5340.0339.5339.0338.5
Magnetic field (mT)(A)
(B) Magnetic field (mT)
0.5 mT
620 eV
560 eV
435 eV
380 eV
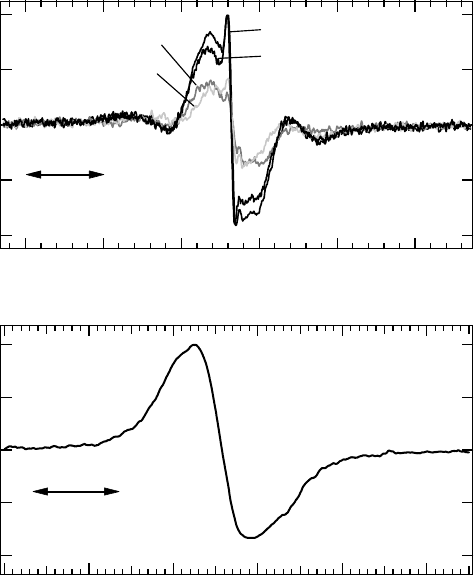
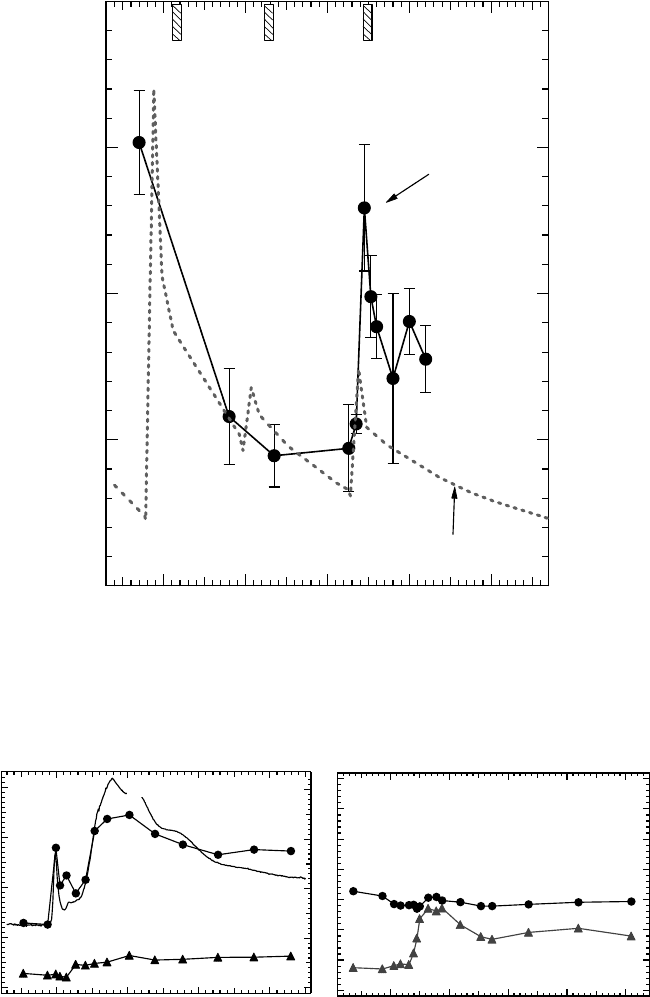
Figure 20.13 (A) EPR spectra of short-lived unpaired electron species obtained by in situ EPR measure-
ment. Thin lms of calf thymus DNA were irradiated with various energies of soft x-rays at 298K under
vacuum. (B) EPR spectrum of stable radical induced in a DNA pellet sample obtained after 150kVp soft x-ray
irradiation.
(Data from Yokoya, A. etal., Int. J. Radiat. Biol., 84, 1069, 2008a.)
564 Charged Particle and Photon Interactions with Matter
0.20
0.15
0.10
0.05
0.00
EPR signal intensity(relative)
700600500400300
Photon energy(eV)
Photoabsorption cross section of DNA
Photoabsorption cross section(relative)
O K-edgeN K-edgeC K-edge
EPR
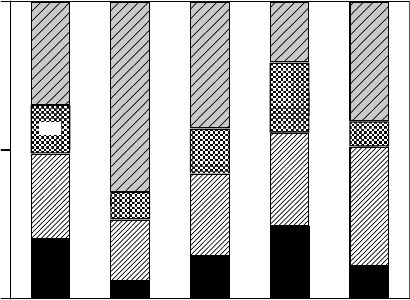
Figure 20.14 Dependence of the spin concentration of short-lived unpaired electron species induced in thin
lms of calf thymus DNA on soft x-ray photon energy at 298K under vacuum. The dashed line shows the pho-
toabsorption cross section of DNA. (Reproduced from Yokoya, A. et al., Int. J. Radiat. Biol., 84, 1069, 2008a.
With permission.)
570560550540530
Photon energy(eV)Photon energy(eV)
After exposure to water vapor
Adenine, O
K-edge region
Intensity(relative)
430420410400
(A) (B)
XANES
After exposure to water vapor
Adenine, N K-edge region
Figure 20.15 Dependence of the spin concentration of short-lived unpaired electron species induced in an
evaporated adenine thin lm on the soft x-ray photon energy around the nitrogen (A) and oxygen (B) K-edges
(⦁) at 298K under vacuum (Yokoya etal., 2009b). The solid red line in (A) shows the XANES spectrum of an
adenine thin lm cited from Fujii etal. (2004b). The XANES spectrum around the oxygen K-edge is not shown
in (B) because adenine has no oxygen atoms. The spin concentration obtained by irradiation of the sample with
soft x-rays after exposing the sample to water vapor at low pressure of water vapor (∼10
−5
Pa for over 6h) is plot-
ted for comparison (▴). (Reproduced from Yokoya, A. et al., Radiat. Phys. Chem., 78, 1211, 2009b.)
Spectroscopic Study of Radiation-Induced DNA Lesions 565
lesions are satisfactorily induced above the oxygen K-edge region. In particular, Nth-sensitive sites
are the most probable damage induced by irradiation with 760eV photons. The characteristic dis-
tribution of damage depending on the photon energy might offer a new technique for studying
enzymatic
DNA repair processes in living cells.
20.6 role oF water moleCules surrounding dna and
photoeleCtron
s
peCtrosCopy
F
or
dna s
olutions
As described above, water molecules, in particular, the hydration layer that is located very close to
the DNA, are heavily involved in the induction of nucleobase lesions as well as strand breaks and
non-DSB types of clustered damage. The geometric structures of DNA with hydrated water mol-
ecules in solution and their electronic states are of great importance in exploring the interaction of
radiation with DNA and in understanding the mechanisms of damage induction and of spatial- and
time-propagation of unstable species in solution. The primary processes induced by the interaction
of radiation with biochemical molecules in water solution can provide a proper prototype of the
above processes in cell-mimetic conditions. It can be safely assumed that the conformational struc-
ture of hydrated DNA is determined by the hydrogen-bonded “hydration network” of the water mol-
ecules surrounding it. These conformational structures further determine the electronic energies of
the DNA–water complex and, presumably, the chemical reaction pathways from bulk water to DNA.
Becker etal. (1994) and La Vere etal. (1996) proposed for the rst time that the hydration network
surrounding DNA plays an important role in terms of the hole transfer from the rst glassy hydration
layer to DNA and hydroxyl radical formation in the surrounding water layers. This assumption also
allows us to have the following thermodynamic perspective on the damage caused by radiation to
DNA and its electronic or chemical restoration.
The intact genomic DNA in a cell is in its thermochemically and biologically stable state
dened by the hydration-network-aided natural conformation, namely, the B-form or the so-called
Watson–Crick conformation. The primary irradiation products, that is, the ionic and excited sites
of DNA induced by the primary processes during interaction with radiation, are regarded as both
the cores of the remaining radiation damage and localized excess energies. Some of the excess
270 eV
Branching ratio
1.0
0.5
0
AP
SSB
Nth
Fpg
760 eV560 eV435 eV380 eV
Figure 20.16 Dependence of the relative yields of DNA lesions on soft x-ray photon energy at 298K under
vacuum. The total yield of damage is normalized to one. SSB, single-strand breaks; Nth, base lesion excised
by Nth protein; Fpg, base lesion excised by Fpg protein. The yield of the common substrates AP sites was
estimated using the data obtained by simultaneous treatment with Nth and Fpg (Fujii etal., 2009), according
to the following equation: yield of common substrates = Nth-sensitive sites + Fpg-sensitive sites − (yield of
additionally induced SSB by Nth + Fpg treatment).
566 Charged Particle and Photon Interactions with Matter
energies diffuse into the surroundings with time, which either gives rise nally to a metastable
thermal state involving stable damage or restores the original state of the natural molecular form.
The hydration network plays an important role both as the intermediary of heat transport to the
environment and as the structural memory of the original hydrogen-bonded conformation in
the restoration processes. It is important to note the necessity of experimentally observing the
stable and/or dynamic hydrated structures of DNA in solution from the perspective of its elec-
tronic properties. The hydration of DNA should also inuence the eigenstate energy of the excited
molecular orbital more than the valence or inner-shell orbitals, so that the photoabsorption spec-
trum, presenting the excited orbitals, should show an environmental “chemical shift” for mol-
ecules in water solution. Recently, a new method of spectroscopy for molecules in liquid solution
using free liquid jet samples in vacuum has been proposed combined with soft x-ray synchrotron
radiation. The technique of liquid beams in vacuum (Fuchs and Legge, 1979; Faubel etal., 1988;
Faubel and Kisters, 1989; Faubel and Steiner, 1992) has been employed in several experiments
using physicochemical spectroscopy (Siegbahn, 1974; Faubel etal., 1997, 1999; Morgner, 1998).
However, biological applications are few.
The function of the liquid jet technique is to delay the actual phase transition and achieve a
practical size for liquid samples used during experiments in vacuum vessels. Let us briey consider
the phase state of the liquid water jet sample in the vacuum, which will be further examined below
along with the result of the temperature-dependent experiment. Figure 20.17 illustrates the phase
diagram of water and the change in the thermal state of the liquid water beam. The liquid water
introduced through the micrometer-sized orice into the vacuum with a signicant amount of stag-
nation pressure nds itself in the gas phase because of the abrupt decrease in atmospheric pressure.
However, because the amount of internal energy required to release each molecule in the liquid
phase into the gas phase is not contained in the thin liquid beam, substantial evaporation occurs
–50 0 50
T (°C)
100
10
–1
10
–2
10
–3
10
–4
1
P (atm)
Ice Water
Vapor
Supercooled
liquid
Superheated
liquid
• •
• •
•
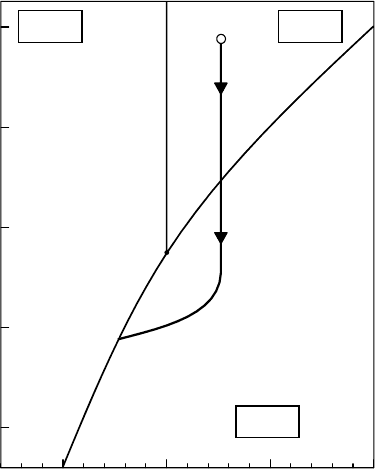
Figure 20.17 Schematic phase diagram for a liquid water beam. Thin solid curves indicating the surface
between the phases are calculated theoretically on the basis of the Clausius–Clapeyron equation. The thick
solid curve approximates the thermal state of a liquid water beam starting at 1 × 10
5
Pa and 300 K in the liquid
phase
and ending in the solid phase in vacuum.
Spectroscopic Study of Radiation-Induced DNA Lesions 567
only from the surface of the beam. The release of a certain amount of heat of evaporation gradu-
ally decreases the temperature of the liquid as a function of the ight distance of the liquid beam
in vacuum (see Fuchs and Legge, 1979; Faubel etal., 1988; Faubel and Kisters, 1989; Faubel and
Steiner, 1992),
so that most of the water molecules
remain in
the
liquid
or
in
the
superheated
liquid
phase. Further decreases in the temperature moderate the emission of heat of evaporation, and then
the molecules remain in a supercooled state even when they nd themselves in the solid phase.
Thus, from an experimental perspective, a certain retention time or retention distance is available
until
the liquid is nally caught in the solid phase.
For
reference, on the basis of the above consideration of the thermodynamic behavior of a liquid
in vacuum, Ukai etal. (2008) calculated the approximate temperature of the liquid beam as a func-
tion of the ight distance in vacuum. For example, the temperatures of the liquid water admitted
through a 10 μm orice into vacuum with stagnation pressures of 1, 3, or 8MPa at 300K, thus cal-
culated, are shown in Figure 20.18 together with the x-ray absorption near edge structure (XANES)
spectra
for the total photoelectron yields of water beams in the same conditions.
The
total photoelectron yields for a liquid water beam are measured as a function of both the
incident photon energy of 531–547eV and the ight distance of 1–17 mm, which is the distance from
the nozzle of the liquid beam to the point of irradiation on the beam in the vacuum. The results
obtained with a stagnation pressure of 3MPa at 300K using the 10μm nozzle are shown in Figure 20.18.
As described above, they represent the temperature dependence of the XANES spectrum in the
vicinity of the oxygen K-edge. The main features of the XANES spectrum of water are a broad
530 532 534 536 538 540 542 544 546 548 550
Photon energy (eV)
Total photoelectron yields (arb. units)
7 mm
12 mm
17 mm
5 mm
3 mm
2 mm
1 mm
1 mm
gas
Downstream distance from nozzle
0 1 2 3
220
240
260
280
300
Temperature (K)
3.0 MPa
φ10 μm nozzle
Initial; T =300 K
8 MPa
3 MPa
1 MPa
Distance (cm)
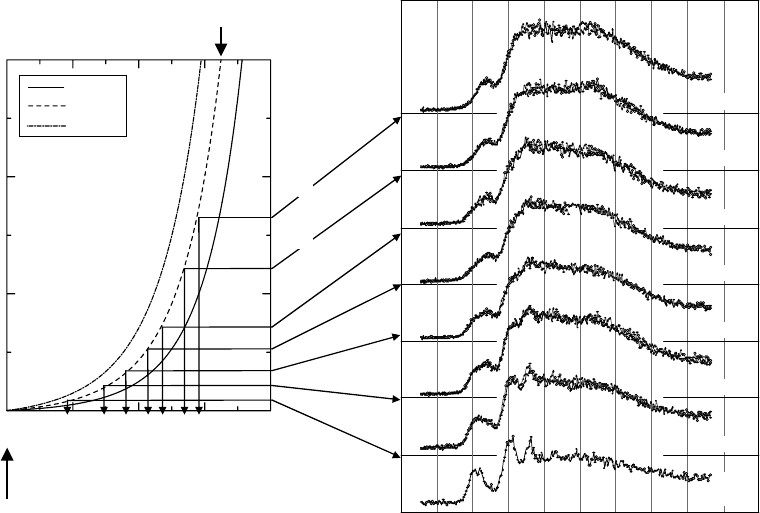
Figure 20.18 Left: Approximate temperature of a liquid water beam emitted through a 10 μm nozzle with
stagnation pressures of 1, 3, and 8MPa at 300 K. Right: The XANES spectra for a liquid water beam as a func-
tion of ight distance of it at 1, 2, 3, 5, 7, 12, and 17 mm downstream from the nozzle in vacuum. The XANES
spectrum for water vapor is also shown at the bottom, which was obtained at a distance at 1 mm downstream
from
the nozzle and at 1
mm
off the liquid beam.
568 Charged Particle and Photon Interactions with Matter
enhancement of the yields at photon energies of 537–540eV and a small enhancement at the photon
energy around 535 eV, the latter of which is called the “pre-edge peak,” although the K-shell ioniza-
tion potential of water in liquid has not yet been reported. It is readily seen that the appearance and
diminished size of the post-edge peaks and the shift of the pre-edge peak are the result of “contami-
nation” from the coexisting water vapor when the temperature of the liquid beam is relatively high
(close to 300K). At a ight distance of more than 10 mm, a new peak appears to grow at the photon
energy of 541eV. The XANES spectrum for ice presents a strong peak at this energy (Wernet etal.,
2004), so the appearance of the new peak signies the growth of micro ices in the liquid beam. It is,
therefore, concluded that at a ight distance of a few millimeters, the water in the present beam is in
the liquid phase, where the present XANES spectrum is in good accord with the previous XANES
spectra obtained by a total photoelectron measurement (Wilson etal., 2004) and by a total x-ray
uorescence
measurement (Kashtanov etal., 2004).
In
this liquid water beam condition, Ukai etal. (2008) reported the rst results of the total
photoelectron yields for a DNA constituent molecule in water. They measured the yield of guano-
sine 5′-monophosphate (GMP)/water as a function of the incident photon energies of 396–416 eV
(shown in Figure 20.19), from which a much greater fraction of continuous electron yields is sub-
tracted. Small enhancements are seen in the electron yields, the intensities of which are about 15%
of the total electrons. The photoionization cross sections of a water molecule monotonously decrease
with increasing photon energy in the region of 396–416 eV (Berkowitz, 2002). Furthermore, the
absorption edge for the carbon K-shell is at a much lower energy by about 100eV and that for oxygen
is much greater by 130eV. It is therefore natural to conclude that the features observed in the present
spectra of photoelectron yields are due to the photoabsorption and photoionization of the nitrogen
K-shell electrons in the guanine site of GMP, so that the features are regarded as the XANES spec-
trum
of GMP in water solution in the vicinity of the nitrogen K-edge.
Let
us briey consider the ratio of the electron yields from the guanine site to the continuous
yields for other elements obtained in the XANES spectrum in the vicinity of the nitrogen K-edge.
The continuous electron yields are the result of the ionization of the valence orbital electrons of
water and the ionizations due to the 2s and 2p orbitals of the oxygen atoms, the 1s orbital of the
400 405 410 415
Photon energy(eV)
Photoelectron yields (arb. units)
GMP
Solution
Film
P
CH
2
O
–
–
O O
O
H
2
N
O
N
N
N
N
O
H
H
H
H
OH OH
Figure 20.19 Left: Structure of GMP (guanosine-5′-monophospate). Right: The XANES spectrum
for GMP in the vicinity of the nitrogen K-edge. “Solution” shows the XANES spectrum for the beam of a
10g/100 mL GMP solution in water. The large amplitudes of continuous electron yield from water molecules
and
other elements are subtracted. “Film” shows the XANES spectrum for a solid lm for reference.
Spectroscopic Study of Radiation-Induced DNA Lesions 569
carbon atoms, and the 2s and 2p orbitals of the phosphorus atom in GMP. Although the molecular
photoabsorption cross sections for water or the nucleotide in liquid water have not yet been obtained
experimentally or theoretically, the sum of the atomic photoabsorption cross sections can provide
reasonable estimates. Because the cross section for light atoms in the region of K-shell absorption
is about 1Mb (10–18cm
2
) at maximum, the photoabsorption cross sections for other atoms at a
photon energy around 400eV are about 0.5 Mb for carbon, 0.06Mb for oxygen, and 0.7Mb for
phosphorus (Yeh and Lindau, 1985). If we consider the case of a mass concentration of GMP/water
of 10g/100mL (corresponding to a molecular ratio of 1/213), the net absorption cross section for
continuous electron yields relative to one guanine unit containing ve nitrogen atoms amounts to
18Mb. Thus, a value of about 15% for the ratio of the electron yields due to nitrogen atoms in GMP
to the continuous electron yields due to water and other elements is reasonably explained by the fact
that GMP molecules are not concentrated in the middle or the surface of the liquid beam, but are
equally
dispersed in water.
The
main features of the XANES spectrum for GMP in water solution consist of relatively sharp
peaks at photon energies of 400 and 402eV and a much broader peak in the region of photon energy
around 407eV. The peaks at 400 and 402eV are normally ascribed to the excitation of nitrogen K-shell
electrons into the vacant antibonding orbital(s). The XANES spectrum for GMP in the form of a thin
solid lm is also shown in Figure 20.19. The GMP in the solid lm is thought to be neat GMP or GMP
containing the fewest number of water molecules under vacuum. The XANES spectrum for a GMP/
water solution is, in general, very similar to that for the solid lm. A similar reference is also available
showing the XANES spectrum for a guanine thin solid lm (Fujii etal., 2004b), which is also similar
to the present XANES spectra for the GMP/water solution and GMP in the solid lm.
Because excitation in the region near the nitrogen K-edge takes place mainly in the frame of the
guanine site, the XANES spectrum is thought to be sensitive to the inuence of water molecules sur-
rounding the guanine site. Both the K-shell and antibonding orbitals of GMP in solution are subject
to the inuence of the external Coulombic eld from the surrounding water molecules. Because the
antibonding orbital is thought to be widely spread around the nucleobase site, its energy is more sensi-
tive to the external Coulombic eld than that of the K-shell orbital; thus, strong hydration should give
rise to an environmental chemical shift. However, a signicant chemical shift is not observed in the
peak energies in the XANES spectrum for GMP in a water solution when compared with those for a
solid lm. Fujii etal. (2004b), therefore, tentatively conclude that strong attractive interactions at the
guanine site from the water molecules are absent, which is consistent with the common understand-
ing of the hydrophobic properties of nucleobases, although the guanine site is thought to be embed-
ded in the bulk water molecules (as discussed above) because of the ratio of the yield of electrons
ejected from nitrogen atoms in GMP to those from other elements. In other words, the hydrophobic
interaction of GMP at the guanine site should increase the interaction energy with an approaching
water molecule and repel the water molecule at a relatively large intermolecular distance, which is
accessible with thermal energy. However, this tentative conclusion as regards the interaction of the
guanine site in GMP with water molecules should be more clearly examined with higher-resolution
chemical-shift measurements. Photoelectron emission spectra from DNA-related molecules in solu-
tion should be analyzed in future using an electron energy analyzer combined with the liquid water
beam technique. Ukai etal. (2009) have developed a photoelectron energy analyzer for liquid beam
samples. They reported that partial electron yields for the K
−1
1b
1
1b
1
Auger transition were obtained
for the rst time by measuring the electrostatically dispersed electron kinetic energy spectra as a
function of photon energy.
20.7 summary
Experimental evidence introduced in this chapter suggests the following: (1) The yield of DNA dam-
age demonstrates a rather complicated relationship when compared with that induced by other radi-
ations, because the yield strongly depends on the radical scavenging conditions during irradiation.
