Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

5.2 Rings and Strain 189
is cyclopropane’s 60°. Along with this relief,however, comes increased torsional strain,
as there are eight pairs of hydrogens eclipsed as opposed to cyclopropane’s six pairs
(Fig. 5.8). One might estimate the maximum torsional strain in planar cyclobutane
at 8 kcal/mol (1 kcal/mol strain for each pair of eclipsed carbon–hydrogen bonds).
=
H
H
H
H
H
H
H
H
H
H
H
H
WEB 3D
FIGURE 5.8 There are eight pairs of
eclipsed carbon–hydrogen bonds in
cyclobutane and therefore about
8 kcal/mol of torsional strain.
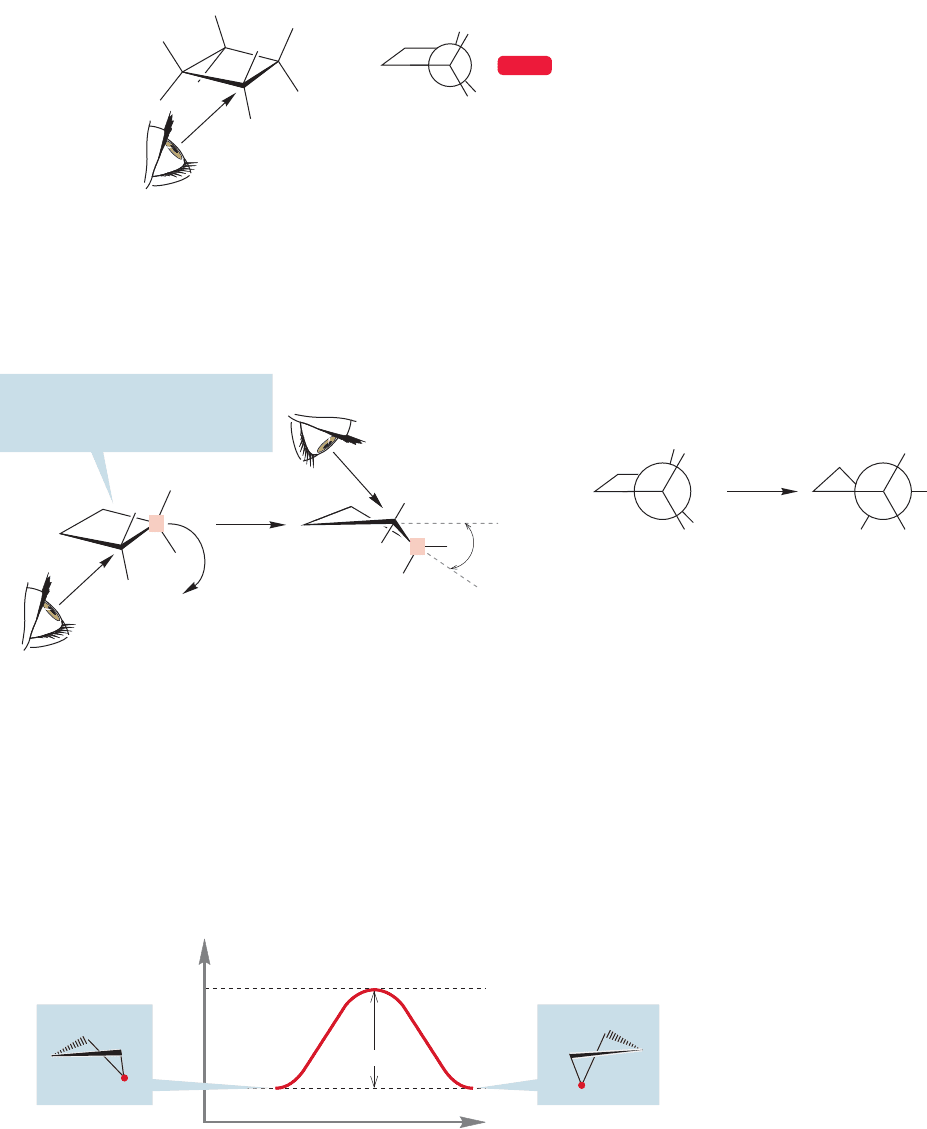
Unlike cyclopropane, cyclobutane has a way to balance torsional and angle
strain. The four-membered ring need not be planar but can distort, or “pucker”
somewhat if this will result in an energy lowering. Let’s look at the consequences
of puckering the ring by moving one methylene group out of the plane of the other
three (Fig. 5.9).
34
H
H
H
H
H
H
H
H
H
H
H
H
In these Newman projections the
red carbon is hidden
Puckering the ring involves
keeping three carbons coplanar
and moving the red carbon down
C
H
H
H
H
C
FIGURE 5.9 Puckering relieves
torsional strain in cyclobutane.
As the ring puckers, torsional strain is reduced, but angle strain is increased. A
balance between the two effects is struck in which the ring puckers about 34° and the
angle closes to about 88°. This form is not static, however; the ring is in
motion through rotation about the carbon–carbon bonds, much as are the acyclic
alkanes.The planar form of cyclobutane is like the eclipsed form of ethane and lies not
in an energy well, but at the top of an energy barrier separating a pair of puckered
cyclobutanes. This barrier is very small, about 1.4 kcal/mol (Fig. 5.10). Cyclobutane
is a mobile, not static, molecule in which different nonplanar forms rapidly intercon-
vert.In Figure 5.10, the bending is exaggerated—the flap is really only 34° out of plane.
C
O
C
O
C
Energy
a
b
1.4 kcal/mol
a
The out-of-plane, “down” carbon
is indicated by a red dot
b
FIGURE 5.10 Mobile cyclobutane.
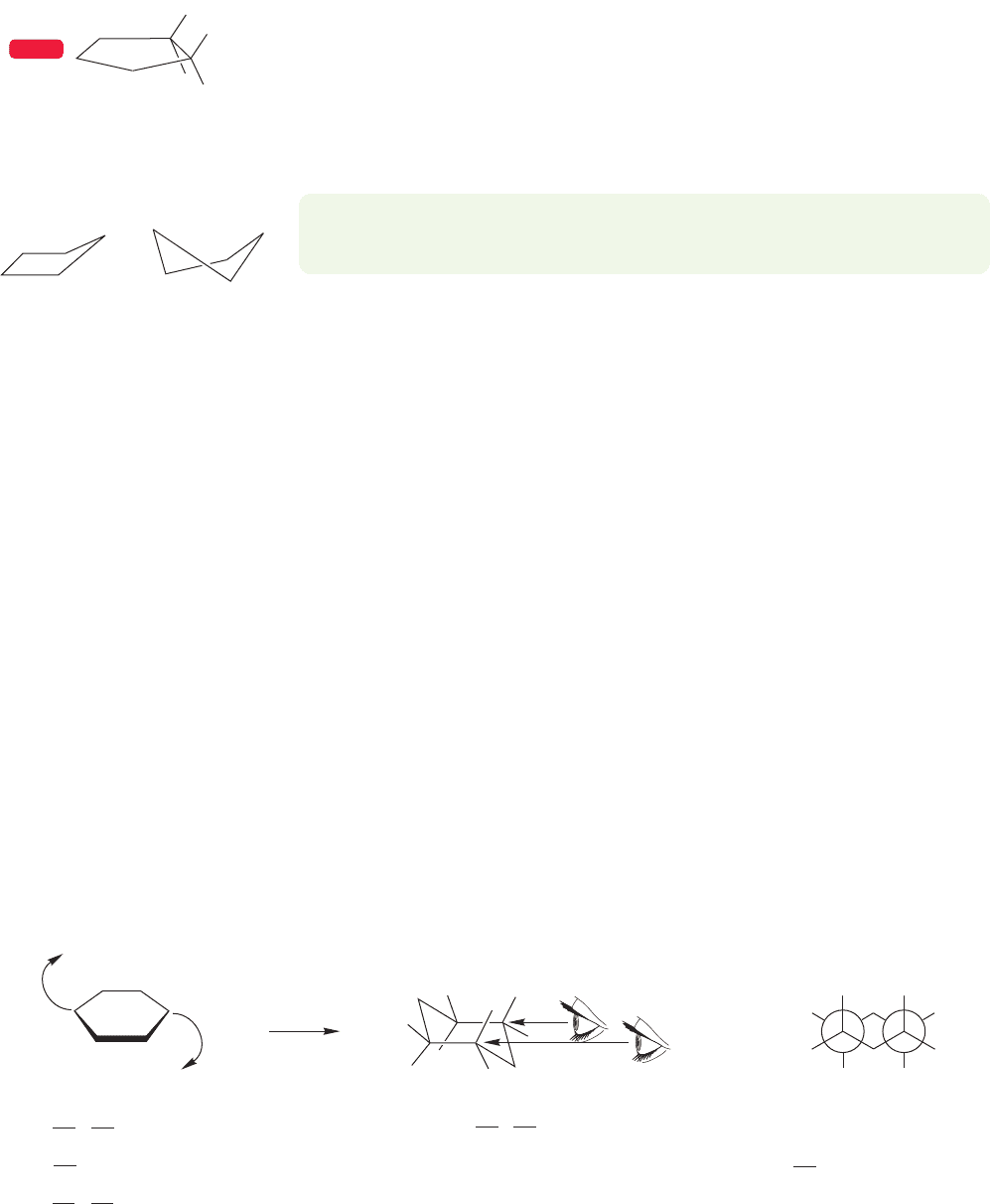
Let’s now look at cyclopentane, (CH
2
)
5
,the next cycloalkane,and see how it dis-
torts in a similar way. Were cyclopentane planar, it would suffer the torsional strain
induced by ten pairs of eclipsed hydrogens. The internal angle in a planar pentagon
is 108°, however, so angle strain would be quite small. As in cyclobutane, the ring
distorts from planarity, relieving some eclipsing at the cost of increased angle strain.
For nonplanar cyclopentane, there are two forms of comparable energy, the “enve-
lope” form and the “twist” form (Fig. 5.11).
190 CHAPTER 5 Rings
PROBLEM 5.1 Draw a Newman projection looking down one of the carbon–
carbon bonds in planar and envelope cyclopentane. Use models!
Neither the puckered envelope form nor the twist form of cyclopentane is
static. In the envelope form the “flap” moves around the ring, generating the five
possible puckered isomers. This motion requires only a series of rotations around
carbon–carbon bonds and closely resembles the motions in cyclobutane shown in
Figure 5.10. A model will help you to visualize this motion. Hold two adjacent
carbons, sight down the bond that connects them, and convert one form of the
envelope into another.
By now you must be expecting that a similar distortion from planarity will be
present in cyclohexane, (CH
2
)
6
, but you are probably prepared for neither the mag-
nitude of the effect nor its consequences for the structure and stability of all cyclo-
hexanes. In contrast to the smaller rings, distortion from planarity in cyclohexane
relieves both the angle and torsional strain of the planar structure. The internal
angle in a planar hexagon is 120°, larger, not smaller, than the ideal sp
3
angle of
109.5°. Deviation from planarity will decrease this angle and thus decrease angle
strain. Torsional strain from the twelve pairs of eclipsed carbon–hydrogen bonds
in planar cyclohexane can be expected to contribute about 12 kcal/mol of strain,
which is also decreased in a nonplanar form. So in cyclohexane, both angle and
torsional strain will be relieved by relaxing from a planar structure. Remarkably,
this relaxation produces a molecule in which essentially all of the torsional and
angle strain is gone. The formation of the energy-minimum cyclohexane, called
the “chair” form, is shown in Figure 5.12. The internal angle in real cyclohexane
is 111.5°, close to the ideal angle in a simple straight-chain alkane
(112° in propane), and the carbon–hydrogen bonds are nicely staggered. Newman
projections looking down the carbon–carbon bonds show this, as will (even bet-
ter) a look at a model.
C
O
C
O
C
H
Planar cyclopentane
showing two pairs of
eclipsed hydrogens
The two forms of
nonplanar cyclopentane
Envelope Twist
H
H
H
WEB 3D
FIGURE 5.11 Two distorted
cyclopentanes.
A double Newman projection o
f
the chair form of cyclohexane;
note the perfect staggering of
the C
H bonds
HH
HH
H
H
H
H
Chair cyclohexane:
111.5 C
C
C bond angles;
about 0 kcal/mol of torsional strain
H
H
H
H
H
H
H
H
Pull up
Pull down
Planar cyclohexane:
120 C
C
C bond angles and
about 12 kcal/mol of torsional strain
from C
H bond eclipsing; there is
also angle strain from the too-wide
120 C
C
C internal angles
FIGURE 5.12 Planar and chair
cyclohexane.
The details of cyclohexane stereochemistry are important enough to warrant a
lengthy discussion (Section 5.4, p. 197), but we can do a few things here in prepa-
ration. First of all, it is necessary to learn to draw a decent cyclohexane. No person
can truly be described as educated unless he or she can do this, and anyone can,
5.2 Rings and Strain 191
regardless of artistic ability. So learn how to do it and the next time your roommate
mentions some obscure European writer, impressing you with his or her erudition
and calling into question your sophistication, confound your tormenter with a
perfectly drawn cyclohexane!
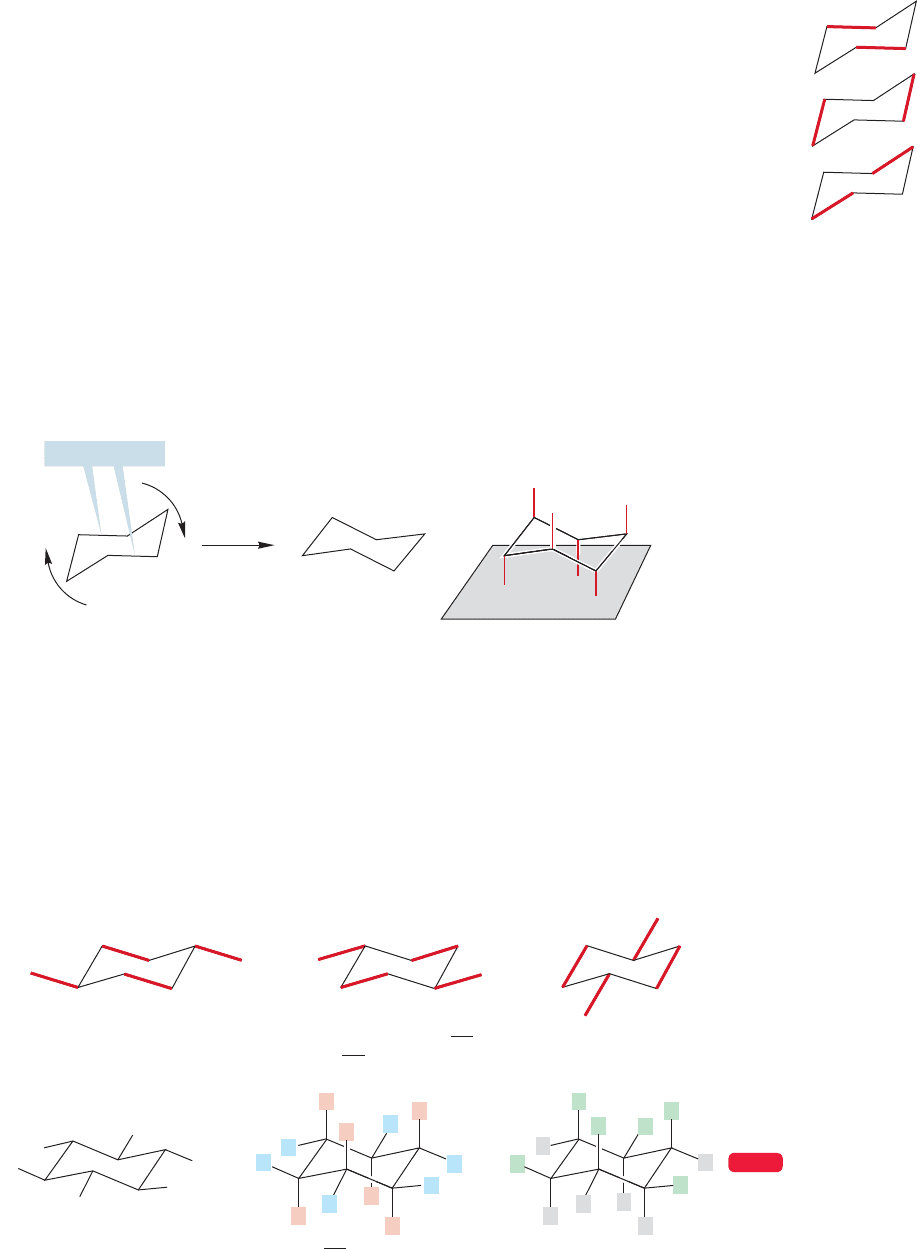
There are a few tricks that make the construction of a perfect drawing easy. First
of all, within the ring, opposite bonds are parallel to each other (Fig. 5.13). With a
little practice, keeping the proper bonds parallel should let you easily draw the
carbon framework of cyclohexane.
It is a bit more difficult to get the hydrogens right. It helps greatly to tip the ring
a little so that the “middle”bonds are not parallel to the top and bottom of the paper
(Fig. 5.14). Now we see the molecule as it would rest on a flat surface. Six of the
carbon–hydrogen bonds are easy to draw; three of them point straight up and three
straight down.The “up”and “down” hydrogens alternate.The hydrogens in these six
bonds are called axial hydrogens, and the bonds to them are called the axial
carbon–hydrogen bonds.
FIGURE 5.13 In chair cyclohexane,
there are three pairs of parallel
carbon–carbon bonds.
=
tip
H
H
H
H
H
H
Middle bonds
FIGURE 5.14 The set of six “straight
up and down” or “axial”
carbon–hydrogen bonds of chair
cyclohexane are shown in red. All
axial carbon–hydrogen bonds are
parallel.
So far, so good—but it is the positioning of the last six hydrogens, the equa-
torial hydrogens, that gives people the most trouble. To get them right, take
advantage of the parallel carbon–carbon bonds shown in Figure 5.13. Each mem-
ber of this set of six equatorial carbon–hydrogen bonds is parallel to the two
carbon–carbon bonds one bond away.Like the six axial carbon–hydrogen bonds,the
equatorial bonds also alternate up and down, but this set points only slightly up or
slightly down (Fig. 5.15).
H
H
The six equatorial hydrogens
of chair cyclohexane
All 12 C
H bonds of chair
cyclohexane; axial hydrogens
are shown in red, equatorial
hydrogens in blue
Up hydrogens in green,
down hydrogens in gray
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
The three pairs of in-plane or equatorial C
H bonds;
note that they are parallel to the C
C bonds “one bond removed”
H
WEB 3D
FIGURE 5.15 The equatorial
carbon–hydrogen bonds are parallel
to the ring carbon–carbon bonds one
bond away.The drawing of a perfect
chair cyclohexane is now complete.
192 CHAPTER 5 Rings
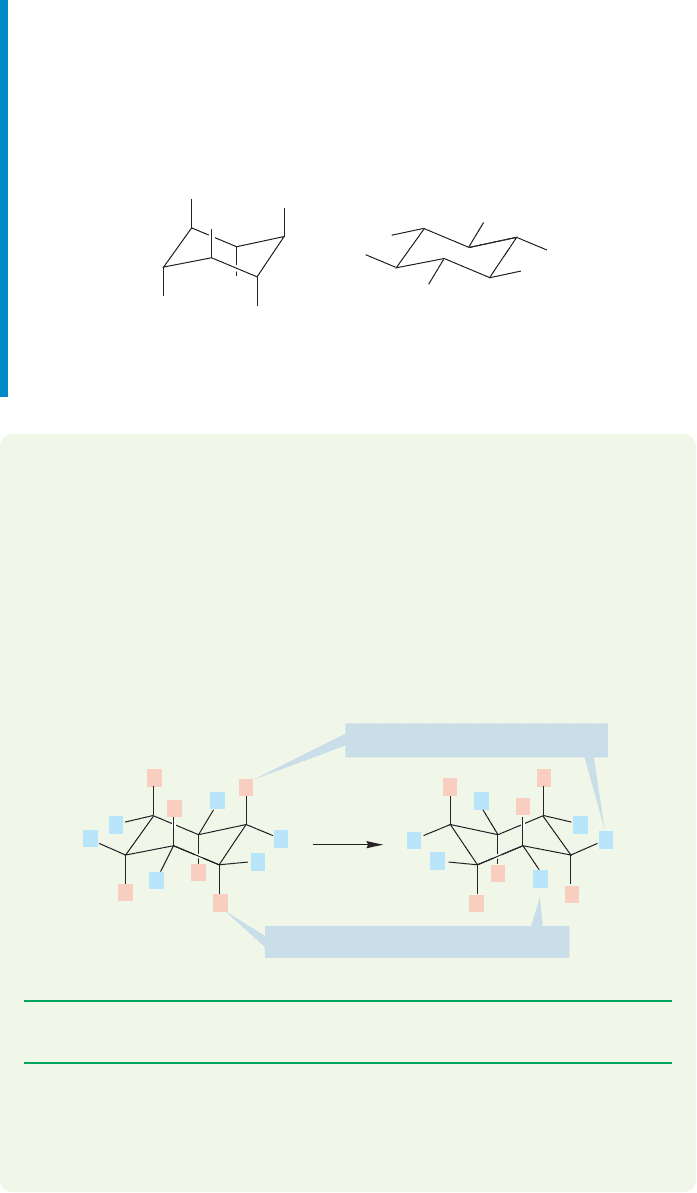
Summary
You have learned to draw a perfect cyclohexane ring in its energy-minimum chair
form.This exercise in drawing lets us see one important thing immediately. Note that
there are two different kinds of hydrogen. There is the “straight up and down” set of
six, which are called the axial hydrogens, and the other set of six, which lie roughly
along the equator of the molecule and are called the equatorial hydrogens (Fig.5.16).
WORKED PROBLEM 5.2 It is possible to convert one chair cyclohexane into another.
Get out your models, grasp the two “end” carbons (at the 1 and 4 positions). Move
the carbon that is “up”downward, and move the one that is “down” upward to gen-
erate another chair. You have just “flipped” the ring. We’ll have much more to say
about this process in Section 5.4.What happens to the set of axial hydrogens when
you convert one chair into another? This problem is important and simple.
ANSWER The set of axial hydrogens becomes the set of equatorial hydrogens!
Whether axial or equatorial, an up hydrogen always remains up,however.
Similarly, a down hydrogen is always down, whether axial or equatorial.
The six axial hydrogens
of chair c
y
clohexane
The six equatorial hydrogens
of chair c
y
clohexane
H
H
H
H
H
H
H
H
H
H
H
H
FIGURE 5.16 Axial and equatorial hydrogens.
Up axial becomes up equatorial
Down axial becomes down equatorial
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
ring flip
H
H
H
PROBLEM 5.3 How many carbons will the
13
C NMR spectrum of cyclohexane reveal?
PROBLEM 5.4 How many hydrogens will the
1
H NMR spectrum of cyclohexane
reveal? Caution: This problem is much harder than Problem 5.3. Try to find two
answers to this question, one correct at high temperature, the other correct at low
temperature.
Larger rings than cyclohexane will also be nonplanar; but no more spectacular
surprises such as cyclohexane’s energy-minimum form remain to be found. Planar
heptagons and octagons have internal angles of 129° and 135°, respectively, and thus
have substantial angle strain. Both seven- and eight-membered rings would have

5.3 Quantitative Evaluation of Strain Energy 193
severe torsional strain were they to remain planar. There is relaxation to nonplanar
forms, but some amount of strain always remains in these medium-sized rings. It is
a complicated business to analyze medium-sized rings completely, and we will not
embark upon it.Rings with minimal angle strain will have severe interactions—often,
but not always, eclipsings of carbon–hydrogen bonds, and thus substantial torsion-
al strain. Relieving those eclipsings usually induces increased angle strain. As in
cyclopentane, there is a compromise to be made between minimizing angle and tor-
sional strain, and Nature does the best it can, finding the lowest energy structural
compromise. Unlike cyclohexane, however, in the medium-sized rings there is no
obvious low-energy solution. Often there are several somewhat different minima,
close to one another in energy, and separated by quite low barriers.
Fragments of chair cyclohexanes are sometimes seen in the structures of medium-
sized rings. Cyclodecane is an example, as Figure 5.17 shows. Several destabilizing
interactions between carbon–hydrogen bonds should be apparent, especially if you
make a model.
As rings get even bigger, the strain energy decreases. The limit is an infinitely
large ring, which resembles an endless chain of methylene groups. In such a species,
it is possible to stagger all carbon–hydrogen bonds, and, of course, in an infinite ring
there is no angle strain. So after cyclohexane, the next strain-free species is reached
when the ring size has grown large enough to approximate an infinitely large ring.
5.3 Quantitative Evaluation of Strain Energy
Strain is an important factor in chemical reactivity.The more strained a compound,
the higher its energy.The higher in energy a compound,the more likely it is to react.
We have just seen how a combination of torsional and angle strains can act to change
the energy of a ring compound, cyclopropane. Now we move on to some quantita-
tive measures of the energies of ring compounds. We introduce some general
techniques and look ahead to our detailed consideration of energy in Chapter 8.
5.3a Heats of Formation To recapitulate material from Chapter 3 (p. 115), the
heat of formation ( ) of a compound is the enthalpy of its formation by the
reaction of its constituent elements in their standard states.The standard state of an
element is the most stable state at 25 °C and 1 atm pressure. For an element in its
standard state, is taken as zero. The more negative (or less positive) a com-
pound’s , the more stable it is. A negative for a compound means that its
formation from its constituent elements would be exothermic—heat would be liber-
ated. By contrast, a positive means that the constituent elements are more
stable than the compound and its formation would be endothermic—energy would
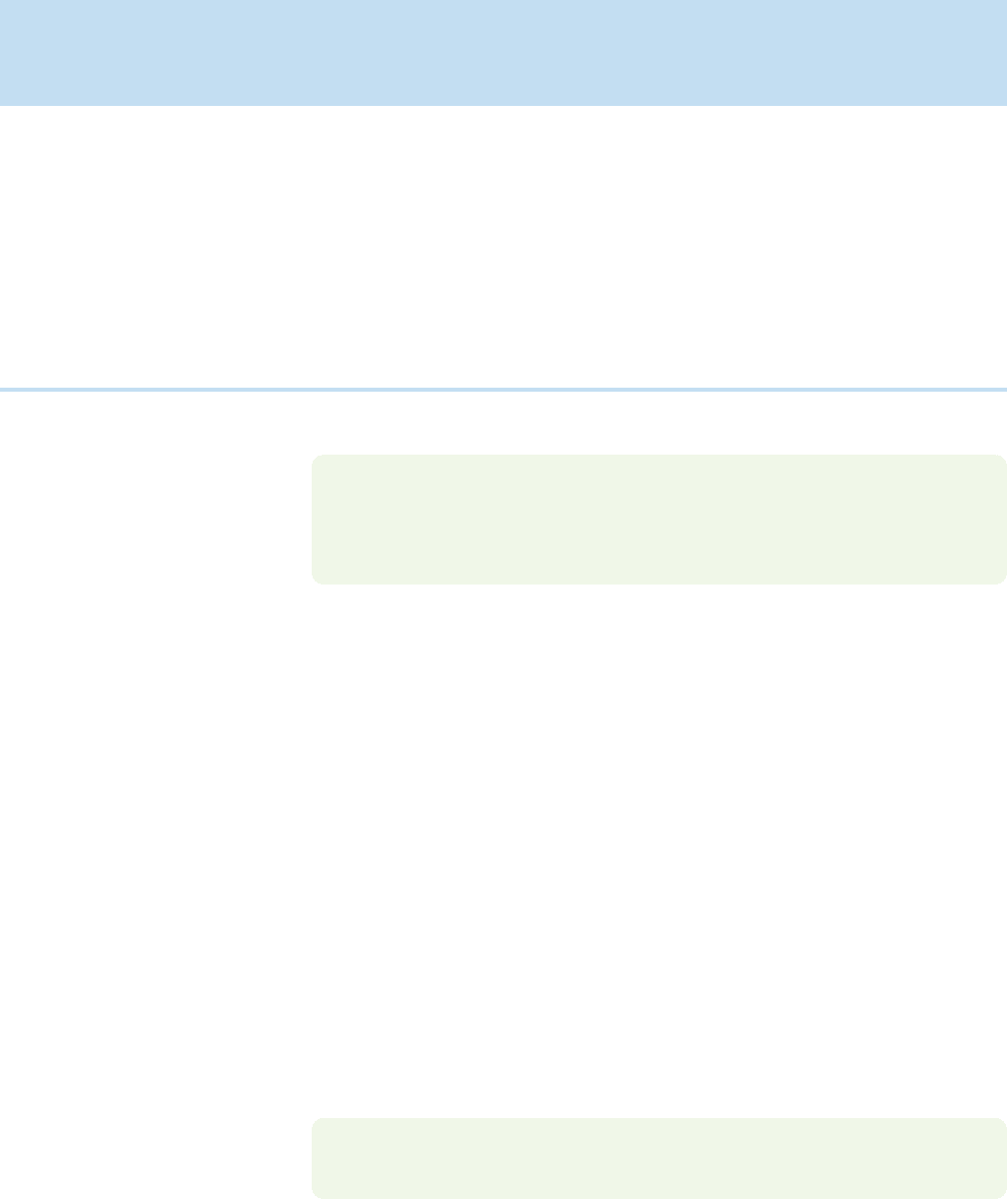
have to be applied (Fig. 5.18).
¢H °
f
¢H °
f
¢H °
f
¢H °
f
¢H °
f
H
Cyclodecane
H
H
WEB 3D
FIGURE 5.17 A medium-sized ring.
Enthalpy
X + Y
X Y
X + Y
X Y
+
–
⌬H ⬚
f
⌬H ⬚
f
Positive sign
means endothermic
Negative sign
means exothermic
FIGURE 5.18 For a compound more
stable than its constituent elements,
is negative. For a compound less
stable than its constituent elements,
is positive.¢H °
f
¢H °
f
194 CHAPTER 5 Rings
Columns 1 and 2 of Table 5.1 collect the heats of formation for some cycloalkanes
and calculate the per methylene group for these hydrocarbons.¢H °
f
PROBLEM 5.5 How would one get the value of for a CH
2
group in an infi-
nite chain, (CH
2
)
? Use the following data to estimate the value of of a
strain-free methylene group in kilocalories per mole: hexane 39.9,
heptane 44.8, octane 49.8, nonane 54.5, decane 59.6 kcal/mol.
¢H °
f
¢H °
f
¢H °
f
Note from the final line of Table 5.1 that the for a methylene (CH
2
) group
in a strain-free straight-chain alkane, (CH
2
)
, is 4.9 kcal/mol.This value is exactly
the we calculate for a methylene group in cyclohexane. Cyclohexane really is
strain-free.We can now use these data to calculate strain energies for the cycloalkanes.
First, calculate (Table 5.1, column 3) the for the ring constructed from strain-
free methylene groups.The difference between the calculated, strain-free (col-
umn 3) and the real,measured (column 1) is the strain energy (strain E,column 4).
For example, cyclopropane has a measured of 12.7 kcal/mol. A strain-free
cyclopropane ring would have a of 3 4.9 kcal/mol 14.7 kcal/mol.
The difference between these two values (27.4 kcal/mol) is the strain energy
(column 4). The strain energy per CH
2
(column 5) is (27.4)/3 9.1 kcal/mol.
Now look at the other rings. Cyclopropane and cyclobutane are about equally
strained, although the strain per methylene is significantly higher for cyclopropane.
Cyclopentane and cycloheptane are slightly strained, and the strain gets worse in
the medium-sized rings until we reach a 12-membered ring, in which strain
decreases markedly. Strain continues to decline in the larger rings until (with some
aberrations) we reach the hypothetical strain-free infinite ring.
¢H °
f
¢H °
f
¢H °
f
¢H °
f
¢H °
f
¢H °
f
¢H °
f
PROBLEM 5.6 Use models to look for the sources of strain in medium-sized
rings.
TABLE 5.1 Strain Energies for Some Cycloalkanes
12 3 4 5
Measured Calcd
per CH
2
for Strain-free Molecule Strain E Strain E per CH
2
Molecule (kcal/mol) (kcal/mol) (kcal/mol) (kcal/mol) (kcal/mol)
Cyclopropane 12.7 4.2 14.7 27.4 9.1
Cyclobutane 6.8 1.7 19.6 26.4 6.6
Cyclopentane 18.7 3.7 24.5 5.8 1.2
Cyclohexane 29.5 4.9 29.4 0.1 0
Cycloheptane 28.3 4.0 34.3 6.0 0.9
Cyclooctane 29.7 3.7 39.2 9.5 1.2
Cyclodecane 36.9 3.7 49.0 12.1 1.2
Cyclododecane 55.0 4.6 58.8 3.8 0.3
(CH
2
)
4.9
≤H °
f
≤H °
f
≤H °
f
5.3 Quantitative Evaluation of Strain Energy 195
5.3b Strain Analyzed by Heats of Combustion Strain energies can also be
determined from an analysis of heats of combustion ( ), the energy released—
or consumed—when a compound reacts with oxygen. This method is attractive for
analyzing strain because the measurements can be made and compared directly.
C
3
H
8
5 O
2
3 CO
2
4 H
2
O Energy
Combustion of a hydrocarbon is an exothermic reaction.The products,H
2
O and CO
2
,
are more stable than the starting hydrocarbon and oxygen.This energy difference, the
exothermicity of the reaction,appears as heat and light (Fig.5.19), which is easily appre-
hended by looking at the light and feeling the heat evolved by a propane stove.
U
¢H °
c
530.6 kcal/mol = exothermicity
of this reaction
CH
3
CH
2
CH
3
5 O
2
+
3 CO
2
4 H
2
O+
Energy
FIGURE 5.19 The combustion of
propane to give CO
2
,H
2
O, and
energy.
CONVENTION ALERT
The conventions are confusing here. When writing energy as the product of a
reaction it is conventional to show it as “ energy.”The enthalpy of this exothermic
reaction, ΔH, is negative, however! So, when writing an equation for the reaction,
we say:
C
6
H
12
9 O
2
6 CO
2
6 H
2
O 937 kcal/mol
but, when indicating the enthalpy for the reaction, we say:
C
6
H
12
9 O
2
6 CO
2
6 H
2
O ΔH 937 kcal/mol
U
U
Combustion of hydrocarbons can often be used to establish energy differences
between molecules. Compare, for example, the heats of combustion for octane and
an isomer, 2,2,3,3-tetramethylbutane (Fig. 5.20).
2
2
A reviewer of this book, RMM, who sat with me (MJ) in chemistry classes at Yale, many, many years ago,
reminds me that we were once told that this molecule, the only isomer of octane with a melting point above
room temperature (mp 104 °C), was known as “solid octane.”We were then told that this was likely to be the
only fact we retained from our organic chemistry course! Now you are stuck with this knowledge.
(solid octane)
O
2
O
2
C
8
H
18
+ 12.5 O
2
8 CO
2
+ 9 H
2
O
CO
2
H
2
O+
CO
2
H
2
O+
FIGURE 5.20 Combustion of two
isomeric octanes.
196 CHAPTER 5 Rings
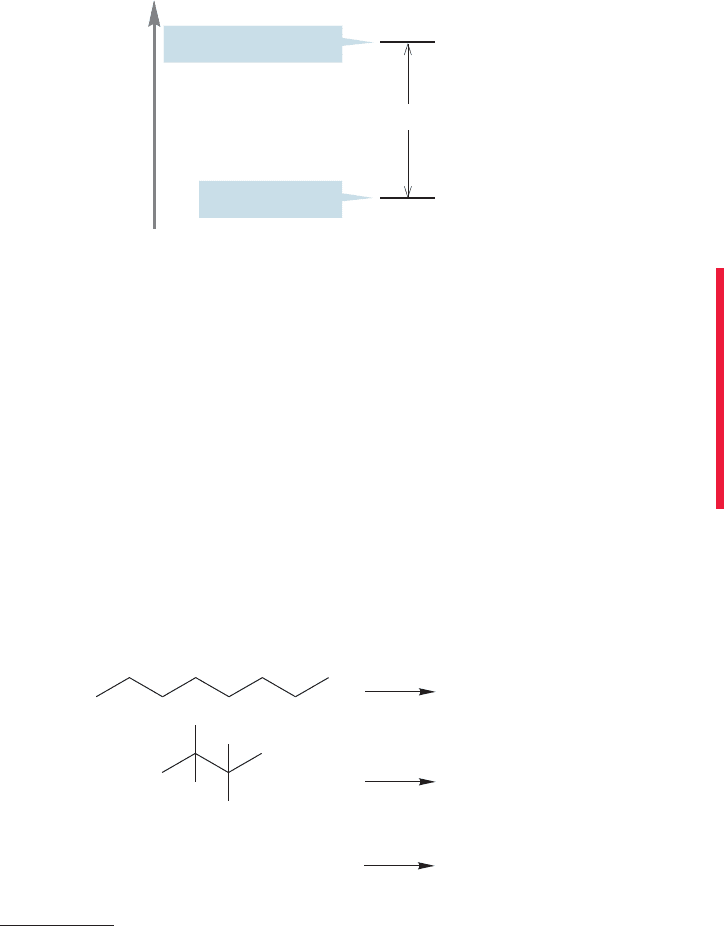
The measured heats of combustion of octane and 2,2,3,3-tetramethylbutane are
1307.60 kcal/mol and 1303.04 kcal/mol, respectively. Figure 5.21 graphically shows
the relationship between these values and the energies of the molecules. Because
these isomeric molecules produce exactly the same products on burning, the differ-
ence in their heats of combustion is the difference in energy between them. Of
course, this analysis also tells you which isomer is more stable, and by how much.
PROBLEM 5.7 The heats of combustion of heptane, 3-methylhexane, and
3,3-dimethylpentane, respectively, are 1149.9, 1148.9, and 1147.9 kcal/mol.
Carefully draw a diagram showing the relative stabilities of these molecules.
We can also use heats of combustion to measure the relative strain energies of
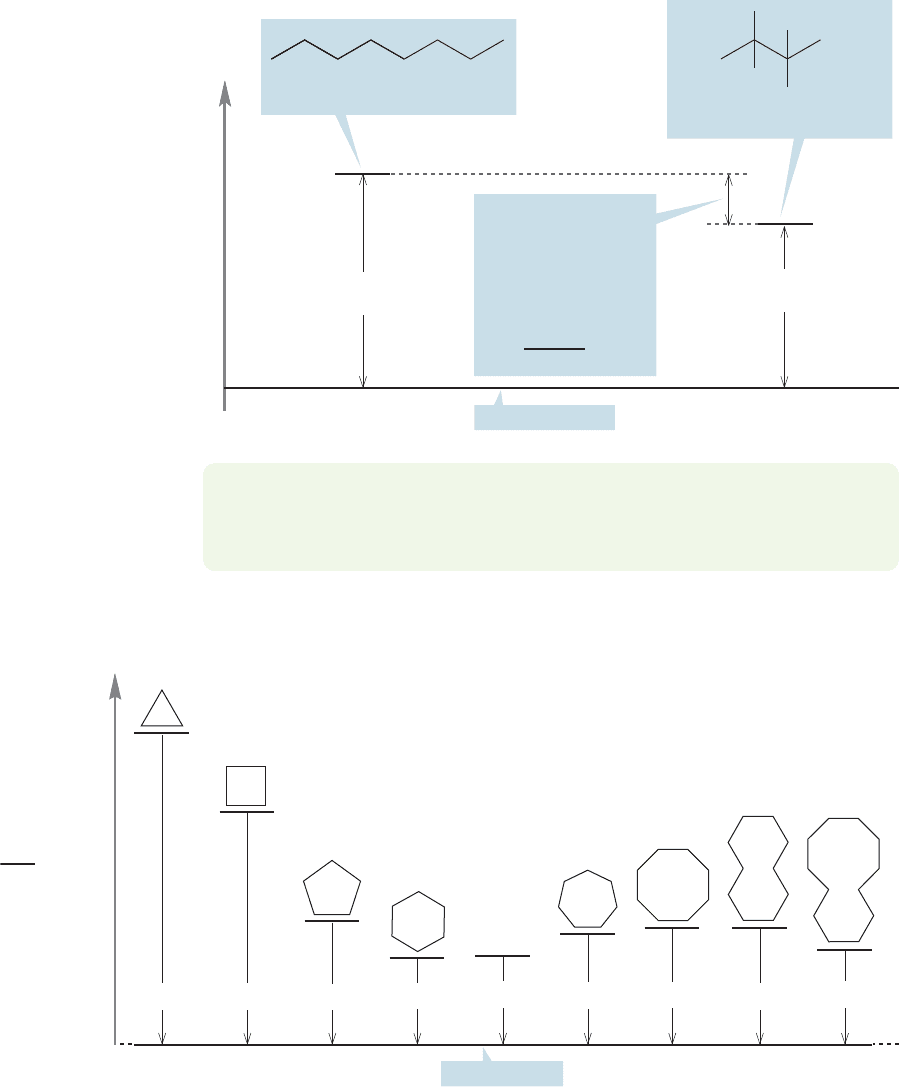
the cycloalkanes. The higher the , the less stable the compound (Fig. 5.22).¢H
°
c
8 CO
2
+ 9 H
2
O
Octane
C
8
H
18
+
12.5 O
2
Heat of combustion ( )
1307.60 kcal/mol
Heat of combustion ( )
1303.04 kcal/mol
2,2,3,3-Tetramethylbutane
also, C
8
H
18
+
12.5 O
2
This gap is the
energy difference
between these two
isomeric compounds:
4.56 kcal/mol
Energy
1307.60
1303.04
4.56
–
⌬H ⬚
c
⌬H ⬚
c
FIGURE 5.21 A quantitative picture
of the combustion of two octanes.
H
2
O + CO
2
CH
2
166.3
163.9
158.7
157.4
157.5
(CH
2
)
∞
158.3
7
158.6
8
158.6
10
157.8
12
(kcal/mol)
H
c
FIGURE 5.22 The heats of combustion per CH
2
for a series of cycloalkanes and a strain-free sequence of methylene
(CH
2
) groups.The higher the heat of combustion, the less stable the compound.

5.4 Stereochemistry of Cyclohexane: Conformational Analysis 197
Once more we need a baseline,and we use the of a strain-free methylene group
in an infinite chain of methylenes,157.5 kcal/mol.This value is determined in much
the same way as was used to get the for a strain-free methylene (p. 194).Then
we measure the values for the series of cycloalkanes, finding the per meth-
ylene for each ring. Subtraction of 157.5 kcal/mol, the of a strain-free meth-
ylene group, gives the strain energy per CH
2
and, therefore, the strain energy of the
ring compound. As Table 5.2 shows, there is a good correspondence between the
strain energies measured from and . Actually this correspondence is no
surprise, because values are often derived from .¢H
°
c
¢H °
f
¢H °
c
¢H °
f
¢H °
c
¢H °
c
¢H °
c
¢H °
f
¢H °
c
TABLE 5.2 Strain Energies for Some Cycloalkanes from and
Measured Strain E per Strain E Strain E
per CH
2
CH
2
( −157.5) from from
Molecule (kcal/mol) (kcal/mol) (kcal/mol) (kcal/mol)
Cyclopropane 166.3 8.8 26.4 27.4
Cyclobutane 163.9 6.4 25.6 26.4
Cyclopentane 158.7 1.2 6.0 5.8
Cyclohexane 157.4 0.1 0.6 0.1
Cycloheptane 158.3 0.8 5.6 6.0
Cyclooctane 158.6 1.1 8.8 9.5
Cyclodecane 158.6 1.1 11.0 12.1
Cyclododecane 157.8 0.3 3.6 3.8
(CH
2
)
157.5
≤H°
f
≤H °
c
≤H °
c
≤H °
c
≤H °
f
≤H °
c
5.4 Stereochemistry of Cyclohexane: Conformational
Analysis
Earlier, when we were considering the ways in which ring compounds distort so as
to minimize strain, we saw that cyclohexane adopted a strain-free chair conforma-
tion. Now it is time to look at cyclohexane in detail. Six-membered rings are most
common in organic chemistry, and a great deal of effort has been made over the
years at understanding their structure and reactivity. One chair form of cyclohexane
can easily be converted into another. We are now going to look in detail at this
transformation and estimate the energies of the various intermediates and transi-
tion states along the path from one chair to another.This process is an example of
conformational analysis.
A little manipulation of a model will show the overall conversion, but it’s not
easy to come to the actual pathway for the process. If we move carbons 1, 2, 3, and
4 in Figure 5.23 into one plane, with carbon 6 above the plane and carbon 5 below
the plane, we come to a “half-chair” structure.
Chair Half-chair
(transition state)
Twist
or twist-boat
C
6
C
5
C
4
C
3
C
2
1
C
C
6
C
2
1
C
C
3
C
4
C
5
C
4
C
3
C
6
C
2
C
5
1
C
FIGURE 5.23 Conversion of
the energy-minimum chair
cyclohexane into the half-
chair and then the twist form.
198 CHAPTER 5 Rings
The half-chair conformation does not represent a stable molecule, but is instead
a picture of the top of the energy “mountain pass” (a transition state) leading to a
lower energy molecular “valley” called the twist conformation or, sometimes the
twist-boat conformation (Fig. 5.23). The half-chair contains many eclipsed
carbon–hydrogen bonds,which become staggered somewhat in the twist conforma-
tion, and angle strain is partially relieved as well. The twist conformation can pass
through a second half-chair to give another chair cyclohexane.Kinetic measurements
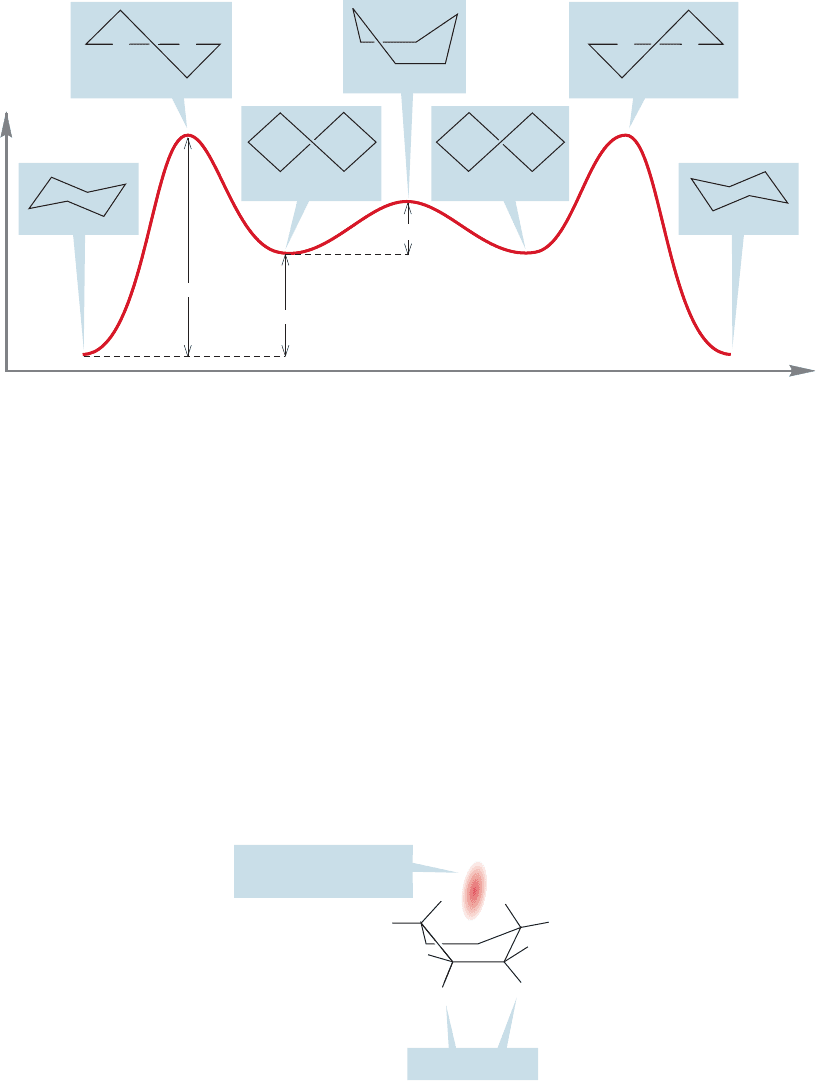
allow an evaluation of the energies involved (Fig. 5.24).The half-chair and the twist
lie 10.8 and 5.5 kcal/mol above the chair, respectively.
Energy
Chair
Chair
Twist
Half-chair
10.8 kcal/mol
5.5 kcal/mol
1.5 kcal/mol
Full-boat
C
C
C C
Twist
Half-chair
FIGURE 5.24 The interconversion of two chair cyclohexanes. The two chairs and the two twist forms
are intermediates (energy minima), and the two half-chairs and the full-boat are transition states
(energy maxima).
Do not confuse the twist with the full-boat shown at the top of a “mountain” in
Figure 5.24. The full-boat is not an energy minimum but is, like the half-chair, an
energy maximum. It lies at the top of the energy barrier (like the half-chair, it, too,
is a transition state) separating two twist forms. The full-boat is only 1.5 kcal/mol
higher than the twist (7.0 kcal/mol higher than the chair), and can never be isolat-
ed because it is not an energy minimum.
Both the half-chair and the full-boat suffer from the kinds of strain we have seen
before. In the half-chair, much of the ring is planar, and there is both angle and tor-
sional strain. The full-boat also has angle and torsional strain, but there is another
hydrogen–hydrogen interaction that is destabilizing.This new interaction is between
the “prow” and “stern” carbons and between the two “inside”hydrogens at the prow
and stern of the boat.This new kind of strain is induced when two atoms come too
close to each other and is called van der Waals strain (Fig. 5.25).
“Boat” cyclohexane
Torsional strain
Steric repulsion:
van der Waals strain
H
H
H
H
H
H
H
H
FIGURE 5.25 van der Waals strain in
the full-boat form of cyclohexane.
Don’t confuse this strain with
attractive van der Waals forces (p. 87).
