Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

(a)
(b)
D
D
Δ
D
D
Δ
(c)
Δ
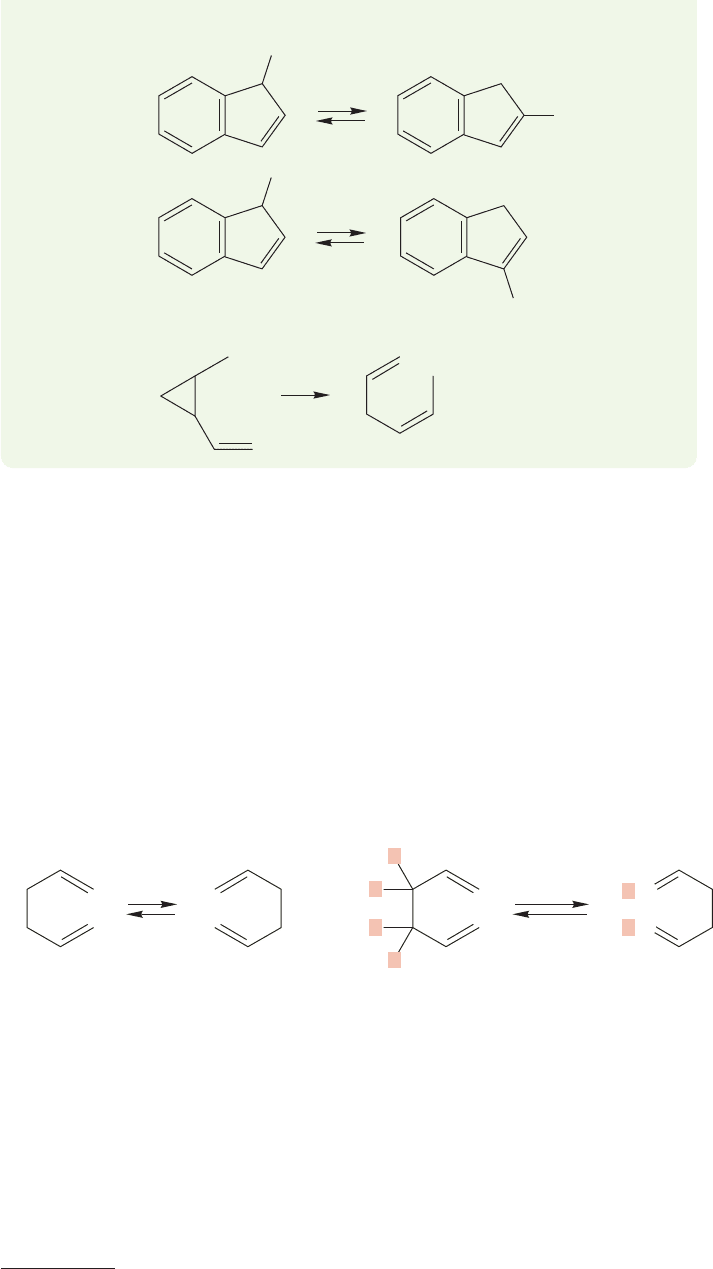
20.6 The Cope Rearrangement 1059
20.6 The Cope Rearrangement
As we saw in Problem 20.13, not all shift reactions are [1,x] shifts. One very com-
mon sigmatropic shift reaction is called the Cope rearrangement, and is named for
Arthur C. Cope (1909–1966, the same Cope of the Cope elimination, p. 914) who
spent most of his professional life at MIT.
4
The Cope rearrangement is a [3,3]
sigmatropic shift that occurs in almost any 1,5-hexadiene. In this sigmatropic shift
the new bond is formed between the two number 3 atoms, which is the reason it is
called a [3,3] shift (Fig. 20.49). In its simplest form, the Cope rearrangement is
degenerate and must be revealed through a labeling experiment.
4
Cope didn’t actually discover this reaction; C.Hurd of Northwestern University (1897–1998) apparently did.
It was Cope’s research group that thoroughly studied the reaction, however.
This degenerate,
“invisible” reaction…
…is revealed by this
labeling experiment
A [3,3] shift—a Cope rearrangement
Δ
2
2
31
31
2
2
31
31
D
D
D
D
D
2
C
D
2
C
90 ⬚C
FIGURE 20.49 The degenerate Cope rearrangement, a [3,3] shift, can be revealed by a
labeling experiment.
Like the [1,5] shifts we looked at first, the Cope rearrangement is intramol-
ecular, uncatalyzed, and shows no strong dependence on solvent polarity.The acti-
vation energy for the Cope rearrangement is about 34 kcal/mol, a value far below
what one would estimate for the simple bond breaking into two separated allyl
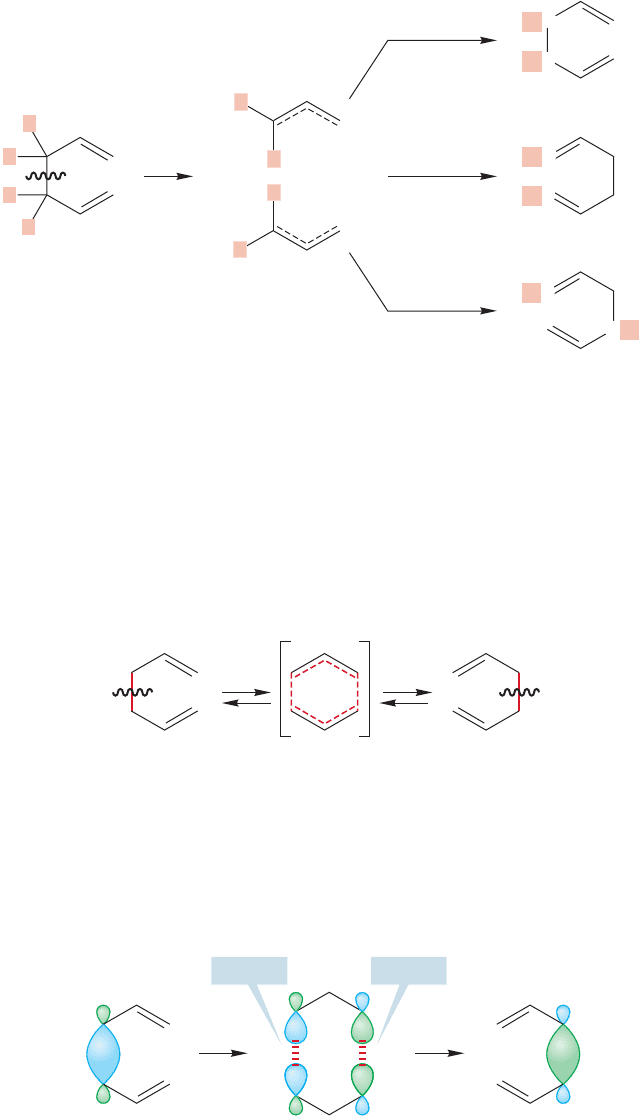
1060 CHAPTER 20 Reactions Controlled by Orbital Symmetry
radicals (Fig. 20.50). If the mechanism of the Cope rearrangement did involve two
independent allyl radicals,the labeled 1,5-hexadiene would have to give two prod-
ucts, which it does not.
.
.
2
2
1
1
3
3
D
D
D
D
1
1
3
3
1
1
3
3
Two independent
allyl radicals
(starting material)
recombine
3,3
recombine
1,1
recombine
Not found
1,3
D
D
D
D
D
2
C
D
2
C
D
2
C
D
2
C
D
2
C
CD
2
FIGURE 20.50 A labeling experiment
shows that the Cope rearrangement
does not involve formation of a pair
of free allyl radicals because the
radical recombination products are
not all formed.
Reattachment at the two C(3) positions is allowed because the interaction of
the two lobes on the two C(3) carbons is bonding.This picture is consistent with
the near ubiquity of the Cope rearrangement when 1,5-dienes of all kinds are
heated (Fig. 20.52).
The Cope rearrangement has a nonpolar transition state (Fig. 20.51), but this
time it is not a simple hydrogen atom that migrates but a whole allyl radical. An
analysis of the symmetry of the orbitals involved shows why this reaction is a
relatively facile thermal process but is not commonly observed on photochemical
activation. As we break the bond, the phases of the overlapping lobes
must be the same.The HOMO of the allyl radical is
2
,and that information allows
us to fill in the symmetries of the two allyl groups making up the transition state.
£
C(1)
O
C(1)
Transition state
(migration of an
allyl radical)
Δ
.
.
FIGURE 20.51 The transition state
for the Cope rearrangement involves
the migration of one allyl radical
across another.
2
2
1
1
1
1
3
3
3
3
Bonding Bonding
Transition state (two partially
bonded allyl radicals—Φ
2
is
the singly occupied HOMO
for each)
FIGURE 20.52 An orbital symmetry
analysis shows that the thermal Cope
rearrangement is allowed.
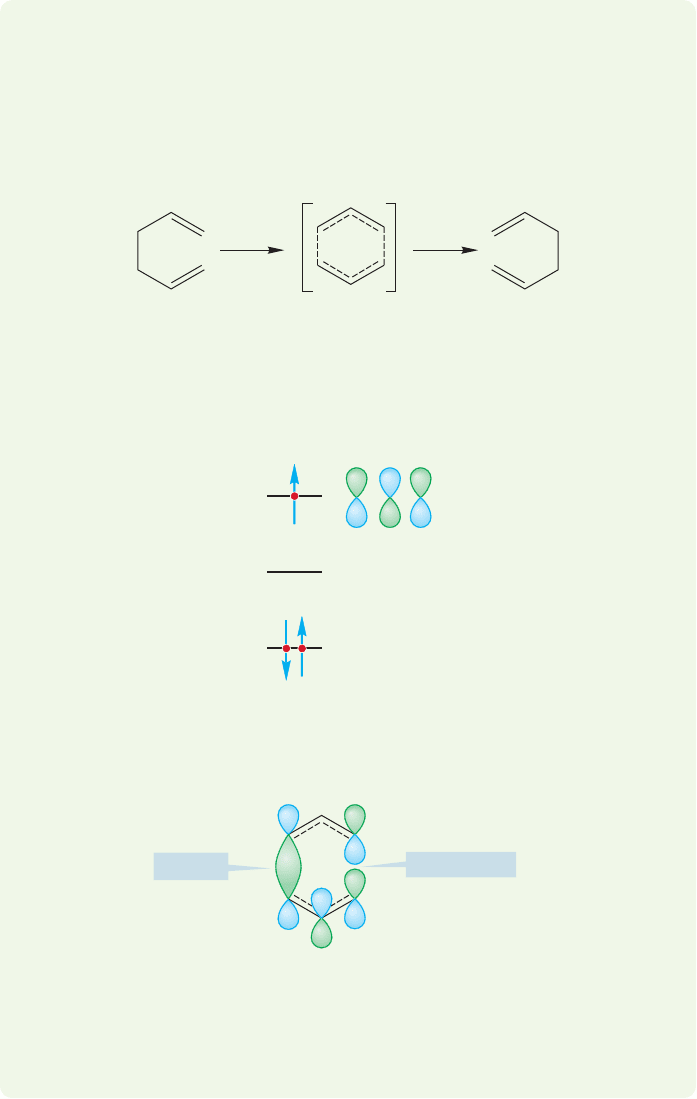
20.6 The Cope Rearrangement 1061
So we now have a general picture of the transition state for the Cope rearrange-
ment. But much more detail is possible if we work a bit at it. How might the two
migrating allyl radicals of the transition state arrange themselves in space during the
reaction? Two things are certain. First, the two C(1) atoms must start out close
together, because they are bonded. Second, the two C(3) positions must also be
within bonding distance, because in the transition state there is a partial bond
between them (Fig. 20.52).
Transition state
for the [3,3] shift
.
.
WORKED PROBLEM 20.17 Use orbital symmetry to analyze the photochemical
[3,3] shift in 1,5-hexadiene. Be careful! Photons are absorbed one at a time.It
requires specialized equipment to form a singly excited molecule and then have it
absorb a second photon. So we need only consider the singly excited state.
ANSWER The transition state for the [3,3] shift involves two interacting allyl
radicals.
If one electron is promoted on irradiation, the transition state must have one
electron in
3
, the photochemical HOMO.£
Φ
3
Φ
3
Φ
2
Antibonding!
Bonding
If a rotation were energetically feasible, if one C(3) carbon could rotate, a bond-
ing interaction could be achieved where the figure notes the antibonding inter-
action. However, for these sp
2
hybridized carbons, this rotation involves a large
energy cost.
Now an orbital analysis shows that there is no easy path for the photochemical
[3,3] shift. One antibonding interaction is always present.
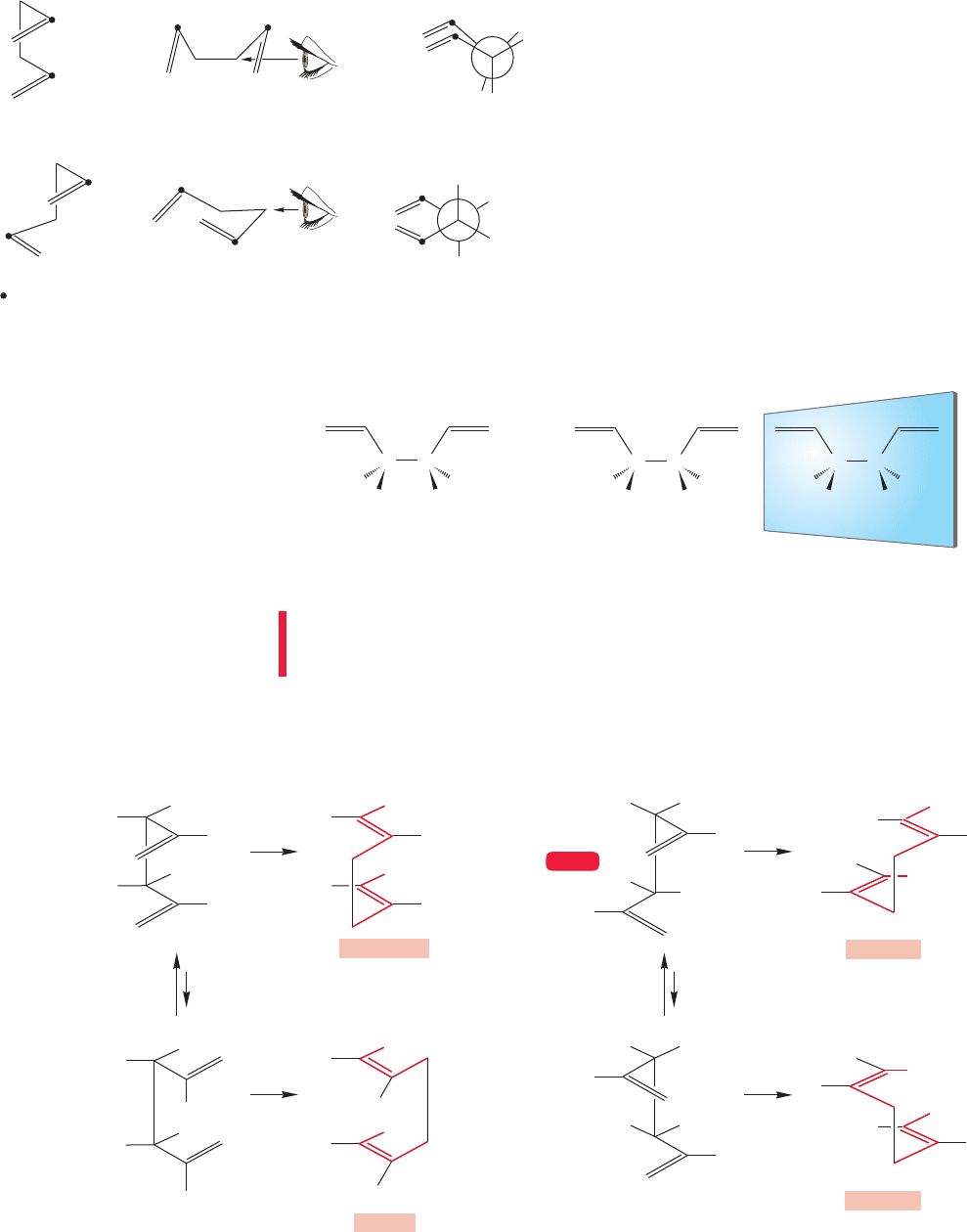
1062 CHAPTER 20 Reactions Controlled by Orbital Symmetry
The question now comes down to determining
the position of the two C(2) atoms in the transition
state.Two possibilities are shown in Figure 20.53.The
two C(2) atoms might be close together as in the boat-
like transition state originally called the six-center
transition state. Or, they can be rather far apart, as in
the chairlike or four-center transition state. The fig-
ure shows a number of views of these two possibili-
ties, but this is probably a time to get out the models.
In 1962, W. von E. Doering and W. R Roth car-
ried out a brilliant experiment that determined which
of these two possible transition states was favored for
this [3,3] sigmatropic shift.They used a stereolabeled
molecule, 3,4-dimethyl-1,5-hexadiene (Fig. 20.54).
Notice first that there are three forms of this molecule,a meso,achiral form and a racemic
pair of enantiomers (p. 156). It all depends on how the methyl groups are attached.
2
H
H
Boatlike, or “six-center” arrangement
2
2
3
3
1
1
H
H
H
H
H
H
22
2
33
11
1
= C(2)
Chairlike, or four-center arrangement
2
2
3
3
1
1
2
2
2
2
3
3
3
3
1
1
1
3
3
=
=
=
=
FIGURE 20.53 Several views of the
chairlike and boatlike arrangements
for the transition state of the Cope
rearrangement.
HH
H
3
CCH
3
CC
HCH
3
H
3
CH
CC
H
CH
3
H
H
3
C
CC
Mirror
Racemic mixture
Meso form
FIGURE 20.54 The meso and
chiral forms of 3,4-dimethyl-1,5-
hexadiene.
In the following figures,we have drawn only one enantiomer of the racemic pair.
Be sure to keep in mind that the actual experiment used a 50:50 racemic mixture of
the two enantiomers.
Both the meso and racemic forms of 3,4-dimethyl-1,5-hexadiene can adopt
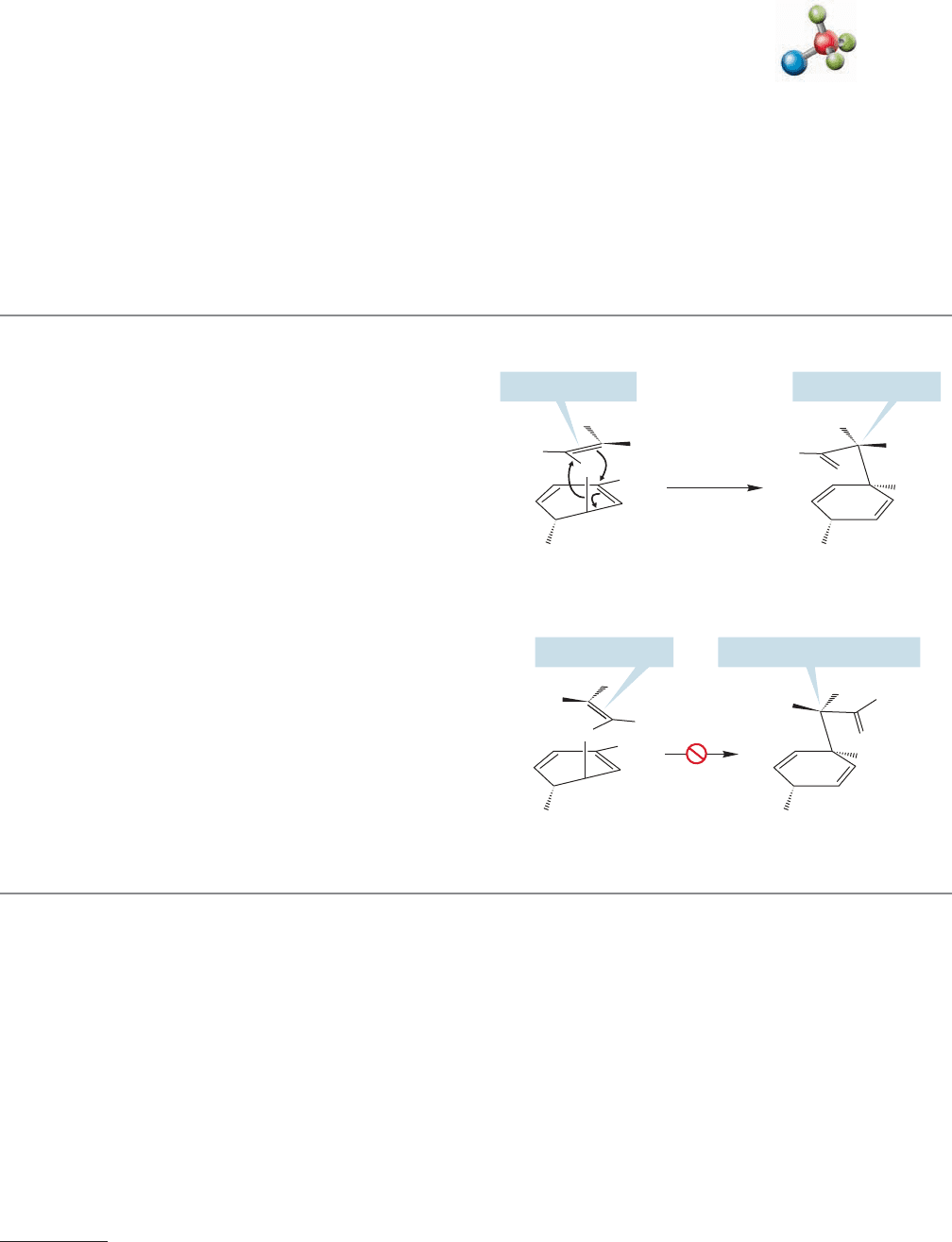
either the boat or chair arrangement. Figure 20.55 shows the results for the
CONVENTION ALERT
CH
3
CH
3
H
H
Meso compound in the boatlike arrangement
Δ
H
H
trans,trans
(E,E )
Δ
cis,cis
(Z,Z )
Meso compound in the chairlike arrangement
CH
3
CH
3
H
H
CH
3
CH
3
H
H
H
H
CH
3
CH
3
H
H
Δ
H
H
H
H
trans,cis
(E,Z )
H
CH
3
CH
3
CH
3
CH
3
Δ
H
H
H
H
cis,trans
(Z,E )
H
H
H
H
H
CH
3
CH
3
H
H
H
H
CH
3
CH
3
H
H
WEB 3D
FIGURE 20.55 The products formed from the boatlike and chairlike arrangements of meso-3,4-dimethyl-1,5-hexadiene.
20.7 A Molecule with a Fluxional Structure 1063
meso compound. The key to Doering and Roth’s experiment is that the chair
and boat arrangements give different products for both the meso and chiral
forms.
The results showed that it is the chairlike form that is favored,and that it is pre-
ferred to the boatlike arrangement by at least 6 kcal/mol. Presumably, some of the
factors that make chair cyclohexane more stable than the boat form (p. 198) oper-
ate to stabilize the related chairlike transition state.
There are many things that are important about this work. First of all, it is a
prototypal example of the way labeling experiments can be used to dig out the
details of a reaction mechanism.The second reason is more subtle,but perhaps more
important. As we will see in Section 20.7, the study of the Cope rearrangement
leads directly to an extraordinarily intellectually creative idea.
CHORISMATE TO PREPHENATE: A BIOLOGICAL COPE
REARRANGEMENT
The Cope rearrangement is by no means restricted to the
laboratory. Nature uses Cope-like processes as well. For
example, in bacteria and plants a critical step in the
production of the essential amino acids tyrosine and
phenylalanine is the rearrangement of chorismate to
prephenate, catalyzed by the enzyme chorismate mutase.
At the heart of this rearrangement is a Cope-like process
called the Claisen
5
rearrangement. The research groups of
Jeremy Knowles (1935–2008) at Harvard and Glenn
Berchtold (1932–2008) at MIT collaborated on a tritium
(
3
H T, a radioactive isotope of hydrogen) labeling exper-
iment, related to that used by Doering and Roth, to deter-
mine that the enzymatic reaction also proceeds through a
chairlike transition state. Notice that the chairlike transi-
tion state must take the compound with a (Z) double bond
to a product in which the stereogenic carbon is (S), not
(R). The conversion of chorismate into prephenate is a
Cope-like rearrangement. The chair transition state is
shown in the top reaction and a boat transition state is
shown in the bottom reaction.
(Z ) Double bond
This carbon is (S)
This carbon would be (R )
OH
O
T
H
T
H
–
OOC
COO
–
chorismate
mutase
Chorismate
Chairlike transition
state for chorismate
Prephenate
OH
–
OOC
COO
–
O
(Z ) Double bond
OH
O
T
H
T
H
COO
–
COO
–
...the tritium-labeled
carbon would be (R)
lf this boatlike transition
state were used…
OH
–
OOC
COO
–
COO
–
O
20.7 A Molecule with a Fluxional Structure
We spent some time in Section 20.6 working out the details of the structure of the
transition state for the Cope rearrangement. This rearrangement is a most general
reaction of 1,5-hexadienes, and appears in almost every molecule incorporating the
1,5-diene substructure.In this section we will examine the consequences of the Cope
rearrangement and will work toward a molecule whose structure differs in a funda-
mental way from anything you have seen so far in this book. At room temperature
it has no fixed structure; instead it has a fluxional structure,in which nearest neigh-
bors are constantly changing. Along the way to this molecule there is some extraor-
dinary chemistry and some beautiful insights. This section shows not only clever
ideas and beautiful chemistry, but also how much fun this subject can be.
5
The same Claisen of the Claisen condensation (p. 987) and the Claisen–Schmidt reaction (p. 984).
Cope rearrangement
1064 CHAPTER 20 Reactions Controlled by Orbital Symmetry
The degenerate
Cope rearrangement
of 1,5-hexadiene
Δ
An electrocyclic
rearrangement, a
modified Cope
rearrangement
1,3-Cyclohexadienecis-1,3,5-Hexatriene
Δ
H
H
FIGURE 20.56 The electrocyclic ring closure of cis-1,3,5-hexatriene to 1,3-cyclohexadiene is similar to a Cope
rearrangement.
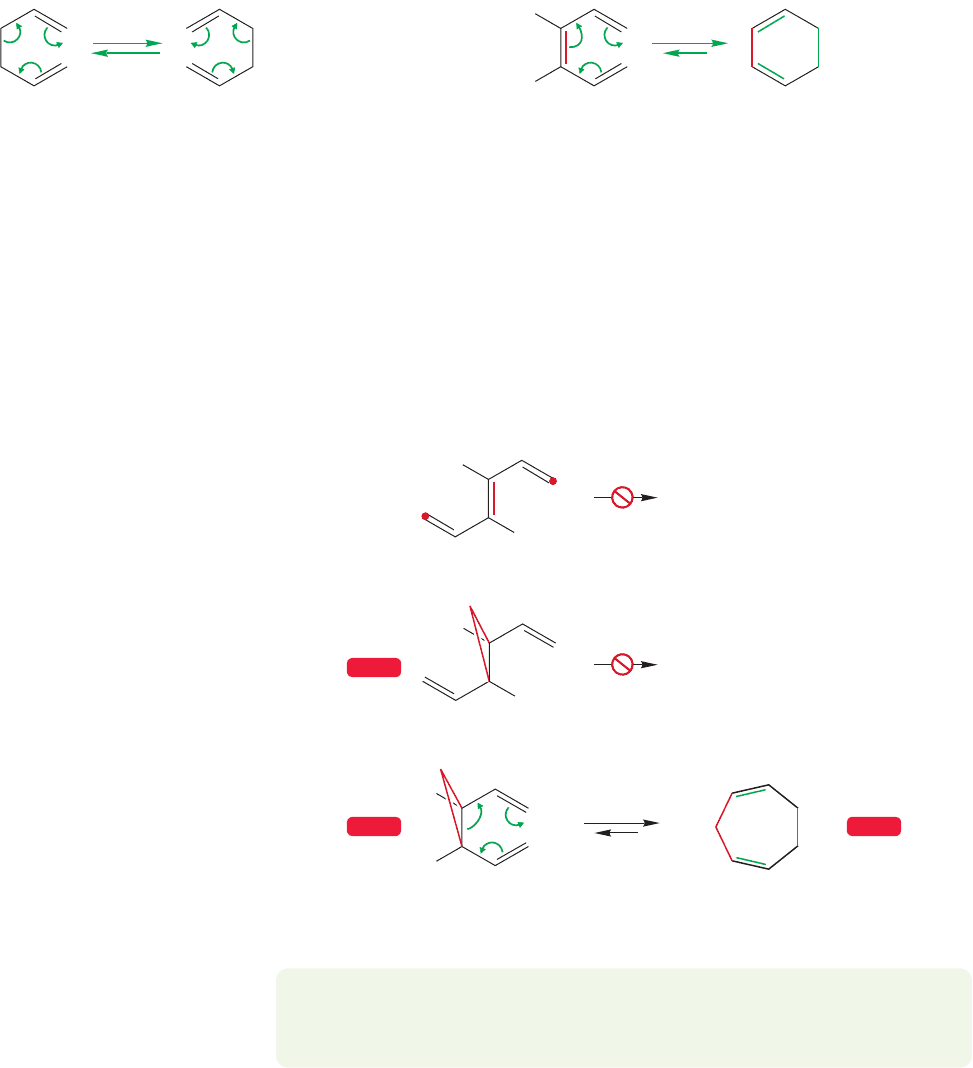
The simple 1,5-hexadiene at the heart of the Cope rearrangement can be elab-
orated in many ways, and in fact we’ve already seen one. If we add a central π bond
we get back to cis-1,3,5-hexatriene (Fig. 20.56), a molecule we saw when we con-
sidered electrocyclic reactions (p.1040).The arrow formalism of the Cope rearrange-
ment leads to 1,3-cyclohexadiene.
trans-1,3,5-Hexatriene
H
H
(a)
(b)
1,4-Cycloheptadienecis-1,2-Divinyl-
cyclopropane
Δ
H
H
trans-1,2-Divinyl-
cyclopropane
H
No Cope
No Cope
H
WEB 3DWEB 3D
WEB 3D
FIGURE 20.57 (a) trans-1,3,5-
Hexatriene cannot undergo this
rearrangement because the ends
of the molecule (dots), where
bonding must occur, are too far apart.
(b) trans-1,2-Divinylcyclopropane
cannot undergo the Cope rearrange-
ment, but the cis isomer can.
But there is a stereochemical problem. This reaction is only possible for the
stereoisomer with a cis central double bond.In the trans isomer, the ends of the triene
system are too far apart for bond formation (Fig. 20.57a). That’s an easy concept,
and it appears again in a slightly different modified 1,5-hexadiene: 1,2-divinylcy-
clopropane (Fig. 20.57b). The Cope rearrangement is possible in this molecule and
here, too, it is only the cis molecule that is capable of rearrangement. In the trans
compound, the ends cannot reach.
PROBLEM 20.18 The Cope rearrangement of cis-1,2-divinylcyclopropane is very
easy. It is rapid at room temperature. Why should it be so much easier than the
other versions we have seen?
There is another stereochemical problem in the reaction of cis-1,2-divinyl-
cyclopropane that is a bit more subtle. The most favorable arrangement for
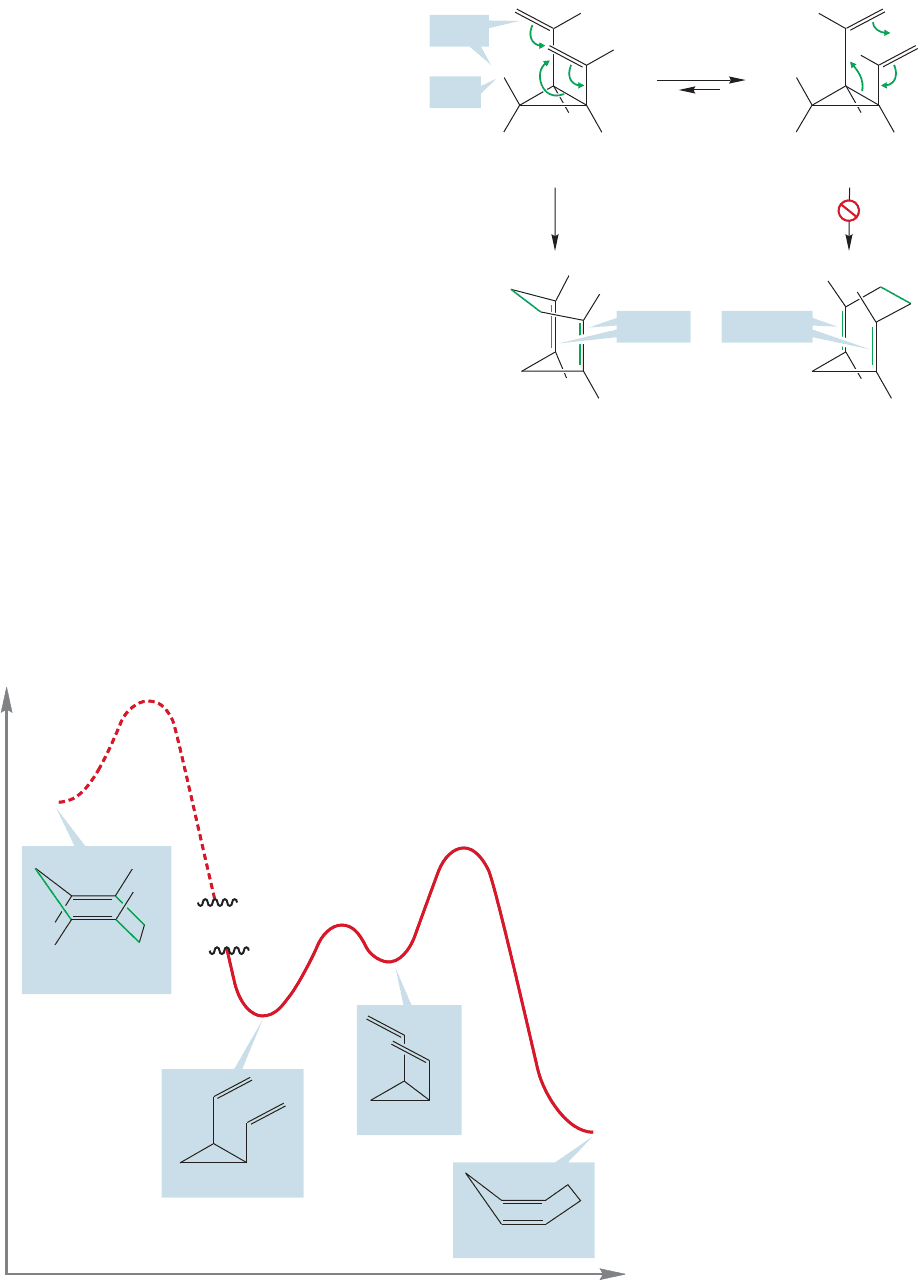
20.7 A Molecule with a Fluxional Structure 1065
cis-1,2-divinylcyclopropane is shown in Figure 20.58.
The two vinyl groups are directed away from the
ring, and in particular, away from the cis ring hydro-
gen.Yet this extended form cannot undergo the Cope
rearrangement at all! The less stable, coiled form
must be adopted where the two vinyl groups point
back toward the ring and are closer to the offending
cis hydrogen.
The problem is that the extended form must pro-
duce a 1,4-cycloheptadiene product with two trans
double bonds. Although the paper puts up with these
trans double bonds without protest, the molecule
will not. Remember, trans double bonds in small
rings are most unstable. trans-Cycloheptene is a barely
detectable reactive intermediate that can be intercept-
ed only at low temperature.The constraints of the ring
lead to severe twisting about the π bond and destabi-
lization. A seven-membered ring containing two trans double bonds is unthinkably
unstable.
By contrast, the higher energy coiled form yields a seven-membered ring con-
taining two cis double bonds, and that presents no problem. Be certain you see why
the two forms give different stereochemistries. Figure 20.59 shows the Energy ver-
sus Reaction progress diagram for the Cope rearrangement of cis-1,2-divinylcyclo-
propane.
Less stable, “coiled” form
Bump
cis H
rotate
Cope Cope
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
More stable, “extended” form
H
H
H
H
H
Both trans
Both cis
FIGURE 20.58 Only the less stable,
coiled arrangement of cis-1,2-
divinylcyclopropane can undergo the
Cope rearrangement.The more
stable, extended form must produce a
hideously unstable trans,trans-
cyclohepta-1,4-diene.
Energy
Reaction progress
Coiled form
Extended form
cis,cis Diene
trans,trans Diene
H
H
H
H
FIGURE 20.59 The energy diagram
for the Cope rearrangement of cis-
1,2-divinylcyclopropane.
1066 CHAPTER 20 Reactions Controlled by Orbital Symmetry
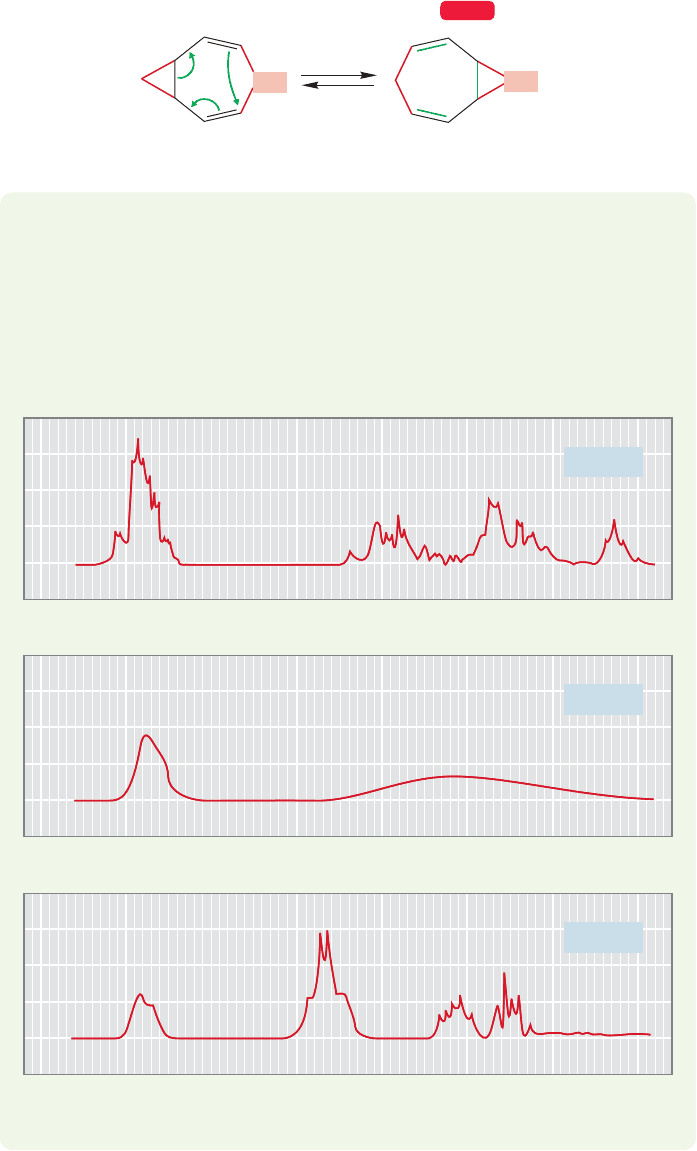
Still homotropilidene
Homotropilidene
Δ
CH
2
CH
2
WEB 3D
FIGURE 20.60 The degenerate Cope
rearrangement of homotropilidene.
Now let’s make another addition to the 1,5-diene system. Let’s add a methyl-
ene group joining the two double bonds to give the molecule named “homotropili-
dene.” We can still draw the arrow formalism, and the Cope rearrangement still
proceeds rapidly. As Figure 20.60 shows,we are back to a degenerate Cope rearrange-
ment, because the starting material and product are the same.
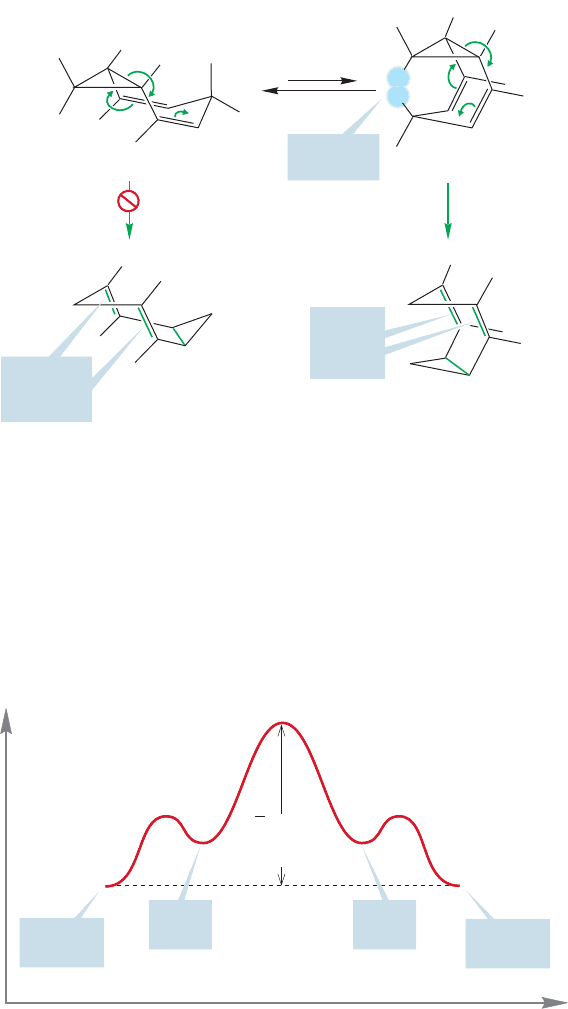
PROBLEM 20.19 The rapid Cope rearrangement of homotropilidene led to prob-
lems for W. R Roth, who first made the molecule. Can you explain in general
terms the three perplexing
1
H NMR spectra for this molecule that are shown?
Note that both low- and high-temperature spectra have sharp peaks, whereas at
an intermediate temperature the spectrum consists of two “blobs.” You are not
required to analyze the spectra in detail—just give the general picture of what
must be happening.
76543210
0
7654321
0
7654
Chemical shift (
δ) (ppm)
321
–50 ⬚C
20 ⬚C
180 ⬚C
20.7 A Molecule with a Fluxional Structure 1067
There are also stereochemical problems for homotropilidene (Fig. 20.61). Like
1,2-divinylcyclopropane, the most stable extended form of homotropilidene is unable
to undergo the Cope rearrangement, and it must be the higher-energy coiled
arrangement that is active in the reaction. Here the destabilization of the coiled
arrangement is especially obvious, because the two methylene hydrogens badly
oppose each other. As before,the extended form must give an unstable molecule with
a pair of trans double bonds incorporated into a seven-membered ring.The less sta-
ble, coiled arrangement has no such problem, because it can give a product contain-
ing two cis double bonds.
H
Bad steric
interaction
Extended form
H
H
rotate
H
H
H
H
Coiled form
Cope Cope
H
H
H
H
H
H
H
H
H
H
H
H
H
Two trans
double
bonds
H
H
H
H
Two cis
double
bonds
FIGURE 20.61 Only the higher-
energy, coiled form of
homotropilidene can give a stable
product in the Cope rearrangement.
The lower-energy, extended form
would produce a product containing
two trans double bonds in a seven-
membered ring.
Although cis-1,2-divinylcyclopropane and homotropilidene undergo the Cope
rearrangement quite rapidly even at room temperature and below, one might con-
sider how to make the reaction even faster. For the reaction to occur, an unfavorable
equilibrium between the more stable but unproductive extended form, and the less
stable but productive coiled form must be overcome. The activation energy for the
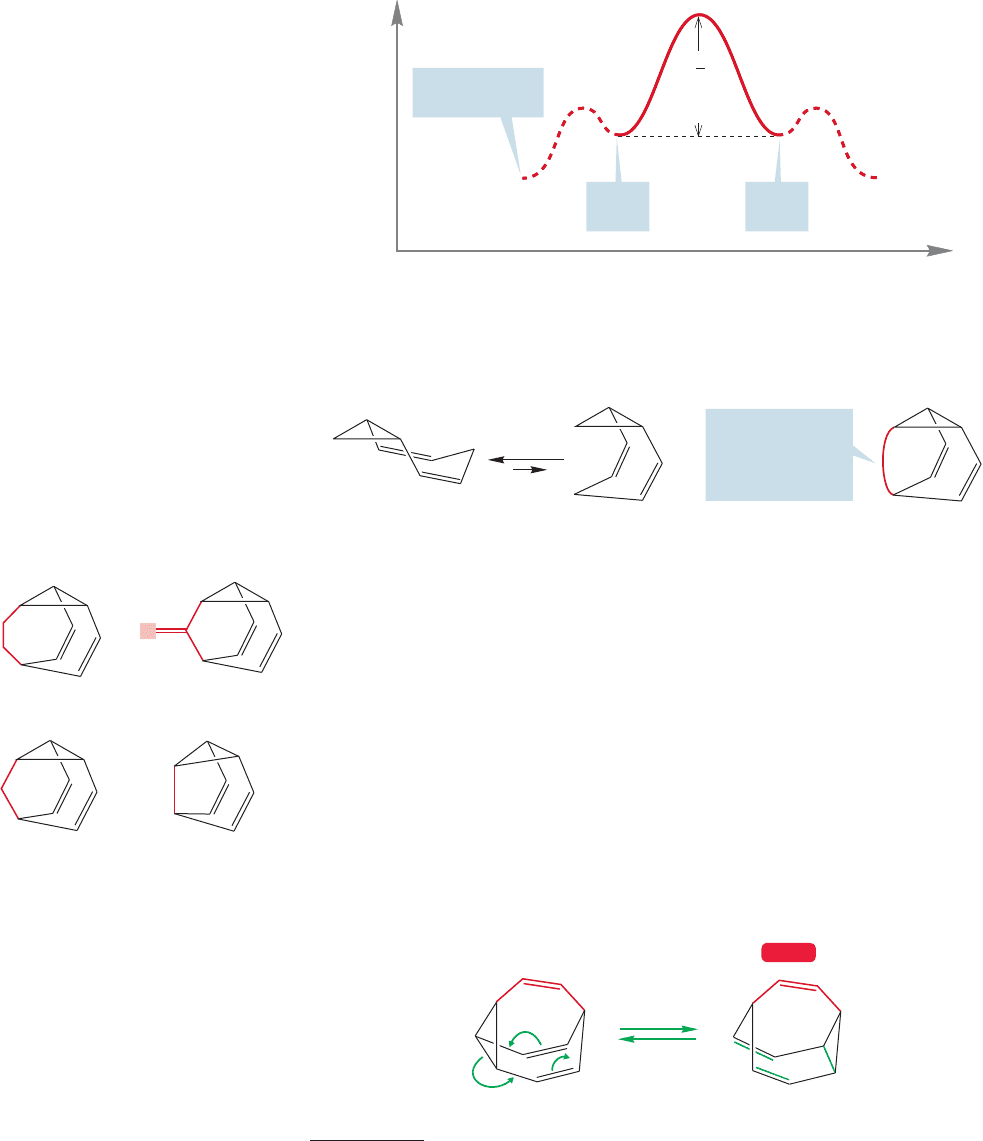
reaction includes this unfavorable equilibrium (Fig. 20.62).
Energy
Reaction progress
ΔG for Cope
rearrangement
from extended form
Extended
form
Extended
form
Coiled
form
Coiled
form
†
FIGURE 20.62 An Energy versus
Reaction progress diagram for
the Cope rearrangement of
homotropilidene.The activation
energy (ΔG
‡
) for the reaction
includes the energy difference
between the extended and coiled
forms.
1068 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Energy
Reaction progress
Coiled
form
Coiled
form
(extended form
not present)
⌬G for
Cope from
coiled form
†
FIGURE 20.63 An Energy versus
Reaction progress diagram for
the Cope rearrangement of a
homotropilidene locked into the
coiled arrangement.The activation
energy is lower than in Figure 20.62
because it is no longer necessary to
fight an unfavorable equilibrium
from the extended form, shown here
as a phantom (dashes) just to remind
you of the previous figure.
If this unfavorable equilibrium could be avoided in some way, and the molecule
locked into a coiled arrangement, the activation energy would be lower, and the reac-
tion faster (Fig. 20.63).
Our next variation of the Cope rearrangement includes the clever notion of bridging
the two methylene groups of homotropilidene to lock the molecule in a coiled arrange-
ment (Fig.20.64).This modification greatly increases the rate of the Cope rearrangement
Extended form
Coiled form
This bridge locks
the molecule
into a coiled
arrangement
FIGURE 20.64 A homotropilidene locked into the coiled arrangement can be achieved if
the two methylene groups are connected with a bridge.
BarbaraloneDihydro-
bullvalene
Barbaralane
Semibullvalene
O
FIGURE 20.65 Four bridged
homotropilidenes. All undergo very
rapid Cope rearrangements.
Cope
Bullvalene Bullvalene
WEB 3D
FIGURE 20.66 If the new bridge
is a carbon–carbon double bond,
the resulting molecule is known as
bullvalene.
because being locked in the coiled form decreases the activation energy (Fig. 20.63). No
longer is it necessary to fight an unfavorable equilibrium in order for the Cope rearrange-
ment to occur; the coiled form is built into the structure of the molecule. Many bridged
homotropilidenes are now known, and some are shown in Figure 20.65.
Now bridging is a clever idea, and it has led to some fascinating molecules and
some very nice chemistry. But it’s only a good idea. What comes next is an extraor-
dinary idea; one of those insights that anyone would envy, and which define through
their example what is meant by creativity. Suppose we bridge the two methylene
groups with a double bond to give the molecule known as bullvalene.
6
At first, this
molecule seems like nothing special. As Figure 20.66 shows, the Cope rearrange-
ment is still possible in this highly modified 1,5-hexadiene.
6
What an odd name! If you are curious about its origin,see Alex Nickon and Ernest F. Silversmith’s nice book
on chemical naming, The Name Game (Pergamon, New York, 1987). You won’t find the systematic naming
scheme in there, but you will find hundreds of stories about the origins of the names chemists give to their
favorite molecules, including bullvalene.
