Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

720 Charged Particle and Photon Interactions with Matter
to be scavenged at acid yields of approximately 5. The observation of abnormality of some diphe-
nyliodonium salts agrees with that in previous studies using γ-ray (Nagahara et al., 2000; Nakano
etal., 2005), EUV (Brainard et al., 2008; Hirose et al., 2008a), and deep UV (Ablaza et al., 2000)
as exposure tools. Side reactions induced by reactive decomposition products have been suggested
as
the reason for the high efciency of DPI-tf.
The
C
37
parameter has been estimated by pulse radiolysis for acid generators. Figure 26.5 shows
representative kinetic traces of the decay of tetrahydrofuran-(THF) solvated electrons with and
without triphenylsulfonium tris(triuoromethansulfonyl)methane (TPS-tsm) (Natsuda et al., 2009).
The decrease in the initial yield and the increase in the decay rate were observed with increasing
TPS-tsm concentration. The increase in the decay rate indicates the reaction of acid generators with
solvated electrons. The rate constants for the reactions of acid generators with solvated electrons
have been used to estimate the effective reaction radii of acid generators (Kozawa et al., 2004).
0
0.01
0.02
0.03
0.04
0.05
0.06
0.07
0.08
0 0.2 0.4 0.6 0.8 1 1.2
Acid generator concentration (M)
Yield of C6 proton adduct (M)
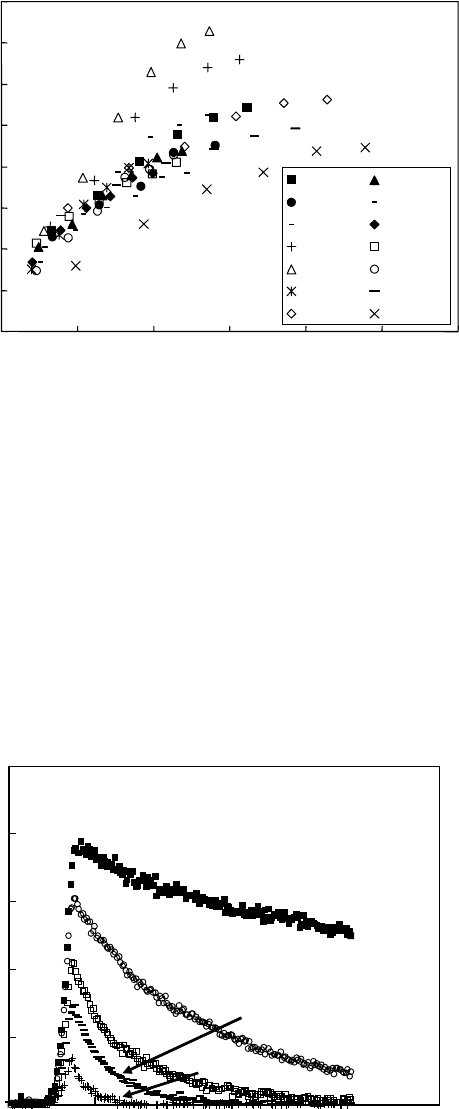
TPS-tf TPS-nf
TPS-PF TPS-Sb
TPS-cpdi TPS-tsm
DPI-tf DPI-nf
BBI-tf BBI-cpdi
BBI-tsm NAI-tf
NDI-tf NDP
Figure 26.4 Relationship between the concentration of acid generators and the yield of C6 proton adducts
(acids).
(From Natsuda, K. et al., Jpn. J. Appl. Phys., 48, 06FC05, 2009. With permission.)
0
0.2
0.4
0.6
0.8
1
0 200 400 600 800 1000
Time (ps)
Optical density
0 mM
10 mM
30 mM
50 mM
100 mM
Figure 26.5 Kinetic traces at 1300 nm in a TPS-tsm solution in THF. The concentrations of TPS-tsm are
0, 10, 30, 50, and 100mM. (From Natsuda, K. et al., Jpn. J. Appl. Phys., 48, 06FC05, 2009. With permission.)
Radiation Chemistry of Resist Materials and Processes in Lithography 721
The radii estimated from the reaction with solvated electrons did not have sufcient accuracy to
explain the difference between acid generators as described above. This is because the acid gen-
erators react not with solvated electrons but with (unsolvated) thermalized electrons in chemically
amplied
resists upon exposure to ionizing radiation.
The
decrease in the initial yield is expressed by the C
37
parameter. Hunt and coworkers (Wolff
et al., 1970, 1975; Aldrich et al., 1971; Lam and Hunt, 1975; Hunt and Chase, 1977) focused on
the decrease in the initial yield of hydrated electrons in water upon addition of various solutes and
demonstrated that the fraction of decrease, f, is expressed by an exponential as a function of solute
concentration:
f
S
C
=
−
exp
[ ]
37
(26.8)
where
[S]
is the concentration of the scavenger
C
37
is a constant that depends on the solvent and the solute
The C
37
parameter can be understood as the solute concentration at which the initial yield of
solvated electrons is decreased to 1/e (37%). C
37
is useful when the concentration is rather high.
When an electron pulse enters the THF solution, THF molecules are ionized and secondary
electrons are emitted. The emitted electrons lose their kinetic energy through their interaction
with surrounding molecules and are nally thermalized. The thermalized electrons are solvated
with THF molecules and become detectable, as shown in Figure 26.5. Considering the time reso-
lution of the pulse radiolysis system used, the initial decrease can be assumed to occur before
solvation. The C
37
parameter is believed to reect the reactions with electrons before solvation
(Hamill, 1969; Lam and Hunt, 1975; Hunt and Chase, 1977; Jonah et al., 1977, 1989; Lewis and
Jonah, 1986; Chernovitz and Jonah, 1988; Saeki et al., 2007). In THF, presolvated states, such
as an excited state of solvated electrons, have not been reported. Therefore, C
37
in THF is likely
related to the reaction with thermalized and possibly epithermal electrons. Figure 26.6 shows
the dependence of the initial yield of THF-solvated electrons on the TPS-tsm concentration
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0 0.05 0.1 0.15 0.2
TPS-tsm concentration (M)
Optical density
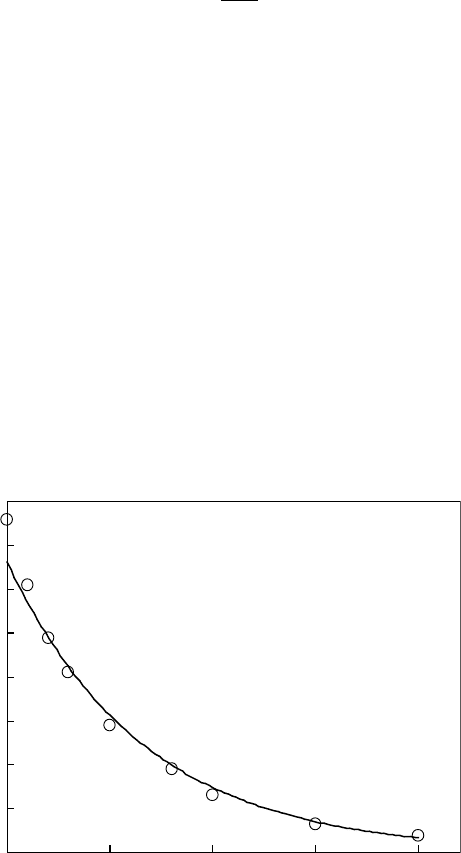
Figure 26.6 Relationship between the initial yield of THF-solvated electrons and TPS-tsm concentration.
(From
Natsuda, K. et al., Jpn. J. Appl. Phys., 48, 06FC05, 2009. With permission.)
722 Charged Particle and Photon Interactions with Matter
(Natsuda et al., 2009). The C
37
parameter can be calculated from the slopes in Figure 26.6. It
has been reported that the C
37
parameter does not correlate to the rate constant for the reaction
with solvated electrons (Natsuda et al., 2008). Figure 26.7 shows the relationships between the
C
37
parameter and the acid yield. The acid yield was evaluated at an acid generator concentra-
tion of 0.4 M. A good correlation was observed between the C
37
parameter and the acid yield,
although chemical reactions in the solid phase are generally different from those in the liquid
phase. Because the thermalization of secondary electrons is completed within 0.1–1ps (Rassolov
and Mozumder, 2001), this thermalization and the immediate reactions of the secondary elec-
trons before solvation are unlikely to be signicantly affected by the translation and rotation of
molecules even in the liquid phase. The C
37
parameter is a good indicator for estimating the reac-
tivity of acid generators with thermalized electrons generated in resist lms. Also, the observed
correlation demonstrated the validity of previously reported reaction mechanisms of chemically
amplied resists (Kozawa et al., 1992).
In EB lithography, a 2–100keV EB is generally used. A single-spur model (Kozawa et al., 2004),
which assumes that each pair does not interact, has been reported to closely reproduce the acid
yields generated in chemically amplied resists upon exposure to a 75keV EB (Kozawa et al.,
2006b, 2007a). The acid generation efciency per ionization is calculated to be 0.74 in a PHS lm
with 10wt% TPS-tf (Kozawa et al., 2005). The single-spur model for chemically amplied EB
resists was constructed on the basis of the fact that it closely reproduces the dynamics of interme-
diate species generated in the model solutions of resist materials upon exposure to a 28MeV EB
(Saeki
et al., 2002; Okamoto et al., 2003, 2007).
The
thermalized electrons migrate in the resist matrix under electric elds produced by charged
species within the Onsager length. Acid generators react with these electrons and release anions.
The mobile electrons are replaced with immobile anions, which act as counteranions of the acid.
20
30
40
50
60
70
40 50 60 70 80 90 100
C
37
(mM)
Acid yield (mM)
N
O S
O
O
O
O
S
+
t-Bu
-C
SO
2
CF
3
SO
2
CF
3
SO
2
CF
3
-C
SO
2
CF
3
SO
2
CF
3
SO
2
CF
3
S
+
SbF
6
S
+
CF
3
SO
3
S
+
C
4
F
9
SO
3
S
+
PF
6
I
+
C
4
F
9
SO
3
I
+
I
+
I
+
I
+
CF
3
SO
3
t-Bu
t-Bu
t-Bu
S
+
O
2
S
-N
O
2
S
CF
2
CF
2
CF
2
O
2
S
-N
O
2
S
CF
2
CF
2
CF
2
t-Bu
t-Bu
N
O
O
N
O
O
OSO
2
CF
3
OSO
2
CF
3
CF
3
SO
3
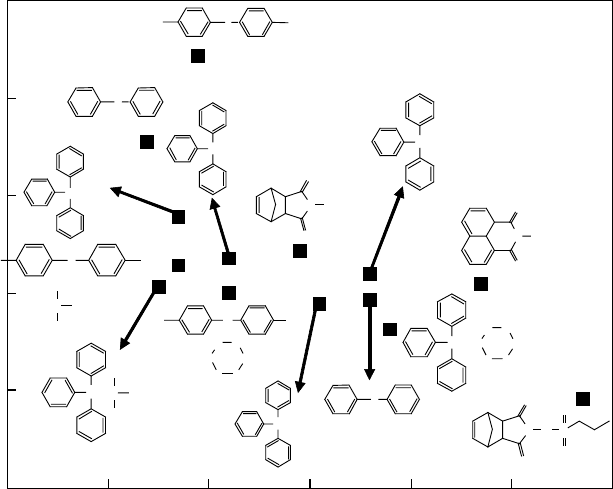
Figure 26.7 Relationship between the C
37
parameter and the acid yield generated in PHS lms with
5wt% C6 and 0.4 M acid generators. (From Natsuda, K. et al., Jpn. J. Appl. Phys., 48, 06FC05, 2009. With
permission.)
Radiation Chemistry of Resist Materials and Processes in Lithography 723
The calculated distribution of counteranions is shown in Figure 26.8 (Kozawa et al., 2009). The
distribution calculated using the single-spur model is also shown in Figure 26.8. The integral of the
graph indicates the acid generation efciency, which decreased when multispurs were taken into
account. The relationship between the average number of overlapped ion pairs and acid generation
efciency (per ionization) was calculated as shown in Figure 26.9. The relationship with the resolu-
tion blur is also shown in Figure 26.9. The acid generation efciency decreases with the increase
in the average number of overlapped ion pairs because of the strong electric elds generated by
multiple cations and the cross recombination. The strong electric elds promote the recombination
between cations and electrons thermalized near cations and decrease the probability of the reaction
of acid generators with electrons. Similarly, the resolution blur increases with the average number
of ion pairs. The acid generation efciency was calculated to be 0.70 per ionization in EB resists at
10wt% TPS-tf loading (0.74 for the single-spur model). The resolution blur was 5.8nm (5.6nm for
the single-spur model).
In nanoscale patterning using a highly sensitive resist, such as a chemically amplied resist,
the statistical effect is not negligible. The fact that ion pairs overlap indicates an increase in the
inhomogeneity of the distribution of intermediate species. However, the long electron migration
in the subexcitational energy region moderates this inhomogeneity, although it induces resolution
blur (Kozawa and Tagawa, 2006). The subsequent proton migration also contributes to reducing the
inhomogeneity
of the acid distribution (Saeki et al., 2006).
26.5 deprotonation oF radiCal Cations
26.5.1 dynaMicS of phS radical cation (phS
•+
)
When onium salts react with low-energy electrons generated by ionization, they decompose into a
neutral radical and an anion. This decomposition path of onium salts is similar to that induced by
the electron transfer from the excited state of polymers observed in photoresists in KrF excimer
laser lithography (Hacker et al., 1992; Cameron et al., 2001). The decomposed products of neutral
triphenylsulfonium radicals were suggested by Dektar and Hacker (1990a). Considering that the
0.00
0.02
0.04
0.06
0.08
0.10
0.12
0 10 20 30
Distance (nm)
Anion generation probability per ionization (nm
–1
)
Single-spur model
Multispur model (EB)
Multispur model (EUV)
Figure 26.8 Distribution of probability of anion generation per spherical-shell thickness (per ionization).
The horizontal axis represents the distance from the rst ionization point (the origin). (From Kozawa, T. et al.,
Jpn. J. Appl. Phys.,
48, 056508, 2009. With permission.)
724 Charged Particle and Photon Interactions with Matter
decomposed products are diphenylsuldes and benzenes (phenyl radicals), it is clear that protons are
not generated from onium salts in this reaction path. Instead, protons are generated from the radi-
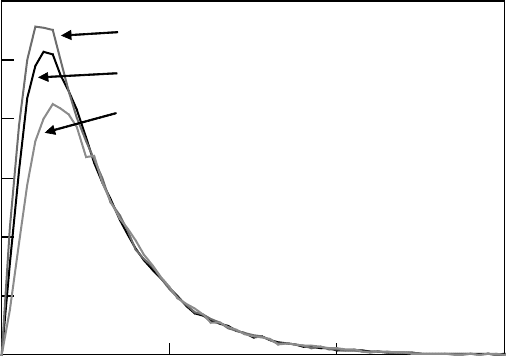
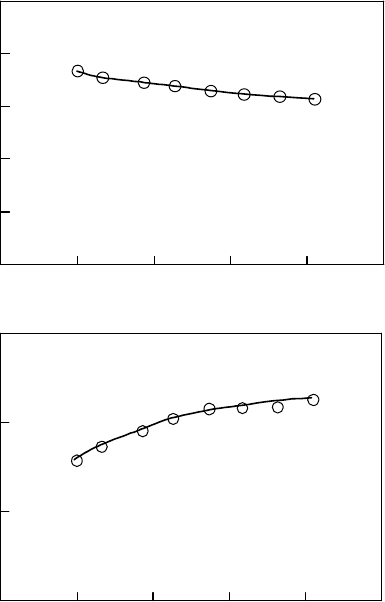
cal cations of polymers (Kozawa et al., 1997). Figure 26.10 shows the transient absorption spectra
obtained in the pulse radiolysis of 100mM of PHS-benzonitrile solution, monitored at the pulse end,
and 200 and 400 ns after the electron pulse (Nakano et al., 2006b). Note that the spectrum at the
pulse end largely overlapped with the transient absorption spectra of solvent intermediates (Tagawa
et al., 1972). An absorption peak was observed at 390nm and 200 and 400ns after the pulse. The
absorption at 390nm does not decay within the observed time range. The absorption from 400 to
550nm shows a slight decay. Comparing the absorptions at 200 and 400ns, this decaying compo-
nent is considered to have an absorption maximum at 430–450nm. By adding triethylamine (TEA)
to the sample, changes in transient spectra were observed, as shown in Figure 26.11 (Nakano et al.,
2006b). Although the absorption intensity decreased, the kinetics at 390nm did not change from
200 to 400nm after the pulse. The decaying component observed between 400 and 550nm almost
disappeared
with TEA addition.
Although
the transient absorption spectra of PHS intermediates have not yet been reported, the
radiation chemistry of phenol derivatives has been well studied. Phenoxy radicals, which are stable
decomposition intermediates of phenols, were rst observed in the 1950s (Porter and Wright, 1955).
Land
et al. reported that phenoxy radicals are very weak bases (Land et al., 1961) and show a char-
acteristic
absorption band at 400 nm (Land and Ebert, 1967). The absorption maxima of phenoxy
4
5
6
7
0 1 2 3 4 5
0.0
0.2
0.4
0.6
0.8
1.0
0 1 2 3 4 5
Average number of ion pairs(a)
(b)
Acid generation eciency
per ionization
Average number of ion pairs
Resolution blur (nm)
Figure 26.9 Relationship of average number of ion pairs in an isolated space with (a) acid generation ef-
ciency (per ionization) and (b) resolution blur. (From Kozawa, T. et al., Jpn. J. Appl. Phys., 48, 056508, 2009.
With
permission.)
Radiation Chemistry of Resist Materials and Processes in Lithography 725
radicals of phenol and p-cresol in water are 400 and 405 nm, respectively (Land and Porter, 1963;
Land and Ebert, 1967). Radical cations of phenol derivatives, which are precursors of phenoxy
radicals in many cases, have been more elusive than phenoxy radicals (Land et al., 1961; Dixon and
Murphy, 1976, 1978; Holton and Murphy, 1979; Maruyama et al., 1986; Tripathi and Schuler, 1987;
Shoute and Neta, 1990; Kesper et al., 1991; Brede et al., 1996). The absorption maxima of radical
cations of phenol and p-cresol are 440 and 430nm, respectively (Ganapathi et al., 2000). These
reports are consistent with the results for PHS. The absorption at 390nm and the decaying compon-
ent
were assigned to the phenoxy radical and the radical cation of PHS, respectively.
The
deprotonation of radical cations of phenol derivatives has been investigated. Dixon and
Murphy reported that the pK
a
values of radical cations of phenol and p-cresol were −2.0 and −1.6,
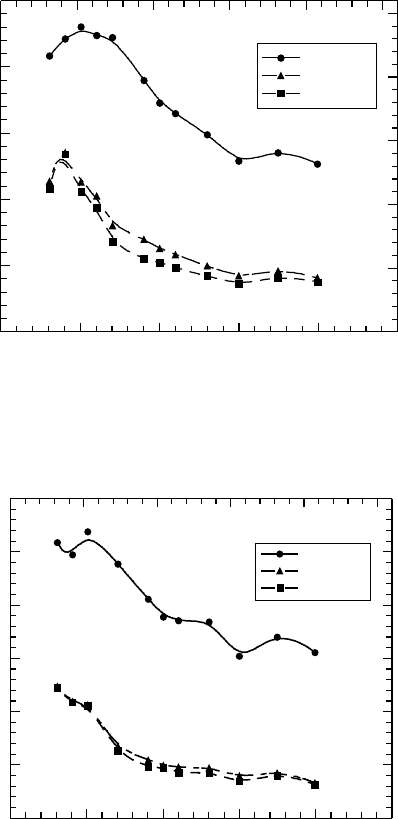
Δ O.D.
350 400 450 500 550 600
Pulse end
200 ns
400 ns
0
0.1
0.2
0.3
0.4
0.5
0.6
Wavelength (nm)
Figure 26.11 Transient absorption spectra obtained in pulse radiolysis of 100mM of PHS solution in ben-
zonitrile with 2.5 mM TEA. The sample was deaerated by SF
6
bubbling before irradiation. (From Nakano, A.
et
al., Jpn. J. Appl. Phys., 45, 6866, 2006b. With permission.)
Δ O.D.
350 400 450 500 550 600
Pulse end
200 ns
400 ns
0
0.1
0.2
0.3
0.4
0.5
Wavelength (nm)
Figure 26.10 Transient absorption spectra obtained in pulse radiolysis of 100mM of PHS solution in
benzonitrile. The sample was deaerated by SF
6
bubbling before irradiation. (From Nakano, A. et al., Jpn.
J.Appl. Phys., 45, 6866, 2006b. With permission.)
726 Charged Particle and Photon Interactions with Matter
respectively (Dixon and Murphy, 1978). Also, Bordwell and Cheng reported that those of phenol and
p-cresol were −8.1 and −7.1, respectively (Bordwell and Cheng, 1991). The radical cation of 2,4-dime-
thoxyphenol was reported to be deprotonated by 2,6-lutidine at a rate constant of 6.5 × 10
8
M
−1
s
−1
in an acetonitrile solution (Gadosy et al., 1999). The radical cation of phenol is deprotonated by
ethanol at a rate constant of 9.16 × 10
10
M
−1
s
−1
(Ganapathi et al., 2001). This is in contrast to the
reaction of the poly(4-methoxystyrene) (PMS) radical cation with ethanol, as discussed in the fol-
lowing text. For p-cresol, the absorption coefcients of the phenoxy radical and the radical cation
are 2.30 × 10
3
and 2.88 × 10
4
M cm
−1
at each peak wavelength (Ganapathi et al., 2000). For the
other phenol derivatives, the absorption coefcients of radical cations observed at 400–600 nm
are generally larger than those of phenoxy radicals observed at 400–500 nm. Therefore, the radi-
cal cation of PHS is considered to be mostly deprotonated 200 ns after the pulse. Considering that
ethanol and p-cresol have similar proton afnities (PAs), the efcient deprotonation of PHS is
probably due to the ion-molecular reaction between base units.
26.5.2 dynaMicS of pMS radical cation (pMS
•+
)
PMS is a model compound of a typical resist polymer (partially protected PHS). The pulse radi-
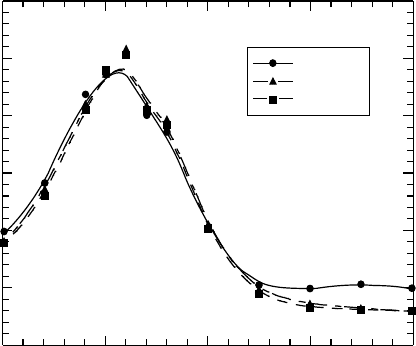
olysis of a PMS solution in dichloromethane has been reported. Figure 26.12 shows the transient
absorption spectra obtained in the pulse radiolysis of 100 mM of PMS solution in dichlorometh-
ane deaerated by Ar bubbling (Nakano et al., 2006b). The transient absorption spectra shown in
Figure 26.12 have a peak at approximately 460 nm. The peak did not decay within the observed
time range. The transient absorption spectra of PMS have not been reported. However, the dynam-
ics of radical cations of methoxylated benzenes, such as methoxybenzene (anisole), methoxytolu-
ene, and dimethoxybenzene, have been intensively investigated using pulse radiolysis (O’Neill
et al., 1975; Holcman and Sehested, 1976; Takamuku et al., 1989; Jonsson et al., 1993), pho-
tolysis (Ito et al., 1989; Baciocchi et al., 1993), chemical oxidations (Forbes and Sullivan, 1966;
Sullivan and Brette, 1975; Schlesener and Kochi, 1984), and electrochemical methods (Ronlan
et al., 1974; Buck and Wagoner, 1980). Radical cations of p-methoxytoluene are observed at 440
(Baciocchi et al., 1993) and 450nm (Ito et al., 1989) in acetonitrile and at 460 nm in 1,2-dichlo-
roethane (Takamuku et al., 1989), and those of methoxybenzene are observed at 430 nm in water
(O’Neill et al., 1975; Holcman and Sehested, 1976; Jonsson et al., 1993). Also, in an aromatic
Δ O.D.
400 450 500 550 600
Pulse end
200 ns
400 ns
0
0.1
0.2
0.3
Wavelength (nm)
Figure 26.12 Transient absorption spectra obtained in pulse radiolysis of 100mM of PMS solution in
dichloromethane.
(From Nakano, A. et al., Jpn. J. Appl. Phys., 45, 6866, 2006b. With permission.)
Radiation Chemistry of Resist Materials and Processes in Lithography 727
compound solution in a halocarbon, a π-molecular complex forms through a reaction with a radical
cation of aromatic compounds with a chlorine anion. The absorption maxima of the π-molecular
complex band of methoxybenzene are 509 nm in dichloromethane (Zweig, 1963) and 524nm in
carbon tetrachloride (Raner et al., 1989). As for methoxytoluene, Takamuku et al. reported that
the π-molecular complex of a p-isomer is not observed in the visible wavelength region, although
those of o- and m-isomers are observed at 550 and 490nm, respectively (Takamuku et al., 1989).
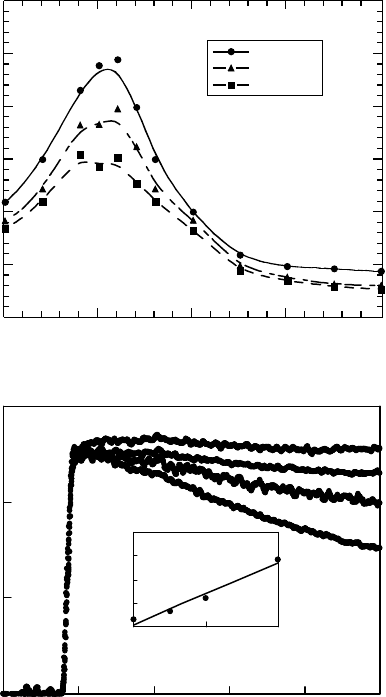
To clarify the assignment of the absorption at 460 nm (Figure 26.12), TEA was added to the solu-
tion. TEA is a well-known cation scavenger. Figure 26.13 shows the transient absorption spectra
of the solution with 10 mM of TEA and the kinetic curves of absorption at 460nm at several
TEA concentrations. The decay of the absorption curve became faster with an increase in the TEA
concentration. The observed rate constants are plotted in the inset of Figure 26.13b. These rate
constants show a linear dependence on the TEA concentration. The bimolecular rate constant cal-
culated from the slope is 1.3 × 10
8
M
−1
s
−1
. By analogy with previous research results mentioned
0
0.1
0.2
0.3
0
100 200 300 400 500
400 450 500 550 600
Pulse end
200 ns
400 ns
Wavelength (nm)
Kinetic curves
Transient spectra
(a)
(b)
∆ O.D. ∆ O.D.
0.1
0.2
0.3
0
Time (ns)
0
1
2
1050
Concentration (mM)
Rate constant
(×10
6
s
–1
)
0 mM
2.5 mM
5.0 mM
10.0 mM
Figure 26.13 (a) Transient absorption spectra obtained in pulse radiolysis of 100 mM of PMS solution
in dichloromethane with 10mM TEA and (b) changes in kinetic curves at 460 nm induced by TEA addition
(0, 2.5, 5.0, and 10.0 mM). The inset shows the relationship between the observed rate constant and the
concentration of TEA. (From Nakano, A. et al., Jpn. J. Appl. Phys., 45, 6866, 2006b. With permission.)
728 Charged Particle and Photon Interactions with Matter
above and from our experimental results, we conclude that the absorption band at 460 nm is
ascribed not to the π-molecular complex but to the radical cation of PMS.
The deprotonation of radical cations of methoxylated benzenes has been investigated (Schlesener
and Kochi, 1984; Baciocchi et al., 1993). The pK
a
of the dimethoxytoluene radical cation is 0.45 in
acetonitrile (Baciocchi et al., 1993), which is signicantly higher than those of phenol and p-cresol.
Dimethoxytoluene radical cations are deprotonated at a rate constant of 3.0 × 10
6
M
−1
s
−1
by 2,6-lutidine
(Baciocchi et al., 1993), whose PA is 955kJ mol
−1
(Lias et al., 1984). This is two orders lower than that
for the reaction of 2,4-dimethoxyphenol radical cations with 2,6-lutidine. It is important to determine
whether the PMS radical cation is deprotonated in the absence of strong proton acceptors, such as
amine. Using ethanol as a proton acceptor, the deprotonation of the PMS radical cation has been inves-
tigated (Nakano et al., 2006b). Ethanol has a PA (788kJ mol
−1
) similar to that of the oxygen atom of
p-cresol (PA = 756kJ mol
−1
) (van Beelen et al., 2004), which is a model compound of PHS. By adding
ethanol to 100mM of PMS-dichloromethane solution, it was examined whether PMS
•+
is deproton-
ated. However, no evident decay was observed within the observed time range. This means that PMS
•+
does not release a proton, at least spontaneously. Also, because the PA of p-methoxytoluene is
801kJ mol
−1
(van Beelen et al., 2004), the deprotonation through ion-molecular reaction with a neigh-
boring unit is considered to be ineffective. This is consistent with the experimental result that PMS
•+
hardly decays within the observed time range, as shown in Figure 26.12. It has been conrmed that the
acid yield in the PHS lm with TPS-tf was about two times higher than that in the PMS lm (Nakano
et al., 2006b). The decrease of the acid yield agrees with the difference in deprotonation dynamics
between PMS and PHS radical cations observed in the pulse radiolysis experiments.
On the basis of the experiments in solutions, the deprotonation mechanism of PHS is suggested
as
follows:
MOH MOH e
+
→ +
−
(26.9)
MOH MOH MO MOH
+
2
+ → +
+
(26.10)
Here, MOH, MOH
•+
, MO
•
, and MOH
2
+
represent a PHS molecule, its radical cation, its phenoxy
radical, and its proton adduct, respectively. This deprotonation mechanism is similar to that of
novolak (Kozawa et al., 1997). The PHS radical cation generated by ionization is deprotonated by
inter- and intramolecular ion-molecular reactions. The reaction mechanism in the solution may be
different from that in the solid lm because of the different conditions of polymers. However, most
PHS hydroxyl groups form inter- and intramolecular hydrogen bonds with a neighboring hydroxyl
group in solid resist lms (Li and Brisson, 1998; Singh et al., 2005). Therefore, reaction (26.10) is
considered
to efciently take place even in solid lms.
26.5.3 pMMa
Upon exposure to ionizing radiation, PMMA molecules are either ionized or excited. For keV
electrons, the cross section of ionization is larger than that for excitation. Through ionization,
a PMMA radical cation and an electron with excess energy are generated. The ejected electron
reacts with PMMA molecules or recombines with its parent radical cation after losing a suf-
cient amount of energy. A PMMA anion radical is formed through the reaction of PMMA with
an electron (Ogasawara et al., 1987; Nakano et al., 2004a). An excited state of PMMA is also
formed through the recombination of an electron with its parent radical cation. The radical cat-
ions (Ichikawa and Yoshida, 1990), radical anions (Tabata et al., 1983; Sakai et al., 1995), and
excited PMMA molecules (Fox et al., 1963; Gupta et al., 1980; Torikai et al., 1990) are reported
to decompose. In either case, the side chain is rst detached and a macroradical is formed.
This macroradical results in β-scission. During the decomposition, protons are considered to be
Radiation Chemistry of Resist Materials and Processes in Lithography 729
generated. It is known that PMMA becomes acidic upon irradiation. Proton generation has been
observed in PMMA lms without an acid generator. The G-value of acid generation is 1.4 with-
out any acid generators (Nakano et al., 2005). Although the counteranion has not been identied,
it is possibly a formic (Tatro et al., 2003) or acetic (Gupta et al., 1980) anion. The main-chain
scission of PMMA is also induced by UV light (Fox et al., 1963; Gupta et al., 1980; Torikai
et al., 1990). PMMA decomposes through the n-π* singlet state. Upon exposure to ionizing
radiation, the contribution of the excited state of PMMA is, however, low because the recombi-
nation reaction (the main generation path for the excited state) is suppressed due to the reaction
of electrons with PMMA. In the presence of acid generators, the generation of an excited state
through a recombination reaction is suppressed even more because acid generators are strong
electron scavengers. The decomposition of PMMA radical anions also leads to main-chain scis-
sion (Sakai etal., 1995). However, the efciency has not been reported. If the decomposition
of PMMA anion radicals is effective, this path causes signicant resolution blur, because the
ejected electrons migrate in the PMMA matrix before they are trapped by PMMA. However,
PMMA is known as the highest-resolution resist. The ultimate resolution of the PMMA resist has
been intensively investigated and is reported to be 3–5 nm (Chen and Ahmed, 1993; Cumming
et al., 1996; Yasin et al., 2001). Therefore, PMMA radical anions are unlikely to decompose
effectively. This is also consistent with the fact that PMMA radical anions are observed to be
relatively stable (Nakano et al., 2004a).
PMMA radical cations, which are obviously a primary product brought about by ionization,
have not been observed even at 4.2K (Tanaka et al., 1990). The identication of PMMA radical
cations was previously reported (Tabata et al., 1983). They later turned out to be PMMA radical
anions (Ogasawara et al., 1987). This elusiveness of PMMA radical cations is widely believed to
be due to their short lifetime. Although the decomposition path has not been completely clari-
ed, some intermediates and products have been identied. Besides PMMA radical anions, neutral
radical species such as main-chain radical –C
•
H–, side-chain radical –COOC
•
H
2
–, and β-scission
products –CH
2
–C
•
(CH
3
)COOCH
3
have been identied (Geuskens and David, 1973). It has been
widely accepted that the main-chain radical leads to main-chain scission. The G-values of main-
chain scission reported by many researchers were scattered. The average of the reported values
is 1.9 ± 0.3 (Chapiro, 1962). The main-chain radical is generated through the detachment of the
side chain. Among these reported neutral radicals, the side-chain radical is a direct product gener-
ated through the deprotonation of PMMA radical cations. The G-value of –COOC
•
H
2
– has been
reported to be approximately 2 (Tanaka et al., 1990). The G-value of triuoromethanesulfonic acids
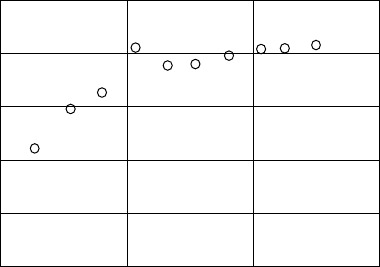
in PMMA with 10wt% TPS-tf is shown in Figure 26.14 (Kozawa et al., 2007a). The G-value of
G-value (molecules/100 eV)
Acid generator concentration (M)
0.0
0.5
1.0
1.5
2.0
2.5
0.0 0.1 0.2 0.3
Figure 26.14 Dependence of the G-value of acid generation in PMMA on acid generator concentration.
(From
Kozawa, T. et al., J. Photopolym. Sci. Technol., 20, 577, 2007a. With permission.)
