Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

5.10 Summary 219
produces “diamantane,” two caps gives us “triamantane,” three caps produces
“tetramantane,” and so on. The ultimate result of the capping process is a
molecule composed of a network of adamantanes, and this is the structure of
diamond.
Adamantane chemistry remains full of astonishing surprises. For years it has
been known that synthetic diamonds could be made from another polymeric form
of carbon, graphite, by treatment at high temperature ( 2300 K) and very high
pressure (7 10
4
kg/cm
2
). However, in the 1980s in the Soviet Union and Japan,
it was discovered that diamond films could be grown at low temperature and pres-
sure by passing a stream of hydrogen gas containing a few percent methane through
an electric discharge (Fig. 5.69).The mechanism of this reaction remains obscure.
Any ideas?
'
H
2
CH
4
electrical
discharge
+
Diamond
FIGURE 5.69 A simple (and
astonishing) synthesis of diamond.
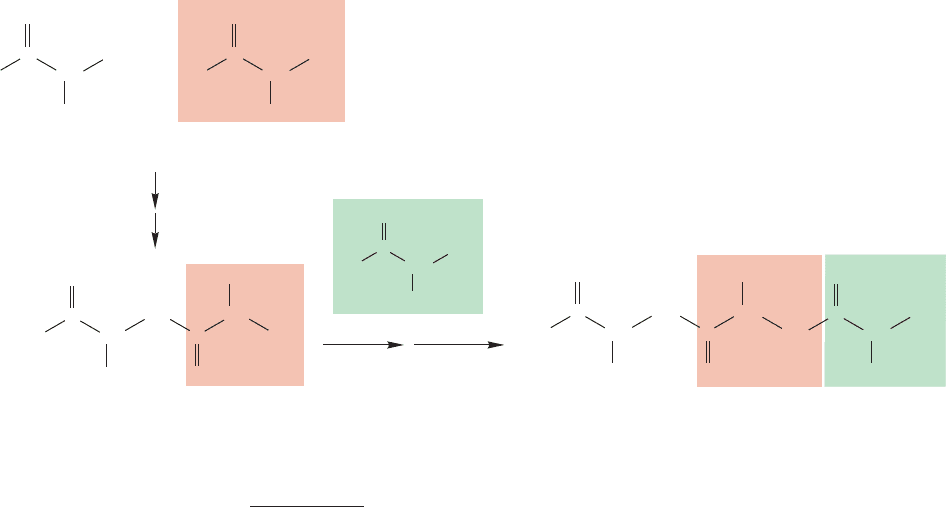
PROBLEM 5.30 What is the empirical formula of diamond? For a large polymeric
molecule such as diamond, the edges of the molecule are insignificant in figuring
out the formula.
PROBLEM 5.31 Can you draw other isomers of tetramantane?
Adamantane remains a favorite of chemists because of its intrinsic symmetry
and beauty, and probably because of the difficulty of working out the mechanism
of its formation. It does not seem of much practical interest. However, some of its
simple derivatives have remarkable properties and some are quite active biologi-
cally. For example, 1-aminoadamantane, a compound easily made from adaman-
tane itself, is one of the few antiviral agents known. This remarkable property
was discovered during routine empirical screening at duPont in the 1960s, and
1-aminoadamantane has since been marketed, mostly as an agent against influenza
A and C. It apparently works by migrating through the cell membrane to attack
the virus within. It is the adamantane cage, acting as a molecular ball of grease,
that helps in the membrane penetration. It has been speculated that other, large,
symmetrical hydrocarbon “blobs” might act in the same way. Unfortunately, they
are not so easy to make.
This chapter deals exclusively with the structural properties of
ring compounds. Two kinds of destabilizing effects on rings are
discussed. Torsional strain, the destabilizing effect of eclipsed car-
bon–hydrogen bonds, was mentioned in Chapter 2 when the
acyclic hydrocarbons were discussed.The planar forms of ring
compounds are particularly subject to torsional strain. In addition,
rings contain varying amounts of angle strain, depending on the
ring size. Any deviation from the ideal tetrahedral angle of 109.5°
will introduce angle strain. Rings adopt nonplanar forms in order
to minimize the combination of torsional and angle strain.
5.10 Summary
New Concepts
The diamond in this impressive bling
is composed of a multi-ring carbon
framework of adamantanes.
220 CHAPTER 5 Rings
angle strain (p. 187)
axial hydrogens (p. 191)
bridged (p. 211)
equatorial hydrogens (p. 191)
fused (p. 211)
spiro (p. 211)
torsional strain (p. 187)
van der Waals strain (p. 198)
Key Terms
The few reactions encountered here are not really new. There is,
however, a somewhat more detailed treatment of the formation
of carbon dioxide and water from hydrocarbons and oxygen
(combustion) than we saw in Chapter 2 (p. 68). There is also a
discussion of the effects of strain on the bond energy of the car-
bon–carbon bond.
Reactions, Mechanisms, and Tools
You are presented in this chapter with the most difficult chal-
lenge so far in visualizing molecules. Moreover, the difficulty in
translating from two dimensions to three is compounded by the
mobile equilibria present in many ring compounds. In analyzing
complicated phenomena, such as the equilibrations of disubsti-
tuted cyclohexanes, be sure to use models, at least at first.
Two small points seem to give students trouble. First, one
hears constantly, I’m no artist, I can’t draw a good chair cyclo-
hexane! Nonsense. It does not take an artist, merely an artisan.
Go slowly, do not scribble, and follow the simple procedure
outlined in this chapter (p. 191ff ). Drawing a perfect chair
cyclohexane is one thing that everyone can do.
Determining which groups are cis and trans in ring com-
pounds causes problems. Remember: cis means “on the same side,”
and trans, “on opposite sides.” Bonds to cis groups do not have to
be parallel, merely both up or both down. Figure 5.70 gives two
common examples, one straightforward, the other not so obvious.
Common Errors
H
X
X
H
X
X
H
H
Up
Up
Down
Down
FIGURE 5.70 In both these compounds the two hydrogens shown
are cis, as are the two X groups.This relationship is easy to see for
the flat cyclopropane, but harder in the nonplanar cyclohexane.
PROBLEM 5.32 By now you have encountered most structural
types and have some experience with rings. Write and name
all the isomers of the formula C
5
H
8
. Stay alert for isomerism
of the cis/trans (Z/E) and (R/S) types. Indicate chiral molecules
with an asterisk. This problem is very hard at this point.
3
There are all sorts of odd structural types among these iso-
mers. It should not be hard to get most of them, but getting
them all is really tough. Think in three dimensions and
organize. Hint: The total number of isomers, not including
enantiomers, is 28.
PROBLEM 5.33 Write out the pairs of enantiomers for the
chiral isomers in Problem 5.32.
PROBLEM 5.34 Assign (R) and (S) configurations for the ver-
sions of the chiral cyclic molecules shown on the left in the
answers to Problem 5.33 given in the Study Guide.
PROBLEM 5.35 Draw the most stable chair conformation of
tert-butylcyclohexane. Put a circle around each of the axial
hydrogens on the cyclohexane. Put a square around each of the
equatorial hydrogens attached to the ring.
5.11 Additional Problems
3
Indeed, this problem constituted the entire first hour examination in MJ’s first course in organic chemistry.
One nonplanar ring deserves special mention.
Cyclohexane avoids torsional and angle strain by adopting a
chair conformation in which a nearly ideal tetrahedral angle
is achieved and all carbon–hydrogen bonds are perfectly
staggered. In cyclohexane, there is a set of six axial hydrogens
that is converted into the set of six equatorial hydrogens
through rotations about carbon–carbon bonds called a
ring flip.
There are many possible quantitative evaluations of strain.
This chapter uses related heats of formation ( ) and heats
of combustion ( ) to arrive at values for the strain energies
of various rings. As rings increase in size, strain initially
decreases, reaching a minimum at the strain-free cyclohexane.
Strain then increases until large ring sizes are reached.
By combining rings in spiro, fused, and bridged fashion,
very complex polycyclic molecules can be constructed.
¢H
°
c
¢H °
f
5.11 Additional Problems 221
PROBLEM 5.36
Draw the two possible chair forms of cis- and
trans-1,4-dimethylcyclohexane. Are the two forms identical,
enantiomeric, or diastereomeric? In each case, indicate which
chair form will be more stable and explain why. Is either of
these molecules chiral?
PROBLEM 5.37 Draw the two possible chair forms of cis- and
trans-1-isopropyl-4-methylcyclohexane. Are the two forms
identical, enantiomeric, or diastereomeric? In each case, indicate
which chair form will be more stable and explain why. Is either
of these molecules chiral?
PROBLEM 5.38 Draw the two possible chair forms of cis- and
trans-1,3-dimethylcyclohexane. Are the two forms identical,
enantiomeric, or diastereomeric? In each case, indicate which
chair form will be more stable and explain why. Is either of
these molecules chiral?
PROBLEM 5.39 Draw the possible chair forms of cis- and
trans-1-isopropyl-3-methylcyclohexane. Are the two forms
identical, enantiomeric, or diastereomeric? In each case, indicate
which chair form will be more stable and explain why. Is either
of these molecules chiral?
PROBLEM 5.40 Draw the double Newman projection as
shown in Figure 5.12 for the following compounds:
(a) 1,1-dimethylcyclohexane looking down the
and bonds.
(b) cis-1,2-dimethylcyclohexane looking down the
and bonds.
(c) the more stable conformation of trans-1,2-dimethylcyclo-
hexane looking down the and
bonds.
(d) trans-1,3-dimethylcyclohexane looking down the
and bonds.
PROBLEM 5.41 In Section 5.6b (p. 205), you saw that for pur-
poses of stereochemical analysis you could treat the decidedly
nonplanar cis- and trans-1,2-dimethylcyclohexanes as if they
were planar. The planar forms represent the average positions of
ring atoms in the rapid chair–chair interconversions. Use planar
representations of the following cyclohexanes to determine
which molecules are chiral.
(a) cis-1,3-dimethylcyclohexane
(b) trans-1,3-dimethylcyclohexane
(c) cis-1-isopropyl-3-methylcyclohexane
(d) trans-1-isopropyl-3-methylcyclohexane
(e) cis-1,4-dimethylcyclohexane
(f) trans-1,4-dimethylcyclohexane
(g) cis-1-isopropyl-4-methylcyclohexane
(h) trans-1-isopropyl-4-methylcyclohexane
PROBLEM 5.42 Draw the planar structure for each of the fol-
lowing compounds. Next, draw the most stable conformation
for each in three dimensions.
(a) trans-1,2-dibromocyclohexane
C(5)
O
C(4)C(1)
O
C(2)
C(5)
O
C(4)C(1)
O
C(2)
C(5)
O
C(4)
C(1)
O
C(2)
C(4)
O
C(5)
C(2)
O
C(1)
(b) cis-1,3-dichlorocyclohexane
(c) trans-1-chloro-4-fluorocyclohexane
PROBLEM 5.43 Which of the molecules in Problem 5.42 are
chiral and which are achiral?
PROBLEM 5.44 Draw a chair conformation of cis-1-bromo-4-
fluorocyclohexane. Draw the ring-flipped structure. Which
structure do you expect to be favored? Why?
PROBLEM 5.45 Draw a chair conformation of cis-1-bromo-3-
chlorocyclohexane. Draw the ring-flipped structure. Which is
favored? Why? Is the molecule chiral?
PROBLEM 5.46 Use the data in Table 5.3 (p. 202) to calculate
the energy difference between the possible isomers of cis- and
trans-1,4-dimethylcyclohexane.
PROBLEM 5.47 Use the data in Table 5.3 (p. 202) to calculate
the energy difference between the possible isomers of cis- and
trans-1-isopropyl-4-methylcyclohexane.
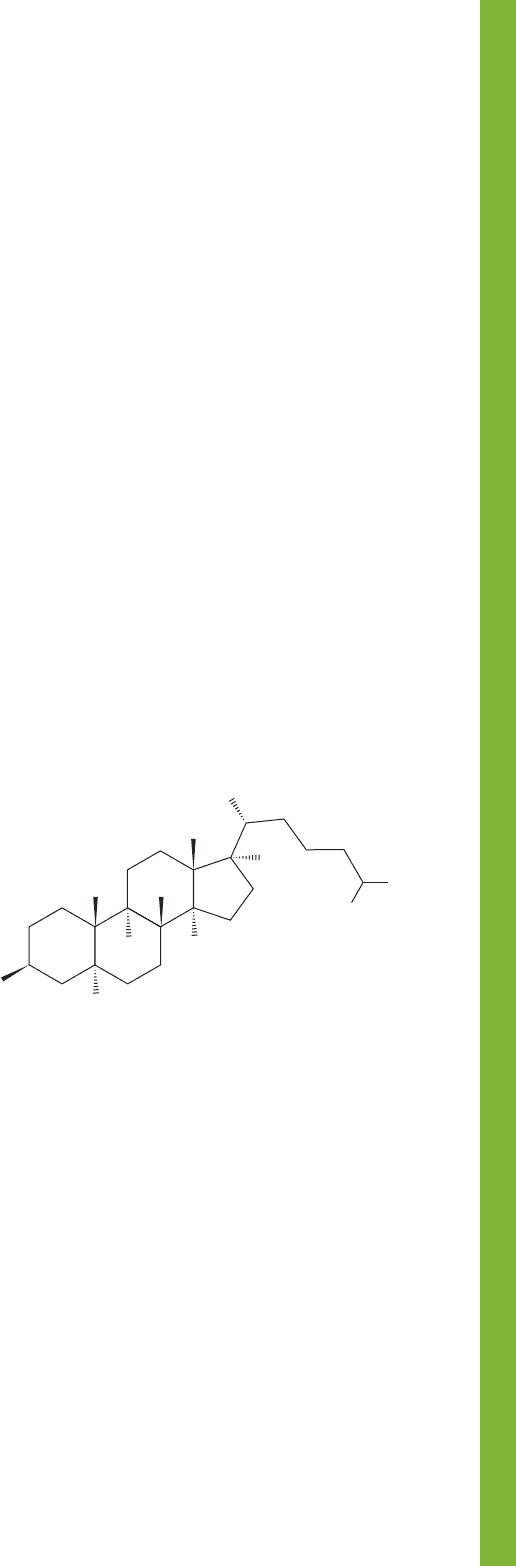
PROBLEM 5.48 Cholestanol (shown below) is a natural prod-
uct found in gallstones and eggs. How many stereogenic atoms
are there in this molecule? Draw the compound with the rings
in their most stable conformation. Hint: The molecule is rela-
tively flat when viewed in three dimensions.
Cholestanol
CH
3
CH
3
H
3
C
H
3
C
H
H
H
CH
3
H
HO
H
PROBLEM 5.49 How many signals will appear in the
13
C
NMR spectrum of 1,1-dimethylcyclohexane?
(a) At low temperature.
(b) At high temperature.
Hint: Start by figuring out what effect the increase in tempera-
ture will have.
PROBLEM 5.50 How many signals will appear for cis-1,3-
dimethylcyclohexane in its
13
C NMR spectrum? How many
signals will appear for trans-1,3-dimethylcyclohexane in its
13
C
NMR spectrum?
(a) At low temperature.
(b) At high temperature.
PROBLEM 5.51 How many signals will appear in the
13
C
NMR spectrum of adamantane (p. 218)?
222 CHAPTER 5 Rings
Use Organic Reaction Animations (ORA) to answer the fol-
lowing questions. In these problems you will encounter new
reactions. Don’t be psyched out or intimidated—follow the ani-
mation carefully and you should be able to get these problems.
PROBLEM 5.59 Observe the reaction titled “Bimolecular
nucleophilic substitution.” Notice that a bromide is being dis-
placed from a carbon atom, in this case C(2) of 2-bromo-
propane. We can imagine a closely related reaction in which
displacement takes place in bromocyclopropane. All we have
(mentally) done is to connect the two end carbons of 2-bromo-
propane with a carbon–carbon bond. Would the reaction with
bromocyclopropane be faster or slower (it can’t be the same!) as
the reaction with 2-bromopropane? Hint: Think about the tran-
sition state for this reaction—what is the hybridization of car-
bon in the transition state?
PROBLEM 5.60 Now consider the same substitution reaction
with axial bromocyclohexane. What would be the conformation
of the product? Would this reaction be faster or slower than the
reaction with 2-bromopropane?
PROBLEM 5.61 Would the rate of reaction with equatorial
bromocyclohexane be faster, slower, or the same as that with
axial bromocyclohexane? Would the reaction with equatorial
bromocyclohexane be faster or slower than the reaction with
2-bromopropane?
PROBLEM 5.62 Observe the reaction titled “Bimolecular elim-
ination.”This reaction also starts with 2-bromopropane. Look
at the orientation of the breaking carbon–hydrogen and carbon–
bromine bonds in this reaction. Now imagine the same
bimolecular elimination occurring in axial and equatorial bro-
mocyclohexane. Which stereoisomer would undergo this reac-
tion faster, the axial or equatorial bromide?
PROBLEM 5.53 Name the following compounds:
CH
3
Cl
CH(CH
3
)
2
(a)
CH
3
Cl
CH(CH
3
)
2
(b)
CH
3
Cl
CH(CH
3
)
2
(e)
CH
3
Cl
CH(CH
3
)
2
(f)
CH
3
Cl
CH(CH
3
)
2
(c)
CH
3
Cl
CH(CH
3
)
2
(d)
PROBLEM 5.52 Draw both chair forms for the following cyclo-
hexanes. Indicate the more stable chair form in each case. Not all
examples will be obvious; some choices may be too close to call.
PROBLEM 5.54 Write structures for the following
compounds:
(a) 2,2-dibromo-3,3-dichloro-5,5-difluoro-6,6-diiodobicy-
clo[2.2.1]heptane
(b) bicyclo[1.1.1]pentan-1,3-diol
(c) hexamethylbicyclo[2.2.0]hexa-2,5-diene
(b)
(c)(a)
F
F
OH
O
HOH
2
C
5
6
4
3
2
1
OH
OH
OH
HO
PROBLEM 5.56 One of the isomers of methylbicyclo[2.2.1]hep-
tane can exist in two diastereomeric forms (we are not count-
ing mirror images, which increases the total number of
stereoisomers to four). Write the possible isomers of methylbi-
cyclo[2.2.1]heptane and explain how one of them can exist in
two forms.
PROBLEM 5.57 (a) Can one of the isomers of methylbicy-
clo[2.2.2]octane also exist in two diastereomeric forms? That is,
does it behave just like the methylbicyclo[2.2.1]heptane men-
tioned in Problem 5.56? (b) Can one of the isomers of methyl-
bicyclo[2.2.2]octane exist in two enantiomeric forms?
PROBLEM 5.58 Draw the favored envelope conformation of
the cyclopentane shown below.
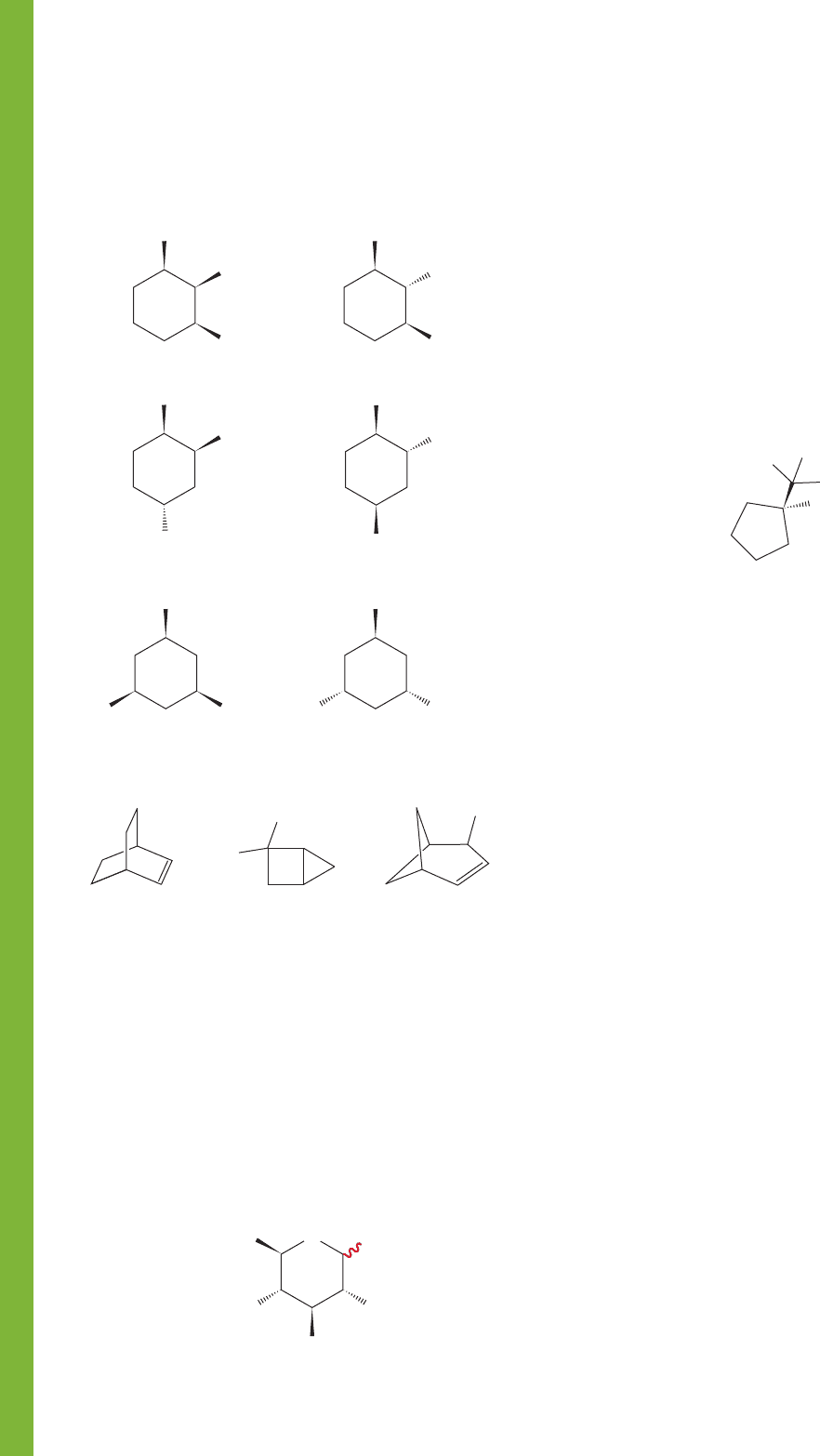
PROBLEM 5.55 The sugar glucopyranose is a six-membered
ring containing an oxygen atom. There are groups attached at
each of the five ring carbons as shown. The red “squiggly” bond
merely means that the hydroxyl group at the 1-position may be
either up or down; that is, there are two structures for gluco-
pyranose. Draw these two molecules in three dimensions.

Alkyl Halides, Alcohols,
Amines, Ethers, and Their
Sulfur-Containing Relatives
223
6.1 Preview
6.2 Alkyl Halides: Nomenclature
and Structure
6.3 Alkyl Halides as Sources of
Organometallic Reagents:
A Synthesis of Hydrocarbons
6.4 Alcohols
6.5 Solvents in Organic Chemistry
6.6 Diols (Glycols)
6.7 Amines
6.8 Ethers
6.9 Special Topic: Thiols
(Mercaptans) and Thioethers
(Sulfides)
6.10 Special Topic: Crown Ethers
6.11 Special Topic: Complex
Nitrogen-Containing
Biomolecules—Alkaloids
6.12 Summary
6.13 Additional Problems
6
CANOLA Canola oil is obtained from the rapeseed plant. Rapeseed also
pumps a significant amount of methyl bromide into the atmosphere.
So, if Sunday you’re free,
Why don’t you come with me,
And we’ll poison the pigeons in the park.
My pulse will be quickenin’,
With each drop of strych-i-nin’,
We feed to a pigeon,
(It just takes a smidgin),
To poison a pigeon in the park.
—TOM LEHRER
1
6.1 Preview
In this chapter, we will see some old friends, alkyl halides and alcohols, as well as
some new molecules rather closely related to them: amines, ethers, thiols, and
thioethers. Both alkyl halides and alcohols can be made through the addition
reactions encountered in Chapter 3 (p. 131). Amines somewhat resemble alcohols,
and understanding the chemistry of amines is a necessary prelude to working
through Chapter 23, which discusses the chemistry of some of the many biolog-
ically important molecules containing nitrogen. A nitrogen atom provides one of
the crucial linking agents in the formation of polyamino acids, called peptides or
proteins (Fig. 6.1). In this chapter, we examine the qualities that make these mol-
ecules similar and prepare for Chapter 7, where we will take a long, detailed look
at four prototypal new reactions in which alkyl halides and alcohols are especially
important.
224 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
+
R
O
C
HO
CH
NH
2
R
O
C
HO
CH
NH
2
R
O
C
HO
CH
NH
2
NH
2
R
R
O
O
C
C
HO
several
reactions
several
reactions
new amino acid
Growing peptide (or protein)
CH
CH
NH
NH
NH
2
R
R
O
O
C
C
HO
CH
R
CH
CH
O
C
NH
Amino acids
FIGURE 6.1 A nitrogen-to-carbon link is vital in the polymeric molecules known as proteins or peptides.
1
Thomas Lehrer (b. 1928) is an American mathematician and songwriter, most of whose work was done in
the 1950s and 1960s. Despite the unfortunate fact that there has been little new material for many years, his
songs, often sardonic and always very funny, retain their appeal. (Lyrics © 1953 Tom Lehrer. Reprinted with
permission.)
6.2 Alkyl Halides: Nomenclature and Structure 225
ESSENTIAL SKILLS AND DETAILS
1. So much of chemistry, including organic chemistry, involves acids and bases. In this
chapter, we review what we know already about this subject, and focus on a measure of
acidity, pK
a
. It is important to have a reasonable idea of the pK
a
values of the general classes
of compounds.There is a table of pK
a
values on the inside of the back cover of this book.
2. A low pK
a
means that the molecule in question is a strong acid, a good proton donor.
Conversely, a high pK
a
means that the compound is a poor acid.
3. We also encounter solvation in this chapter. Being able to choose an appropriate
solvent for a reaction, and to understand how solvents operate, is an important skill
both in the practical world and in this course.
4. The synthetic sequence “alkene halide Grignard reagent” is one you will use
many times in solving synthetic problems.
UU
6.2 Alkyl Halides: Nomenclature and Structure
Alkyl iodides, bromides,chlorides, and fluorides are collected under the name of alkyl
halides. Alkyl halides have the formula C
n
H
2n 1
X, where X F, Cl, Br, or I.
Quite appropriately, alkyl halides are named as fluorides, chlorides, bromides, and
iodides. Both common and systematic nomenclature names are used, although as
the complexity of the molecule increases, the system naturally takes over.The impor-
tant rules to remember for naming saturated halides are (1) minimize the number
given to the substituent, (2) name multiple substituents in alphabetical order, and
(3) base the name on the longest straight chain that contains the halide. If a
carbon–carbon double bond is present, the double bond takes precedence over the
halogen when positional numbers are assigned.Table 6.1 gives some examples with
both common and systematic names given. There are some important common
names that are used very often. For example, haloforms are CHX
3
, vinyl halides are
, and allyl halides are .H
2
C
P
CH
O
CH
2
XH
2
C
P
CHX
TABLE 6.1 Some Alkyl Halides, Names, and Known Properties
Compound Common Name Systematic Name mp (°C)
Methyl fluoride Fluoromethane
Bromoethane
–141.8
–118.6Ethyl bromide
Isopropyl iodide 2-Iodopropane –90.1
tert-Butyl chloride 2-Chloro-2-methylpropane –25.4
CH
2
Br
2
Methylene bromide Dibromomethane –52.7
CHCl
3
Chloroform Trichloromethane –63.5
Vinyl bromide Bromoethene –139.5
Allyl chloride 3-Chloropropene –134.5
2-Bromo-1-chlorobutane
3-Chloro-1-pentene
Br Bromocyclopentane
bp (°C)
–78.4
38.4
89.4
51.0
97
61.5
15.8
45
147
94
138
CH
3
F
CH
3
CH
2
Br
(CH
3
)
2
CH I
H
2
C CHBr
H
2
CCH
2
ClCH
(CH
3
)
3
CCl
Cl
Cl
Br
WEB 3D
WEB 3D
WEB 3D
WEB 3D
WEB 3D
WEB 3D
WEB 3D
WEB 3D
226 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
CC
HH
H
I
I
CHH
2
C
H
3
C
CH I
H
3
C
(CH
3
)
2
CHI
CH
3
Iodomethane
methyl iodide (a methyl halide)
I
(CH
3
)
3
C I
CH
3
CH
2
Iodoethane
ethyl iodide (a primary halide)
2-Iodopropane
isopropyl iodide (a secondary halide)
Iodoethene
vinyl iodide
2-Iodo-2-methylpropane
tert-butyl iodide (a tertiary halide)
I
H
3
C
CH
3
CH
3
I
CI
I
I
FIGURE 6.2 Some alkyl and vinyl
halides at various levels of
abstraction.
means
δδ
C
+
–
X
FIGURE 6.3 A substantial dipole moment exists in the polar
carbon–halogen bond, X F, Cl, Br, or I.
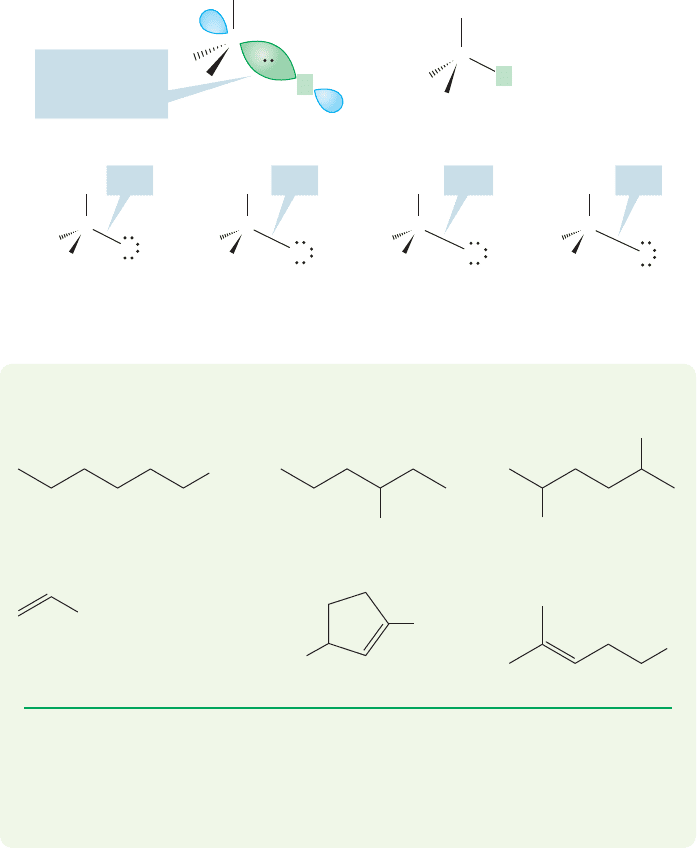
Halides are classified as methyl, primary, secondary, tertiary, and vinyl (Fig. 6.2).
Chapter 2 (p. 76) introduced this nomenclature. Remember: A primary carbon is
attached to only one other carbon, a secondary carbon to two others, and a tertiary
carbon to three others. In a primary halide, the halogen atom is attached to a pri-
mary carbon, and so on. In vinyl halides, there is always a carbon–carbon double bond
to which the halide is directly attached.
TABLE 6.2
Dipole Moments of Some
Simple Alkyl Halides
Alkyl Dipole
Halide Moment (D)
Methyl fluoride 1.85
Ethyl fluoride 1.94
Methyl chloride 1.87
Ethyl chloride 2.05
Methyl bromide 1.81
Ethyl bromide 2.03
Methyl iodide 1.62
Ethyl iodide 1.91
Substituted alkanes are polar molecules.The halogens are relatively electroneg-
ative atoms and strongly attract the electrons in the carbon–halogen bond. Alkyl
halides have dipole moments of approximately 2 debye, abbreviated D (Fig. 6.3;
Table 6.2).
Simple alkyl halides are nearly tetrahedral, and therefore the carbon to which
the halogen is attached is approximately sp
3
hybridized. The bond to halogen
involves an approximately sp
3
carbon orbital overlapping with an orbital on the
halogen for which the principal quantum number varies from n 2 for fluorine
to n 5 for iodine. The bond lengths increase and the bond strengths
decrease as we read down the periodic table. Some typical values for methyl
halides are shown in Figure 6.4, which gives both bond lengths (in
angstrom units) and bond strengths (in kilocalories per mole). Remember:
These values refer to homolytic bond breaking (formation of two neutral radi-
cals, Fig. 1.44).
C
O
X
C
O
X
C
O
X
6.3 Alkyl Halides as Sources of Organometallic Reagents: A Synthesis of Hydrocarbons 227
Bond lengths (A
⬚
)
C
C
=
Overlap of two
singly occupied
orbitals
1.78 1.93 2.14
H
C
H
C
H
C
1.39
H
H
H
C
H
H
H
H
H
H
115 84 72 58
Bond strengths (kcal/mol)
Cl
F
Br
X
X
I
FIGURE 6.4 The bond of an
alkyl halide is formed by the overlap
of a singly occupied carbon sp
3
orbital
with a singly occupied halogen
orbital.
C
O
X
F
Br
(a) (b)
(d)
I
(c)
Cl
Br
Cl
Cl
(f)
(g)(e)
CHI
3
I
PROBLEM 6.1 Name the following compounds:
PROBLEM 6.2 Draw structures for the following compounds:
(a) 3,3-dichlorohexane (d) meso-3,4-dichlorohexane (three dimensions!)
(b) 1-bromocyclohexene (e) (1R,2R)-dichlorocyclopropane
(c) 2-bromopropene
6.3 Alkyl Halides as Sources of Organometallic
Reagents: A Synthesis of Hydrocarbons
Halides have all sorts of uses in the “real” practical world. They appear as anes-
thetics, insecticides, herbicides, the building blocks of polymers such as polyvinyl
chloride (PVC), and myriad other products. As you read this paragraph you are
probably thinking, “Sure, but haven’t there been serious problems with insecticides
such as DDT and herbicides like Agent Orange, and aren’t chlorofluorocarbons
eating up the ozone layer?”The answer is a resounding, Yes! There seems to be no
“free lunch” as unintended consequences often appear when we try to solve prob-
lems with technology. The issues are complex, but it is clear that the chemists of
today and the future need to supply the technical expertise to avoid these problems
while retaining the benefits.
Halides are important in synthetic chemistry because they allow for the forma-
tion of carbon–hydrogen and carbon–carbon bonds. Central to these uses of alkyl
and other halides is their easy conversion into organometallic reagents, molecules
228 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
O
O
O
RX R X
Mg
Diethyl ether
Mg
MgX
2
R
2
Mg
+
FIGURE 6.6 The Grignard reagent
has a complex structure.
An alkyl radical
X = Cl, Br, or I
RRX X
Mg Mg
R
X
Mg
FIGURE 6.7 Formation of a Grignard
reagent.
containing carbon–metal bonds. We look at only one limited use of these synthet-
ically important compounds in this chapter, but many other uses will appear later.
When an alkyl halide (fluorides are generally exceptions) is added to a cold
mixture of magnesium or lithium metal and an ether solvent, the metal begins to
disappear in an exothermic reaction. In general, the solvent used for this reaction,
an ether, has the structure . Diethyl ether or tetrahydrofuran (THF) is
most often used. The end result of the reaction is either a Grignard reagent or an
organolithium reagent (Fig. 6.5).
R
O
O
O
R
R = alkyl or alkenyl
X = Cl, Br, or I
Grignard reagent synthesis
O
O
RX
Mg
RMgX
R MgX
+
–
Organolithium reagent synthesis
RX
RLi
Li
R Li
+
–
FIGURE 6.5 Formation of
organometallic reagents.
Grignard reagents were discovered by P. A. Barbier (1848–1922), and their
chemistry was worked out extensively by his student Victor Grignard (1871–1935),
who received the 1912 Nobel Prize in Chemistry for his work in this area. Neither
the formation of the Grignard reagent nor its structure is an easy subject.The sim-
plest formulation is “RMgX” and should be amplified by the addition of the ionic
resonance form shown in Figure 6.5. The Grignard reagent is also in equilibrium
with a mixture of the magnesium halide and dialkylmagnesium compound.
Moreover, the ether solvent is essential for its formation. Grignard reagents incor-
porate two molecules of solvent, conveniently left out in the traditional formulation
as RMgX (Fig. 6.6). Whatever the detailed structure, the result is a highly polar
reagent that is a very strong Lewis base.
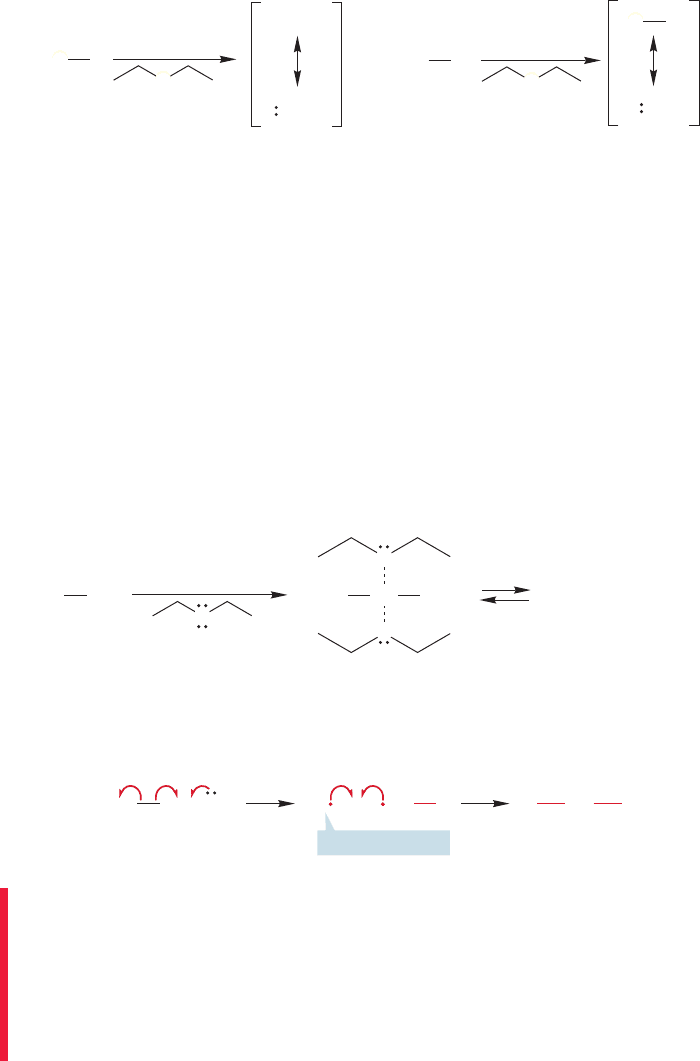
The mechanism of formation of the Grignard reagent involves radical-transfer
reactions in which a transient alkyl radical is formed in the presence of a magnesium-
centered radical (Fig. 6.7).
We met the methyl radical in Chapter 2 (p. 62). Radicals, sometimes called free
radicals, are neutral species that have a single nonbonding electron and can under-
go many reactions, as we will see in Chapter 11. Typical and important reactions
of radicals include combining with other radicals to form bonds.The combination
of two carbon-based radicals is especially important because it results in the for-
mation of a carbon–carbon bond. The arrow formalism for radical reactions uses
single-barbed arrows.
CONVENTION ALERT
