Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

5.5 Monosubstituted Cyclohexanes 199
WORKED PROBLEM 5.8 Draw a Newman projection looking down
the “side” bond of the full-boat cyclohexane.
ANSWER This task is relatively easy. Once again, set your eye slightly
to one side so as to see the carbon–hydrogen bonds on the rear carbon.
H
2
C
O
CH
2
H
H
H
H
=
H
H
H
H
One chair cyclohexane will equilibrate with another as long as the environment
supplies the requisite 10.8 kcal/mol to traverse the energy barrier. Under normal con-
ditions this process is easy, but at very low temperature, we can freeze out one chair
form by lowering the temperature to the point at which there isn’t enough energy
available to cross the energy barrier to the other chair.
How much of the relatively high-energy twist form is there at 25 °C? We will see
many such calculations in Chapter 8, but the chair–twist equilibrium can be treated like
any equilibrium process. A calculation shows that a relatively small energy differ-
ence of ΔG 5.5 kcal/mol results in an enormous preference for the more stable
isomer of the pair, about 10
4
:1.The “take-home lesson”here is that small energy dif-
ferences between two molecules in equilibrium result in a very large excess of the
more stable isomer.
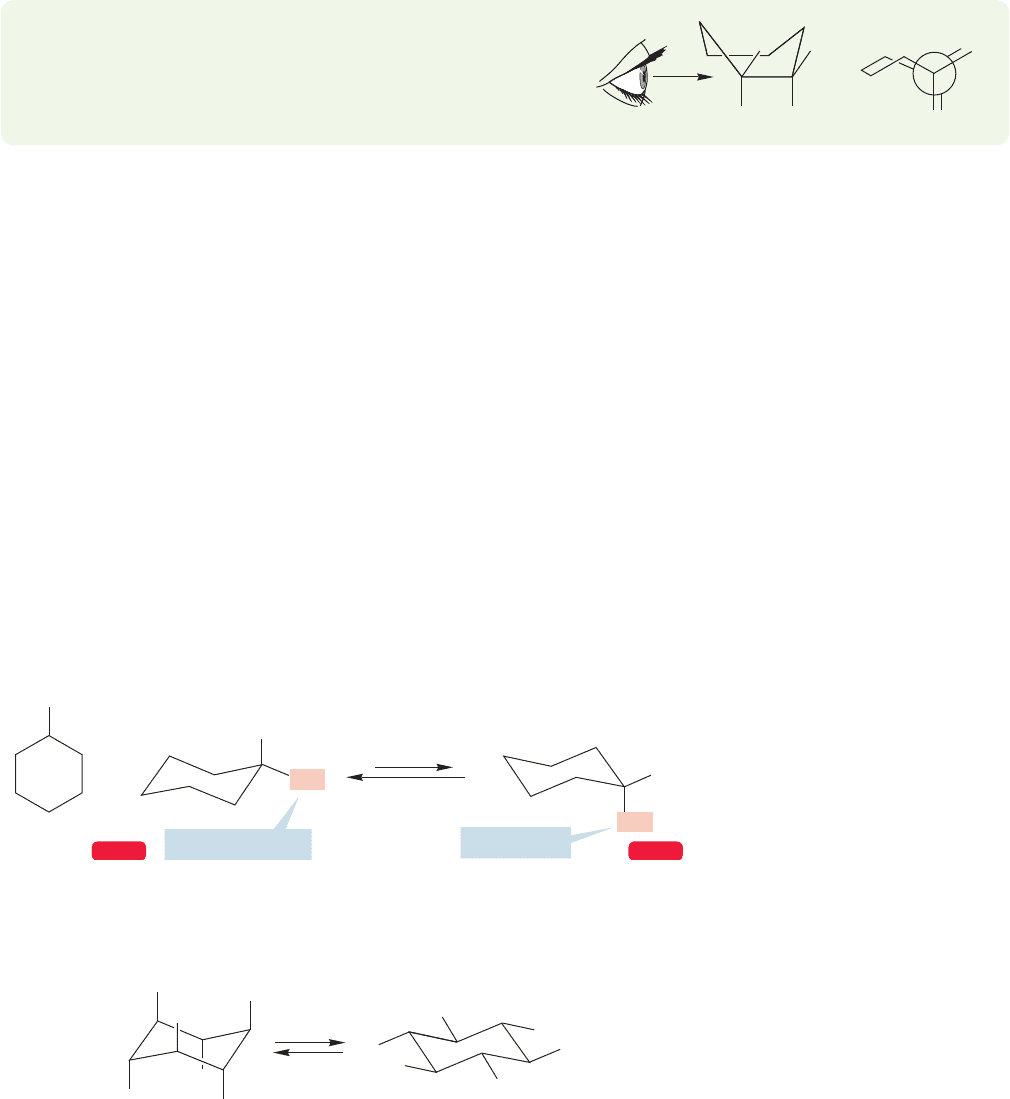
5.5 Monosubstituted Cyclohexanes
The danger of relying too strongly on two-dimensional representations of molecules
is shown nicely by methylcyclohexane.Two-dimensional structures give no hint of the
richness of the complicated, three-dimensional structure of this molecule; they even
hide the presence of two conformational isomers of methylcyclohexane (Fig. 5.26).
CH
3
CH
3
Axial methyl
Equatorial methyl
ring flip
H
H
CH
3
WEB 3D WEB 3D
FIGURE 5.26 The uninformative
two-dimensional structure hides the
existence of two conformational
isomers of methylcyclohexane. In one
isomer, the methyl group is
equatorial, and in the other it is axial.
flip
H
H
H
H
H
H
H
H
H
H
H
H
FIGURE 5.27 In cyclohexane, a ring
flip interconverts axial and equatorial
hydrogens.
Recall (Problems 5.2–5.4, p. 192) that flipping the cyclohexane chair forms intercon-
verts the set of six axial hydrogens and the set of six equatorial hydrogens (Fig. 5.27).
Therefore, a methyl group, or any substituent, can be in either an axial or equatorial
position. These two molecules are diastereomers. (Remember: Diastereomers are
stereoisomers that are not mirror images—see Chapter 4, p. 165.)
Given that two isomers of methylcyclohexane exist, our next job is to determine
which is more stable. As it turns out, we can even make a reasonable guess at the
magnitude of the difference in energy between the two diastereomers. First of all,
notice that axial and equatorial methylcyclohexane are interconverted by a chair flip
(Fig. 5.26). What turns out to be the crucial factor in creating the energy difference
200 CHAPTER 5 Rings
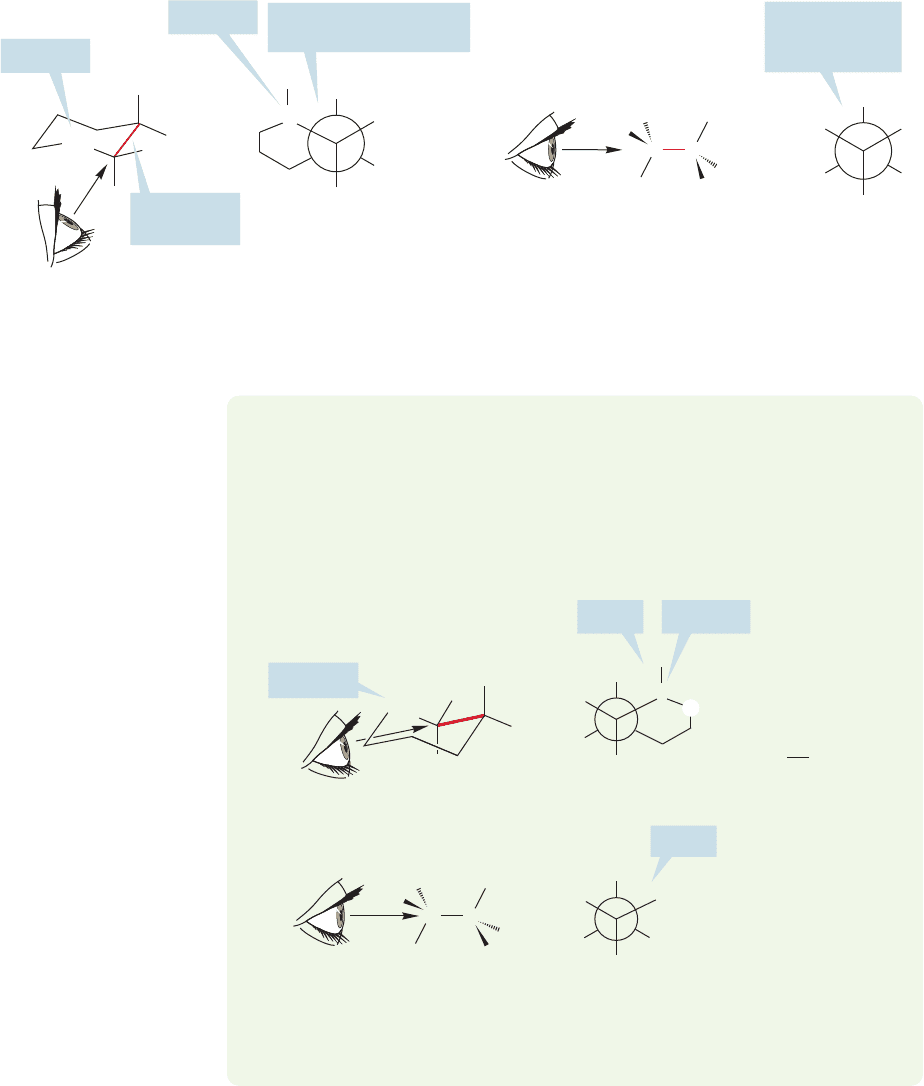
between the two forms is shown by the Newman projections made by sighting down
the bond attaching a ring methylene group to the carbon bearing the methyl group.
In the axial form, there are two gauche interactions between the methyl group and
the nearby ring CH
2
group (Fig. 5.28). Note the destabilizing 1,3-diaxial interac-
tion between the axial hydrogen shown in the figure and the axial methyl group.
Although it is easy to see one of the gauche interactions, the perspective of the
drawing hides the other unless you are careful. No amount of words can help you
here as much as working out this stereochemical problem yourself. It is very impor-
tant to try Problem 5.9. The answer follows immediately.
H
H
H
CH
3
CH
3
CH
3
CH
3
H
One of two gauche
methyl-ring interactions
A gauche
methyl–methyl
interaction
Methylcyclohexane (methyl group axial)
H
gauche-Butane
=
=
H
H
H
H
H
H
H
3
C
H
3
C
H
H
H
H
CC
CH
2
Ring CH
2
Ring CH
2
Look down
this bond
CH
FIGURE 5.28 Methylcyclohexane with the methyl group axial.The Newman projection shows the gauche
interaction with one of the two equivalent ring methylene groups. Each of the two gauche interactions in this
compound resembles a gauche interaction in gauche-butane.
WORKED PROBLEM 5.9 Draw the Newman projection of methylcyclohexane
looking from the other adjacent methylene group toward the methyl-bearing car-
bon. Be sure that you see the second gauche methyl–ring interaction. Next, com-
pare this gauche interaction to that in gauche-butane. Are the two exactly the same?
ANSWER It is difficult to place your “eye”correctly for this view, but if you look along
the red carbon–carbon bond, you can construct the appropriate Newman projection.
One of two
gauche methyl-ring
interactions; this
involves bumping
between CH
3
and the
ring CH
2
CH
2
Ring CH
2
Methylcyclohexane (methyl group axial)
Ring CH
2
Bump
Bump
H
H
H
H
CC
CH
3
CH
3
H
3
C
H
H
H
CH
3
H
H
gauche-Butane
=
=
CH
3
H
H
CH
2
Here, the gauche
interaction is a methyl–
methyl bumping
H
H
CH
3
H
H
CH
CH
2
The two interactions are very similar, but not exactly the same. In butane two
methyl groups bump, whereas in axial methylcyclohexane a methyl group bumps
with a .CH
2
O
CH
2

5.5 Monosubstituted Cyclohexanes 201
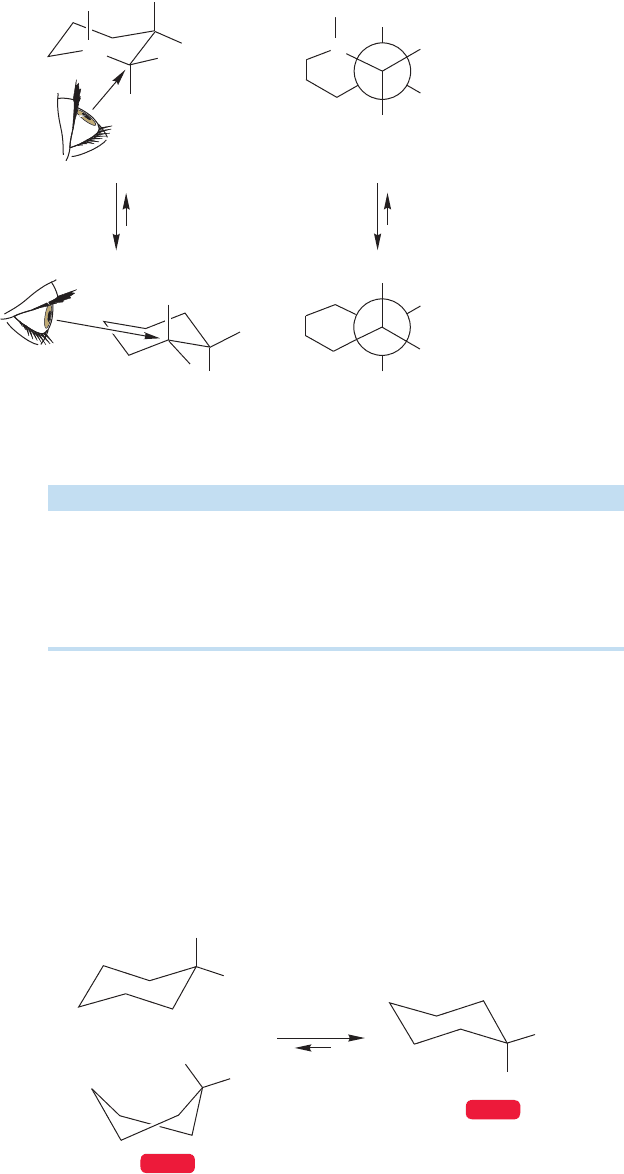
A Newman projection of the conformation of methylcyclohexane with the equa-
torial methyl group made from the same perspective reveals none of these gauche
interactions—this arrangement resembles the anti form of butane (Fig. 5.29).
H
CH
3
H
H
CH
3
CH
3
The two methyl groups
are as far apart as possible
Meth
y
lc
y
clohexane
(
meth
y
l
g
rou
p
e
q
uatorial
)
H
anti-Butane
=
=
H
H
CH
3
H
H
H
H
CC
H
3
C
This ring carbon and the
methyl group are as far
apart as possible
H
HH
H
C
H
3
FIGURE 5.29 Methylcyclohexane with an equatorial methyl group.There are no gauche methyl–ring
interactions.This isomer is 1.74 kcal/mol more stable than the molecule with the methyl group axial.
Note the resemblance to the anti form of butane.
Recall our conformational analysis of butane in Chapter 2 (p. 73).The gauche
form of butane was about 0.6 kcal/mol less stable than the anti form, in which
the terminal methyl groups of butane were as far apart as possible. Accordingly,
we might guess that the isomer with the methyl group equatorial would be
more stable than the axial isomer, and that the energy difference between the
two would be approximately twice the difference between gauche- and anti-butane,
2 0.6 1.2 kcal/mol. We’d be close. The real difference is 1.74 kcal/mol.
PROBLEM 5.10 Why isn’t the difference exactly 1.2 kcal/mol? Point out some of
the differences between the gauche interaction in butane and the gauche interac-
tion in axial methylcyclohexane.
Calculations of the percentage of isomers in the equatorial and axial forms of
methylcyclohexane will become clearer in Chapter 8. For now it’s fair to say that
about 95% of the molecules will have the methyl group equatorial at any given
instant. Once more, a small energy difference (1.74 kcal/mol) has resulted in a
large excess of the more stable isomer. Don’t make the mistake of thinking that
methylcyclohexane consists of a mixture of 95% molecules locked forever in the
conformation with the methyl group equatorial, and 5% of similarly frozen
molecules with their methyl groups axial. These molecules are in rapid equilib-
rium. At any given moment 95% will have their methyl groups equatorial, but
all molecules are continuously equilibrating between equatorial and axial
methyl isomers.
PROBLEM 5.11 The formula for doing equilibrium calculations is ΔG RT lnK,
where R is about 2 cal/deg mol, T is the absolute temperature, and K the equi-
librium constant sought. Calculate the amounts at equilibrium at 25 °C of two
compounds differing in energy by ΔG 2.8 kcal/mol.
#
202 CHAPTER 5 Rings
A group larger than methyl would result in an even greater dominance of the
equatorial conformation over the less stable axial conformation. The 1,3-diaxial
bumpings responsible for the destabilizing gauche interactions will be magnified,
and reflected in the equilibrium constant (Fig. 5.30; Table 5.3).
H
H
H
R
R
R
H
CH
H
H
H
H
H
H
H
H
H
CH
If R is large, this gauche
interaction will be more
destabilizing than the
interaction with a
methyl group
In this form, in which the
R is equatorial, there are
no gauche interactions
between R and the ring
R
H
FIGURE 5.30 The larger R, the more
favored will be the partner in the
equilibrium with the R group
equatorial.
TABLE 5.3 Axial–Equatorial Energy Differences for Some
Alkylcyclohexanes at 25 °C
Compound ≤G (ax/eq) (kcal/mol) K
Methylcyclohexane 1.74 19.5
Ethylcyclohexane 1.79 21.2
Propylcyclohexane 2.21 43.4
Isopropylcyclohexane 2.61 86.0
tert-Butylcyclohexane 5.5 11,916
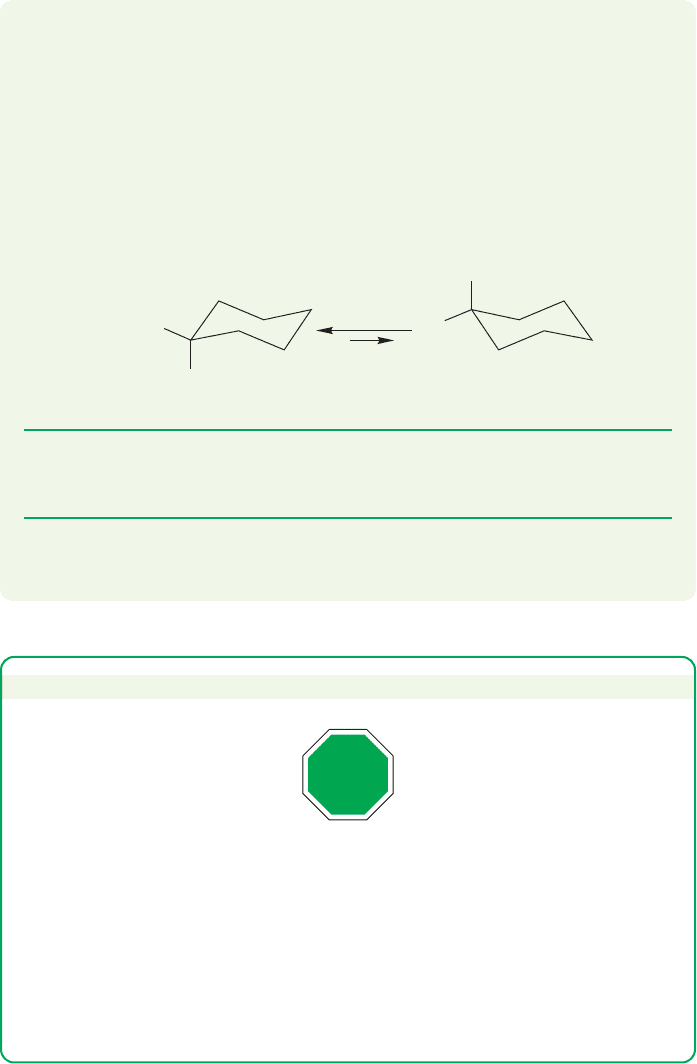
Note the large axial–equatorial (ax/eq) energy difference for the tert-butyl group.
This approximately 5–5.5 kcal/mol translates into an equilibrium constant of almost
12,000 at 25 °C. The situation is complicated for the tert-butyl group, because the
conformation with an axial tert-butyl group is so greatly destabilized that a twist con-
formation, with an energy about 5 kcal/mol higher than the conformation with an
equatorial tert-butyl group,may be lower in energy than the pure chair with an axial
tert-butyl group (Fig. 5.31). Regardless, the conformation of tert-butylcyclohexane
with the tert-butyl group equatorial is favored enormously.
C(CH
3
)
3
H
C(CH
3
)
3
H
(CH
3
)
3
C
or
H
ring flip
Chair
Chair
Twist
WEB 3D
WEB 3D
FIGURE 5.31 The large tert-butyl
group distorts the equilibrium far
toward the much more stable
equatorial form. The geometrical
relationships in the molecule can be
estimated with confidence because
this form dominates the equilibrum
mixture so strongly.
5.5 Monosubstituted Cyclohexanes 203
WORKED PROBLEM 5.12 It is often said that the tert-butyl group locks tert-
butylcyclohexane into the form with the tert-butyl group equatorial.Is this a good
way to put it? Why not?
ANSWER No! The molecule is not locked at all. The forms with the large tert-
butyl group axial and equatorial are in rapid equilibrium, with the equatorial form
greatly predominating. Very few molecules are in the axial form at any one time,
but the equilibrium is mobile. This molecule is not locked into one form and
immobilized forever.
(CH
3
)
3
C
(CH
3
)
3
C
H
H
ring flip
PROBLEM 5.13 How many carbons will the
13
C NMR spectrum of tert-
butylcyclohexane reveal at 25 °C?
PROBLEM 5.14 How many carbons will the
13
C NMR spectra of trans-
1,4-di-tert-butylcyclohexane and all-cis-1,3,5-tri-tert-butylcyclohexane reveal?
PROBLEM SOLVING
In many problems there are clues to the answer that are exceptionally important
to recognize. Here is a perfect example. Whenever you see a tert-butyl group
attached to a cyclohexane, you can be certain that it is there in order to fix the
cyclohexane in one of the possible chairs. tert-Butyl groups are always equatorial!
That observation allows you to fix the positions of any other groups of the ring
with certainty. If the problem involves a tert-butyl-substituted cyclohexane, you
can be sure that the solution will require you to draw the ring in three
dimensions, and that you must put the tert-butyl group in an equatorial position.
GO
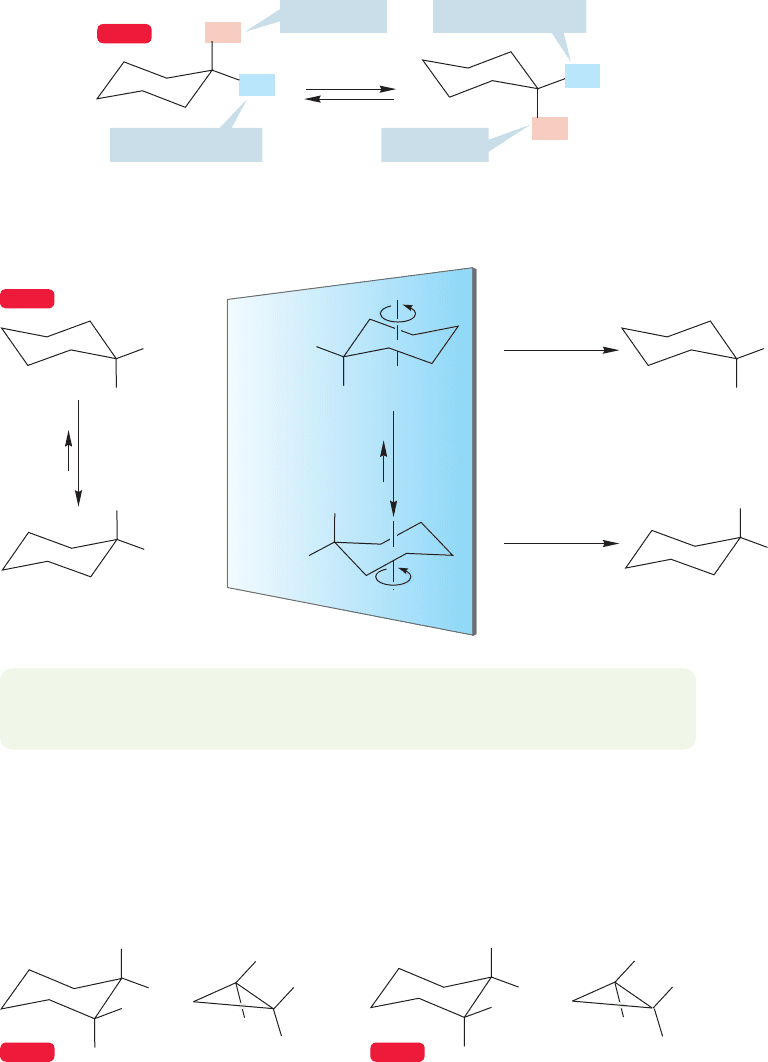
In studies of reaction mechanisms it is often very helpful to know with some pre-
cision the spatial relationships among groups. Ring compounds are quite helpful in
this regard. For example, the rigid frame of cyclopropane allows quite accurate
estimations of angles and distances to be made. But cyclopropanes are so strained
as to be sometimes too reactive for mechanistic work. In fact, the ring is often
opened in chemical reactions. One wouldn’t want one’s rigid framework disappear-
ing as the reaction occurred, but this is all too likely for cyclopropanes. Other rings
are flexible and don’t allow us the firm predictions of angles and distances we want.
204 CHAPTER 5 Rings
However, a large group such as tert-butyl enables us to prejudice the mobile equi-
librium between cyclohexane rings so strongly in favor of the form with the tert-
butyl group equatorial that we can determine with confidence the positions of
other atoms in the molecule (Fig. 5.31). We will see later that this technique is
used frequently.
Summary
All cycloalkanes except cyclopropane distort from planarity so as to minimize
strain.The amount of strain can be measured in a variety of ways,including meas-
urements of heats of combustion or heats of formation. Cyclohexane adopts an
energy-minimum chair form in which there are six hydrogens in the axial posi-
tion and six in the equatorial position. Cyclohexane is a mobile system, as chair
forms interconvert. This interconversion exchanges substituents in the axial and
equatorial positions.
5.6 Disubstituted Ring Compounds
We have already mentioned that cis and trans diastereomers exist for dichlorocy-
clopropanes (Chapter 4, pp. 173–176) and that the trans form is chiral (Fig. 5.32).
Mirror
trans-1,2-Dichlorocyclopropane
(chiral—there is a pair of enantiomers)
cis-1,2-Dichlorocyclopropane
(an achiral molecule, a meso compound)
H
H
CH
2
Cl
Cl
H
H
H
CH
2
Cl
Cl
Cl
H
CH
2
Cl
FIGURE 5.32 cis- and trans-
1,2-Dichlorocyclopropane.
Mirror
trans-1-Bromo-2-chlorocyclopropane
is also chiral, and, of course, there is
a pair of enantiomers
H
H
CH
2
Br
Br
Cl
Cl
H
H
CH
2
FIGURE 5.33 cis- and trans-1-Bromo-
2-chlorocyclopropane.
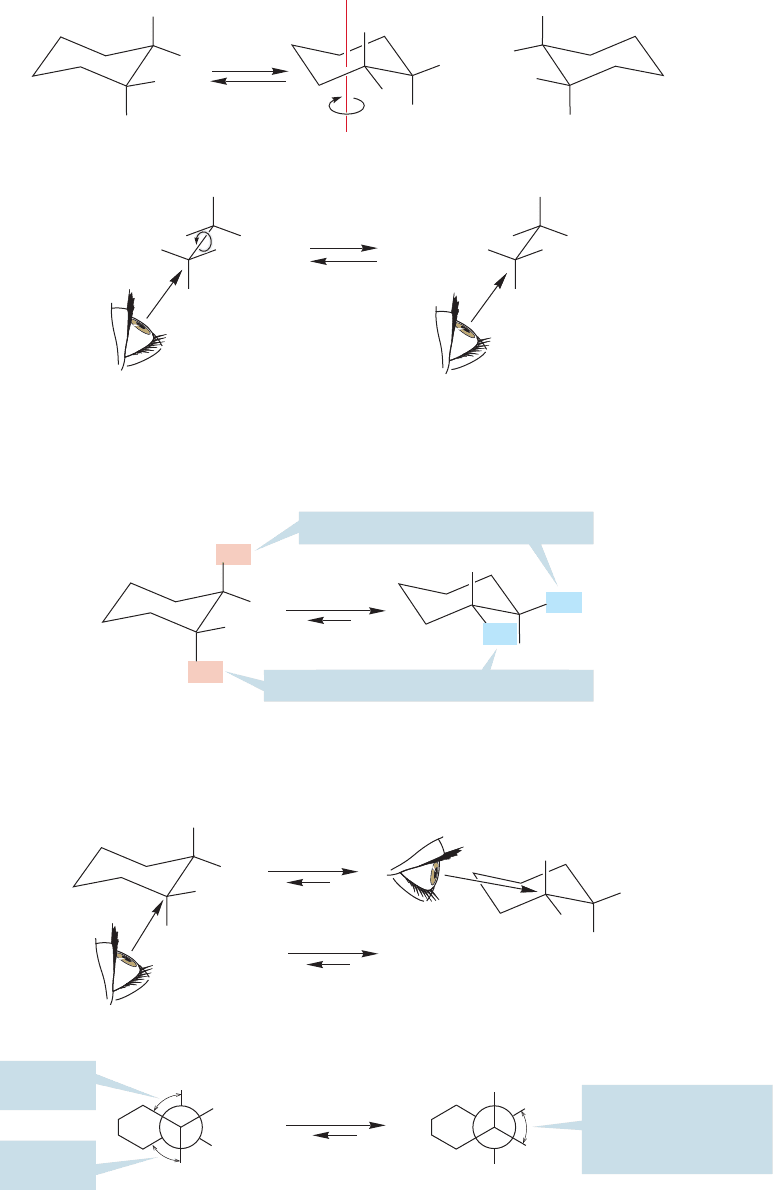
When the two substituents on the ring are different, as in 1-bromo-2-chloro-
cyclopropane, two pairs of enantiomers are possible,and both the cis and trans forms
of this molecule are chiral (Fig. 5.33).
Stereochemical relationships become harder to see in cyclohexanes, where
the molecules are not planar, but they don’t really change. We will first take a
look at 1,1-dialkylcyclohexanes and then at the somewhat more complicated
1,2-dialkylcyclohexanes.
Mirror
cis-1-Bromo-2-chlorocyclopropane
is a chiral molecule—there is
a pair of enantiomers
H
H
CH
2
Br
Br
Cl
Cl
H
H
CH
2
5.6 Disubstituted Ring Compounds 205
1-Isopropyl-1-methylcyclohexane is only slightly more complicated. There are
two isomers, nearly equal in energy, neither of which is chiral (Fig. 5.35).
CH
3
CH
3
flip
K = 1
CH
3
CH
3
Axial methyl
Axial methyl
Equatorial methyl
Equatorial methyl
WEB 3D
FIGURE 5.34 1,1-Dimethylcyclohexane.
WEB 3D
Mirror
CH(CH
3
)
2
CH(CH
3
)
2
(CH
3
)
2
HC
CH(CH
3
)
2
CH
3
H
3
C
CH
3
CH
3
180 rotation
180 rotation
flip flip
180
180
CH(CH
3
)
2
CH
3
CH(CH
3
)
2
CH
3
FIGURE 5.35 Both isomers of 1-isopropyl-1-methylcyclohexane
are achiral—the mirror images are superimposable.
PROBLEM 5.15 Use the data in Table 5.3 to calculate the energy difference
between the possible isomers of 1-isopropyl-1-methylcyclohexane.
5.6b 1,2-Disubstituted Cyclohexanes Just like 1,2-dimethylcyclopropane,
1,2-dimethylcyclohexane can exist in cis and trans forms (as can any 1,2-disubstituted
ring compound), but the chair form of cyclohexane makes the compounds look
somewhat different from the more familiar rigid isomers of cis and trans disubsti-
tuted cyclopropanes (Fig. 5.36).
WEB 3D WEB 3D
H
Methyl groups cis
hydrogens cis
H
CH
3
CH
3
H
H
CH
3
Methyl groups trans
hydrogens trans
H
H
CH
3
H
H
CH
3
CH
3
CH
3
CH
3
FIGURE 5.36 Three-dimensional
representations of cis- and trans-1,2-
dimethylcyclohexane and cis- and
trans-1,2-dimethylcyclopropane.
5.6a 1,1-Disubstituted Cyclohexanes 1,1-Dimethylcyclohexane is a simple,
achiral molecule in which the axial–equatorial equilibration induced by flipping one
chair to the other interconverts equivalent molecules, each of which has one methyl
group axial and one equatorial. The ring flip converts the axial methyl group into
an equatorial methyl group and the equatorial methyl group into an axial methyl
group. There is no net change and, as for cyclohexane itself, the equilibrium con-
stant for the equilibration must be 1 (Fig. 5.34).

206 CHAPTER 5 Rings
PROBLEM 5.16 Figure 5.37b shows only the equatorial–equatorial form of trans-
1,2-dimethylcyclohexane. What is the dihedral angle between the methyl groups
in the much less stable axial–axial trans-1,2-dimethylcyclopropane isomer?
The words cis and trans refer to the “sidedness”of a molecule and do not depend
strictly on the angles between the groups. In a cis disubstituted cyclopropane, the
dihedral angle between the cis groups is 0°, whereas it is 60° in the disubstituted
cyclohexane (Fig. 5.37a). The methyl groups are referred to as cis in either case. In
trans-1,2-dimethylcyclopropane, the dihedral angle between the methyl groups is
120°, whereas in the six-membered ring it is 60° (Fig. 5.37b).
(a) (b)
Two views of cis-1,2-dimethylcyclopropane;
the dihedral angle between the methyl groups is 0
H
0
Dihedral
angle
Two views of trans-1,2-dimethylcyclopropane;
the dihedral angle between the methyl groups is 120
H
120
Dihedral
angle
H
CH
3
H
H
CH
3
CH
3
CH
3
CH
3
CH
3
H
CH
3
CH
3
H
H
Two views of cis-1,2-dimethylcyclohexane;
the dihedral angle between the methyl groups is 60
H
H
CH
3
CH
3
CH
3
H
60
Two views of trans-1,2-dimethylcyclohexane;
the dihedral angle between the
methyl groups is also 60
H
H
CH
3
CH
3
CH
3
CH
3
H
60
CH
3
H
H
FIGURE 5.37 Isomerism in the planar, inflexible cyclopropanes and the nonplanar, flexible cyclohexanes: (a) cis, (b) trans.
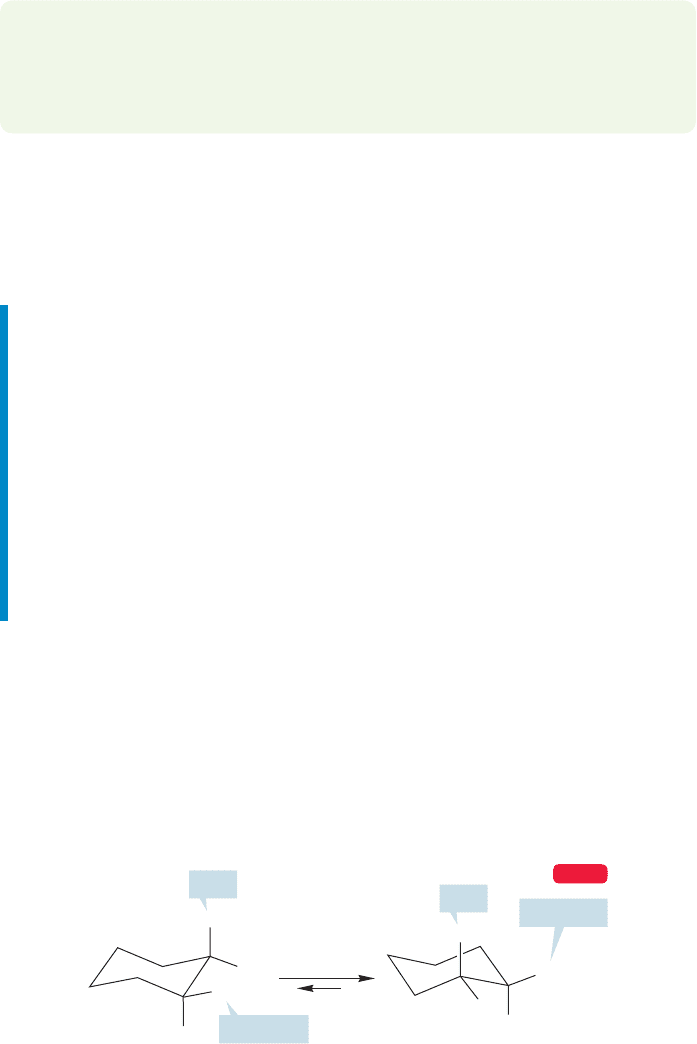
In cis-1,2-dimethylcyclohexane,one methyl group is axial and the other is equa-
torial (Fig. 5.38).The conformational ring flip does not alter the structure—the axial
methyl becomes equatorial, and the equatorial methyl becomes axial. The equilib-
rium constant between these two equivalent compounds must be 1.
ring flip
K = 1
CH
3
H
H
CH
3
H
H
CH
3
This axial methyl becomes equatorial
CH
3
This equatorial methyl becomes axial
FIGURE 5.38 cis-1,2-Dimethylcyclohexane.
Note that the two conformational isomers of cis-1,2-dimethylcyclohexane are
enantiomers.The equilibration between the two conformations produces a racemic
mixture of the two enantiomers.Have we seen a similar situation before? Indeed we
5.6 Disubstituted Ring Compounds 207
have; just recall our discussion of the gauche forms of butane (Chapter 4, p. 163).
The equilibration of the two cis-1,2-dimethylcyclohexanes is very similar to the equi-
libration of the two gauche forms of butane (Fig. 5.39).
ring flip
CH
3
H
H
CH
3
H
=
H
CH
3
CH
3
CH
3
H
3
C
H
H
120° Rotation
120°
rotation
CH
3
H
H
H
H
CH
3
CH
3
H
H
H
H
H
3
C
FIGURE 5.39 The ring flip in cis-
1,2-dimethylcyclohexane converts
one enantiomer into the other. A very
similar process interconverts the two
enantiomers of gauche-butane.
flip
CH
3
H
H
CH
3
H
CH
3
This axial methyl becomes equatorial
This axial methyl also becomes equatorial
H
CH
3
FIGURE 5.40 The ring flip in trans-
1,2-dimethylcyclohexane converts a
molecule with two axial methyl
groups into one with two equatorial
methyl groups.
The trans form of 1,2-dimethylcyclopropane presents a different picture. trans-1,2-
Dimethylcyclohexane can either have both methyl groups axial or both equatorial
(Fig. 5.40).The ring flip converts the diaxial form into the diequatorial form.Let’s apply
conformational analysis to predict which of these two diastereomers is more stable.
As usual, Newman projections are vital, and in Figure 5.41 we sight along the
carbon–carbon bond attaching the two methyl-substituted carbons. In the diequa-
torial isomer, there is one gauche methyl–methyl interaction costing 0.6 kcal/mol.
CH
3
H
CH
3
H
gauche
Interaction
Methyl–methyl gauche
interaction, but no
methyl–ring gauche
interactions
gauche Interactions give
2
1.74 = 3.48 kcal/mol
of destabilization
One methyl–methyl gauche
interaction gives 0.6 kcal/mol
of destabilization
gauche
Interaction
CH
3
CH
3
Diaxial Diequatorial
H
CH
3
H
H
flip
flip
Less stable More stable
flip
H
H
CH
3
H
CH
3
CH
3
FIGURE 5.41 Diequatorial trans-
1,2-dimethylcyclohexane is more
stable than the diaxial isomer.
208 CHAPTER 5 Rings
In the diaxial isomer, the two methyl groups occupy anti positions, and therefore
their interaction with one another is not destabilizing.However, for each axial methyl
group we still have the two gauche interactions with the ring methylene groups (see
p. 199), and this will cost the molecule 2 1.74 kcal/mol 3.48 kcal/mol. We can
predict that the diequatorial isomer should be (3.48 0.6) kcal/mol 2.88 kcal/mol
more stable than the diaxial isomer. In turn, this calculation predicts that at 25 °C
there will be more than 99% of the diequatorial form present.
PROBLEM 5.17 Show that the ring flip of the diequatorial form to the diaxial
form will not racemize optically active trans-1,2-dimethylcyclohexane. What is
the relation between the equatorial–equatorial and axial–axial forms of trans-
1,2-dimethylcyclohexane?
In principle, both the diaxial and diequatorial forms should be separable into a
pair of enantiomers, resolvable (p. 169)—they are both chiral molecules. In practice,
we cannot isolate trans-diaxial-1,2-dimethylcyclohexane; it simply flips to the much
more stable diequatorial form. The diequatorial form can be isolated and resolved.
Summary
Neither cis-1,2-dimethylcyclopropane nor cis-1,2-dimethylcyclohexane can be
resolved, because the cyclopropane is a meso compound (Chapter 4, p. 168), and
the cyclohexane flips into its mirror image. However, both trans-1,2-dimethyl-
cyclopropane and trans-1,2-dimethylcyclohexane can be resolved; they are not
superimposable on their mirror images.The point is that in a practical sense the
planar cyclopropanes and nonplanar cyclohexanes behave in the same way. This
finding has important consequences. In deciding questions of stereochemistry, we
can treat the decidedly nonplanar 1,2-dimethylcyclohexanes as if they were pla-
nar. Indeed, all cyclohexanes can be treated as planar for the purposes of stereo-
chemical analysis,because the planar forms represent the average positions of ring
atoms in the rapid chair–chair interconversions.
Now let’s look at some slightly more complicated molecules in which the two
substituents on the ring are different. In 1-isopropyl-2-methylcyclohexanes cis and
trans isomers exist, and once more we will have to worry about the effects of flip-
ping from one chair form to the other. In the cis compound, there are two differ-
ent conformational isomers (Fig. 5.42). In one isomer, the isopropyl group is axial
and the methyl group is equatorial. When the ring flips, the axial isopropyl group
becomes equatorial and the equatorial methyl group becomes axial.
Equatorial
Equatorial
CH
3
CH(CH
3
)
2
CH(CH
3
)
2
H
H
H
H
Axial
Axial
flip
CH
3
WEB 3D
FIGURE 5.42 cis-1-Isopropyl-
2-methycyclohexane flips from the
conformer with an axial isopropyl
group and an equatorial methyl, to
the conformer with an equatorial
isopropyl group and an axial methyl.
Unlike the dimethyl case, these two compounds are not enantiomeric.They are
conformational diastereomers—conformational isomers that have different physical
