Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

5.6 Disubstituted Ring Compounds 209
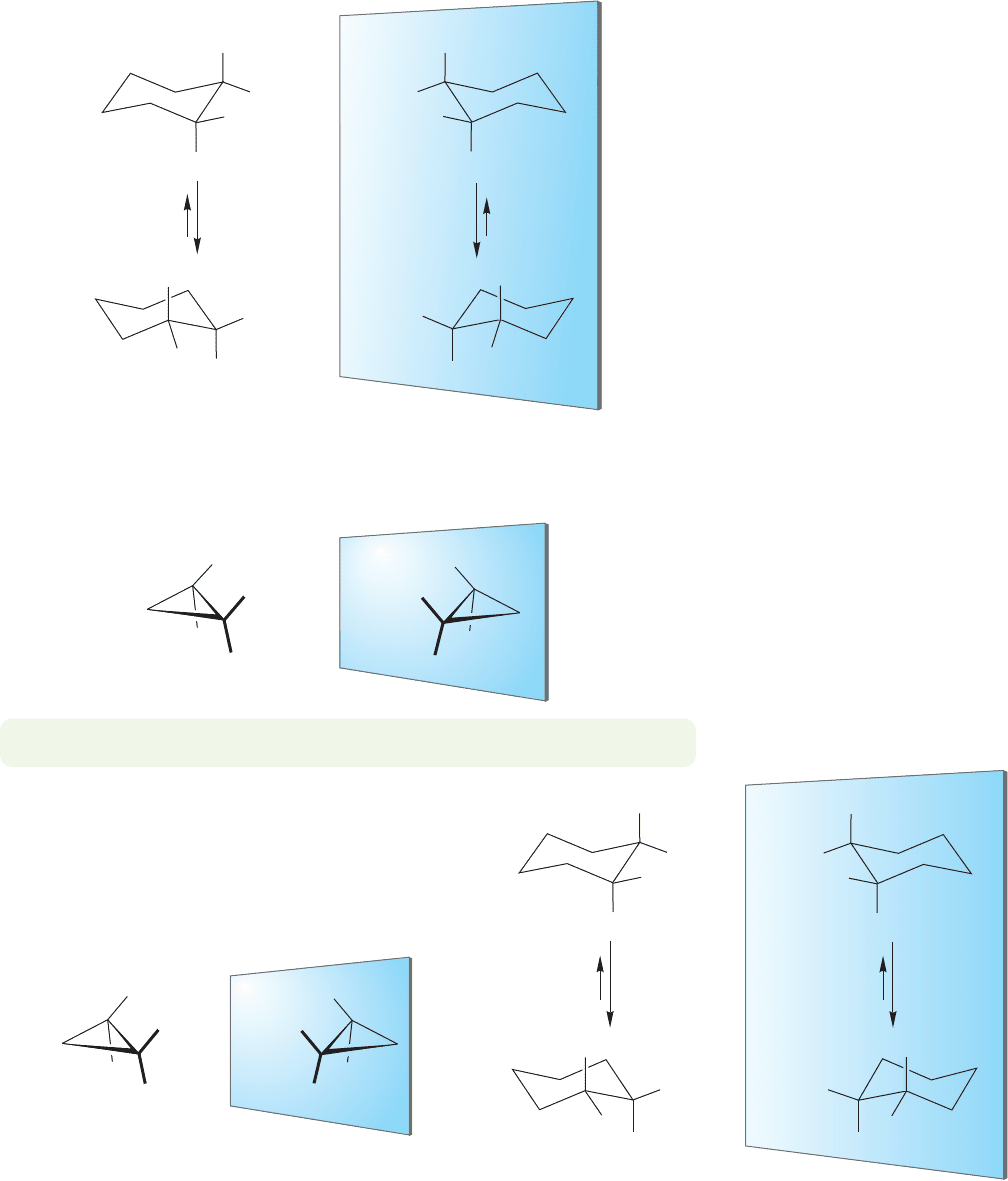
and chemical properties. In principle, each could be isolated and resolved. So there
is a total of four possible cis isomers, the two shown in Figure 5.42 and their mir-
ror images (Fig. 5.43).
Mirror
CH
3
CH
3
CH(CH
3
)
2
CH(CH
3
)
2
(CH
3
)
2
CH
H
H
H
H
H
flip
flip
H
H
H
H
3
C
CH(CH
3
)
2
CH
3
FIGURE 5.43 The four stereoisomers
of cis-1-isopropyl-2-methyl-
cyclohexane. Two pairs of
enantiomers exist.
The rigid molecule cis-1-isopropyl-2-methylcyclopropane cannot undergo any
ring flip, and there is only a single pair of enantiomers. The lack of a possible ring
flip reduces the total number of possible stereoisomers (Fig. 5.44).
Mirror
H
H
CH
3
H
CH(CH
3
)
2
H
H
3
C
(CH
3
)
2
CH
FIGURE 5.44 The two enantiomers of
cis-1-isopropyl-2-methylcyclopropane.
The rigid ring prevents any ring flip.
Mirror
H
H
CH
3
H
3
C
H
CH(CH
3
)
2
(CH
3
)
2
CH
H
FIGURE 5.45 The single pair of enantiomers
of trans-1-isopropyl-2-methylcyclopropane.
Again, the rigid cyclopropane ring prevents
any ring flip.
PROBLEM 5.18 Label the stereogenic carbons of Figures 5.43 and 5.44 as (R) or (S).
The situation is similar in the case of the trans form.
Although the inflexible cyclopropane ring has but a sin-
gle pair of enantiomers (Fig. 5.45), there are two pairs of
enantiomers in the flexible cyclohexane (Fig. 5.46).
Mirror
CH
3
H
3
C
CH(CH
3
)
2
CH(CH
3
)
2
(CH
3
)
2
CH
H
H
H
H
H
flip
flip
H
H
H
H
3
C
CH(CH
3
)
2
CH
3
FIGURE 5.46 The four stereoisomers of trans-1-isopropyl-2-
methylcyclohexane. Two pairs of enantiomers exist.
210 CHAPTER 5 Rings
PROBLEM 5.19 Point out the pairs of enantiomers and diastereomers in Figure
5.46. Assign all the stereogenic carbons as (R) or (S).
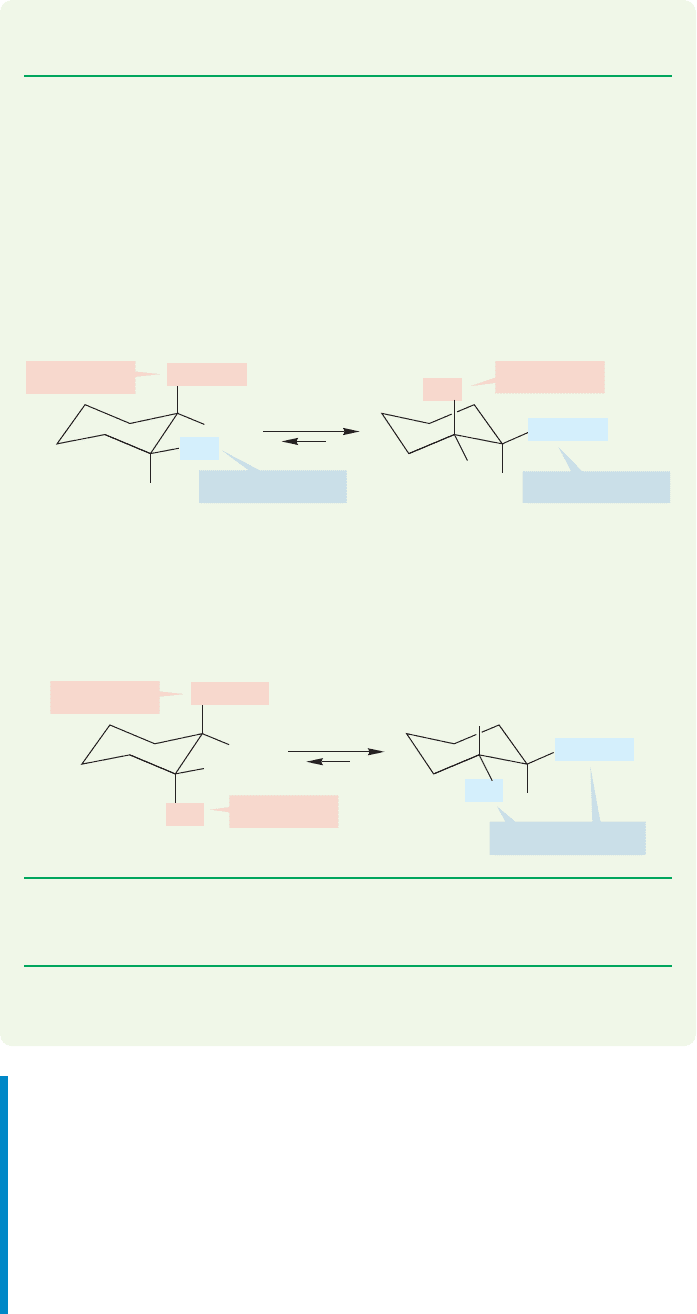
WORKED PROBLEM 5.20 Use the data of Table 5.3 (p. 202) to estimate the energy
difference between (a) the two chair forms of cis-1-isopropyl-2-methylcyclohexane
and (b) the two chair forms of trans-1-isopropyl-2-methylcyclopropane.
ANSWER (a) For the cis compound, ring flipping converts A with an axial iso-
propyl group and an equatorial methyl group into B in which the isopropyl group
is equatorial and the methyl is axial. Table 5.3 tells you that an isopropyl group is
more stable in the equatorial position by 2.61 kcal/mol. A methyl group is more
stable in the equatorial position by only 1.74 kcal/mol. The more stable confor-
mation will be B by (2.61 1.74) kcal/mol 0.87 kcal/mol.
(b) The trans compound has both groups axial (C) or both groups equatorial (D).
Conformation D will be preferred by (2.61 1.74) kcal/mol 4.35 kcal/mol.
However, D suffers a methyl–isopropyl gauche interaction that will be destabiliz-
ing by somewhat more than 0.6 kcal/mol. So, our final answer would be approx-
imately (4.35 0.6) kcal/mol 3.75 kcal/mol.
Equatorial group Equatorial group
H
H
H
H
cis
cis
CH
3
H
3
C
ring flip
CH(CH
3
)
2
Axial groupAxial group
CH(CH
3
)
2
AB
Axial group
Axial group
Equatorial groups
H
H
H
H
trans
trans
CH(CH
3
)
2
CH(CH
3
)
2
CH
3
H
3
C
ring flip
CD
PROBLEM 5.21 Estimate the energy difference between the two conformational
isomers of cis-1,3-dimethylcyclohexane.
PROBLEM 5.22 Does ring flip racemize trans-1,3-dimethylcyclohexane, as it does
cis-1,2-dimethylcyclohexane (Fig. 5.39, p. 207)?
Summary
The “take-home lesson” here is that it takes a careful analysis of the stereoiso-
mers formed by ring flipping of substituted cyclohexanes to see all the possibil-
ities.First,be sure you have made a good drawing (follow the procedure on p. 190).
Next, determine whether or not the isomer is chiral by drawing the mirror image
and seeing if it is superimposable on the original. Now do a ring flip of the orig-
inal isomer. Is the ring-flipped isomer the same as the original? Is it the mirror
image? Is it completely different? Differently substituted cyclohexanes give all
three possibilities.
5.7 Bicyclic Compounds 211
5.7 Bicyclic Compounds
Now that we have dealt with both simple and complicated cycloalkanes (molecules con-
taining a single ring), it is time to look briefly at molecules containing more than one
ring. We have met such structures before, in both Chapter 2 (p. 85) and Chapter 3
(p. 121).
Of course,one can imagine all sorts of molecules containing two separated rings.
There is nothing special about such molecules. However, there are other molecules
in which two rings share a carbon or carbons, and these compounds are more inter-
esting. There are three general ways in which two rings can be connected by shar-
ing carbons.They can share a single carbon,two carbons, or more than two carbons.
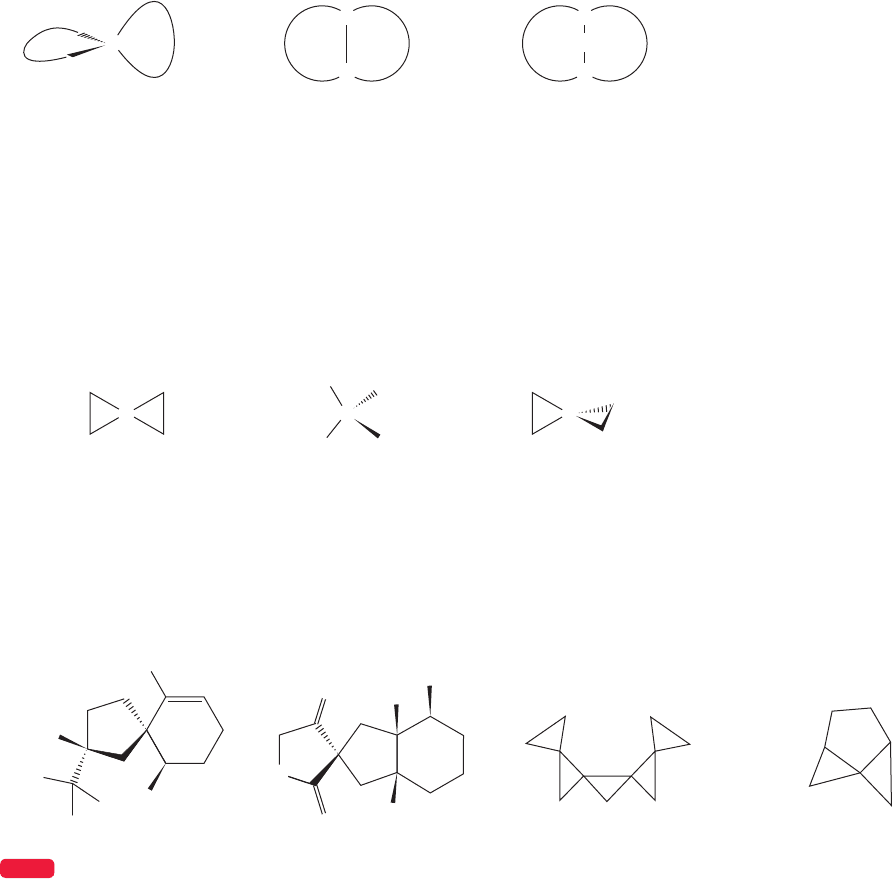
These modes of attachment are called spiro (one carbon shared), fused bicyclic
(two carbons shared), and bridged bicyclic (more than two carbons shared). Fused
is really a special case of bridged, with n 0 (Fig. 5.47).
C
C
Fused substitution
(two carbons are
shared)
Fused bicyclic
Bridged substitution
(more than two carbons
are shared; if n = 0,
the molecule is fused)
(CH
2
)
n
C
C
Bridged bicyclic
C
Spiro substitution
(one carbon is shared)
Spiro
FIGURE 5.47 Rings can share a single
carbon (spiro), two carbons (fused
bicyclic), or more than two carbons
(bridged bicyclic).
C C
The two-dimensional
picture of spiropentane
is highly misleading…
…because the central
carbon must look like
this…nearly tetrahedral
…the two rings
must lie in different,
perpendicular planes
C
FIGURE 5.48 A two-dimensional
picture of spiropentane is misleading.
The two rings lie in perpendicular
planes.
WEB 3D
A [5]triangulane Tricyclo[4.1.0.0
4,6
]heptane
CH
3
H
Bakkenolide-A
CH
3
O
CH
2
O
CH
3
Agarospirol
HO
H
H
3
C
CH
3
CH
3
FIGURE 5.49 Some spiro compounds. Don’t worry about the exotic names.
Spiro substitution is common in both natural products and in molecules so
far encountered only in the laboratory. Note in Figure 5.48 how poorly a two-
dimensional picture represents the real shape of such molecules. Even the sim-
plest spirocyclic compound, spiropentane, is badly served by the flat page. The
central carbon is approximately tetrahedral,and we really must use wedges to give
a three-dimensional feel to this kind of molecule.
Figure 5.49 shows some “natural” (the first two compounds) and “unnatural”
(the second two compounds) spiro compounds. Of course, the distinction is purely
formal—the chemical laboratory is part of Nature.
212 CHAPTER 5 Rings
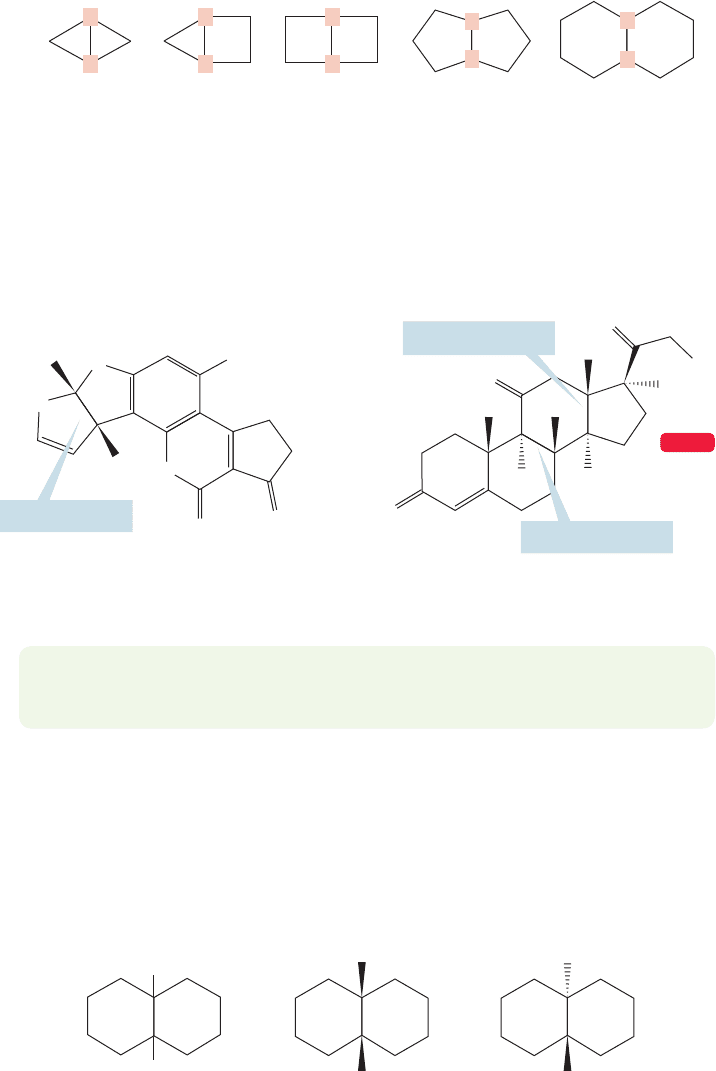
Bicyclic molecules in which two rings share two or more carbons are even
more important than the spirocyclic compounds. The simplest way in which two
rings can share more than one carbon is for two adjacent carbons to be shared
in a fused structure. The “fusion” positions, or “bridgeheads” (p. 121), are shown
in red (Fig. 5.50).
C
C
C
C
C
C
C
C
C
C
FIGURE 5.50 Some schematic, two-
dimensional structures for fused
bicyclic compounds in which two
rings share a pair of adjacent carbon
atoms.
WEB 3D
H
H
O
O
O
Aflatoxin B
1
(very toxic)
HH
OH
H
trans Ring fusion
Cortisone (anti-inflammatory agent)
H
3
C
H
3
C
trans Ring fusion
cis Ring fusion
OCH
3
O
O
O
OH
O
O
FIGURE 5.51 Two fused polycyclic
molecules. Note the cis and trans ring
fusions.
The hydrogens attached at the ring junction,or fusion positions, can be either
on the same side (cis) or on opposite sides (trans). In practice, both stereo-
chemistries are possible for larger rings, but for the small rings only the cis form
is stable (see Problem 5.23). Figure 5.51 shows two compounds that contain
fused rings.
PROBLEM 5.23 Use your models to convince yourself that trans stereochemistry
is not possible for the three molecules on the left of Figure 5.50.
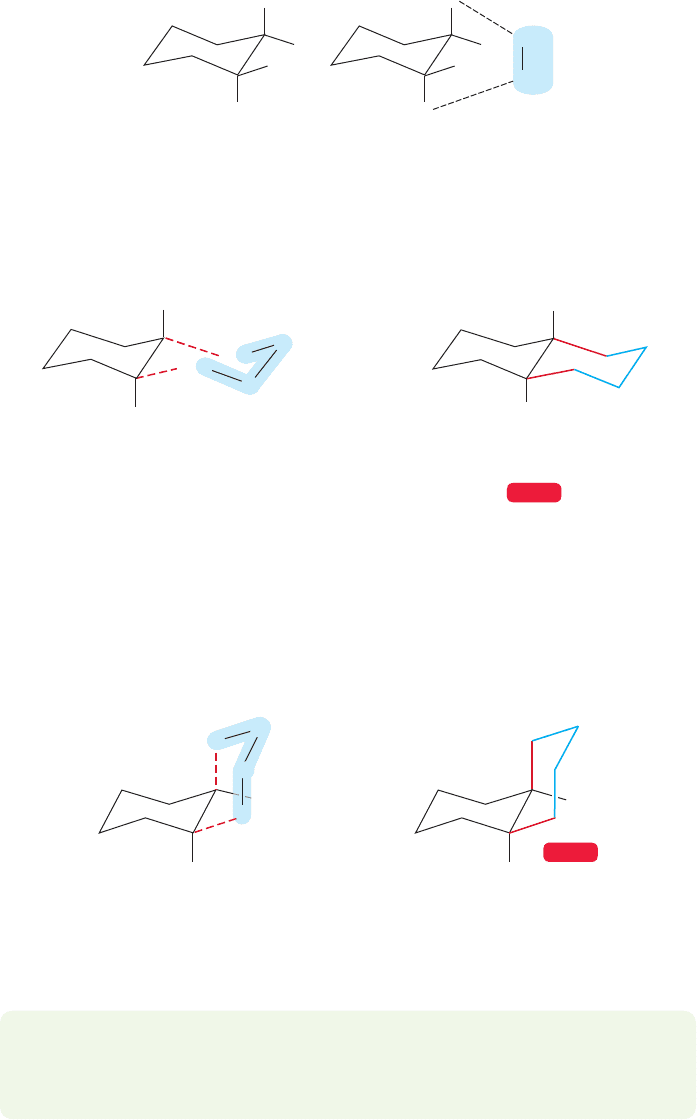
Particularly important are cis- and trans-decalin, the compounds formed by
the fusion of two cyclohexanes. Although Figure 5.52 shows these compounds
in schematic form, by now we can certainly guess that the real shape will be
much more intricate. It’s easy enough to draw them though, if one goes about
it carefully.
Decalin
Schematic picture of
cis-decalin
Schematic picture of
trans-decalin
H
H
H
H
H
H
FIGURE 5.52 In decalin, the two
hydrogens at the bridgeheads can be
either on the same side of the rings
(cis), or on opposite sides (trans).
5.7 Bicyclic Compounds 213
For trans-decalin first draw a single, perfect chair cyclohexane, and put in the
axial and equatorial bonds at two adjacent carbons (Fig 5.53a). As for any trans di-
substituted cyclohexane (p.207), the two rings of trans-decalin will be attached either
through two equatorial bonds or through two axial bonds.As shown in Figure 5.53b,
attachment through two axial bonds is impossible—the distance to be bridged is too
great (try it with models).So we are left with only the two equatorial bonds for trans
(b)
CH
2
CH
2
H
2
C
H
2
C
H
H
(a)
FIGURE 5.53 A futile attempt to construct
trans-decalin by connecting two axial carbons
with a pair of methylene groups. It is not
possible to span the two axial positions with
only four methylene groups; two methylene
groups occupy axial positions leaving only two
others (blue) to complete the ring.
attachment of a second ring, and this time the new chair fits in easily. trans-Decalin
can be made by connecting two adjacent equatorial positions through a chain of four
methylene groups (Fig. 5.54).
It is ea
sy to
conne
ct tw
o
equa
toria
l
positions w
ith a ch
ain of four
m
ethy
lenes;
the hydrog
ens a
t the
fusion
positi
ons (b
ridgeh
eads)
must be axial
trans-D
eca
lin:
tw
o fu
sed c
hair
cyclo
hexan
es
H
H
H
2
C
H
2
C
H
H
CH
2
CH
2
=
WEB 3D
FIGURE 5.54 trans-Decalin.
To m
ake a
cis junc
tion w
e
m
ust connect one
axial
and
one equato
rial po
sitio
n w
ith a
four-c
arbon
chain
H
H
=
H
H
cis-Decalin
The two bridgehead
hydrogens occupy
one axial and one
equatorial position
CH
2
CH
2
CH
2
H
2
C
WEB 3D
FIGURE 5.55 cis-Decalin.
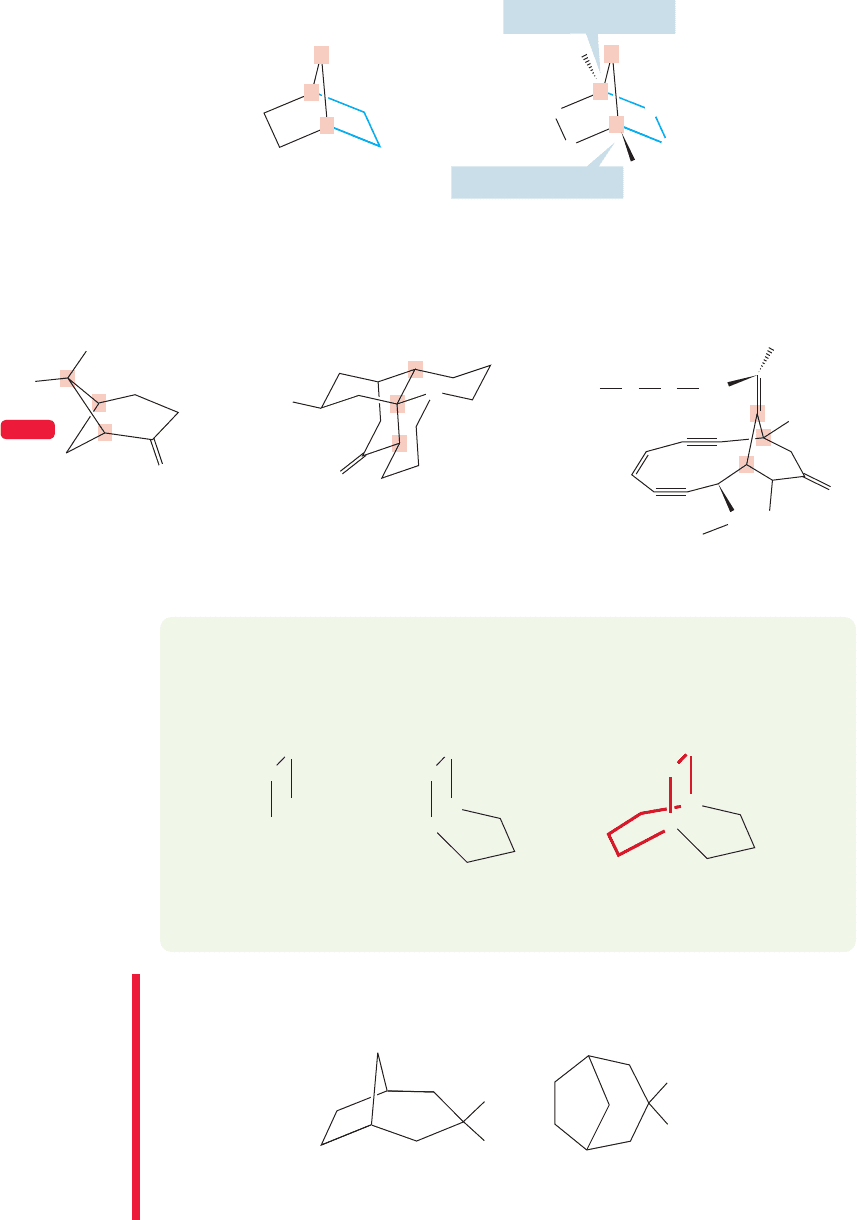
cis-Decalin is quite different because cis substitution must involve one axial and
one equatorial bond in the attachments to the second ring (Fig.5.55). If we pay atten-
tion to the rules for drawing perfect chairs, it’s easy to produce a good drawing for
this molecule. Remember that every ring bond in a cyclohexane is parallel to the
bond directly across the ring (p. 191).
PROBLEM 5.24 Use models to convince yourself that cis-decalin is a mobile mol-
ecule, undergoing easy double chair–double chair interconversions, whereas trans-
decalin is rigidly locked.
214 CHAPTER 5 Rings
Now imagine two rings sharing three carbons in a bridged bicyclic molecule.
Two cyclopentanes, for example, can share two or three carbons. We have seen
the first case before (Fig. 5.50) and the second is drawn in Figure 5.56. In each
case, the carbons at the fusion points are called the bridgehead positions (p. 121).
H
Bridgehead position
Bridgehead position
H
C
C
C
C
C
CH
2
CH
2
H
2
C
H
2
C
CH
2
FIGURE 5.56 Two five-membered
rings sharing three carbons.The
shared carbons are shown in red.
WEB 3D
Lycopodine
H
3
C
N
O
Calicheamicin
O
Several
sugars
O
CH
3
S
CH
2
S
H
S
OH
NHCOOCH
3
β-Pinene
H
3
C
CH
3
CH
2
FIGURE 5.57 Three naturally occurring bicyclic molecules.The
shared carbons are highlighted in red.
The shared carbons are shown in red in Figure 5.56; the carbon–carbon bonds in
blue complete one cyclopentane. A more complete structure for this bicyclic hydro-
carbon is also shown. Such bridged molecules are extremely common. Three com-
pounds found in Nature are shown in Figure 5.57.
WORKED PROBLEM 5.25 Design a molecule in which two rings share four carbons.
ANSWER One sure-fire way to do a problem such as this is to start by drawing the car-
bons to be shared (four, in this example).Then add the remainders of the other rings.
C
C
C
C
Here they are incorporated
into another ring
Here are those four
carbons incorporated
into one ring
The four
carbons to
be shared
C
C
C
C
C
C
C
C
8
5
4
3
2
1
7
6
1
2
3
4
5
6
7
8
CH
3
CH
3
CH
3
CH
3
FIGURE 5.58 Part of the naming protocol for a
typical bicyclic compound.
CONVENTION ALERT
Bicyclic compounds are named in the following way: One first counts the number
of carbons in the ring system. The molecule in Figure 5.58 has eight ring carbons.
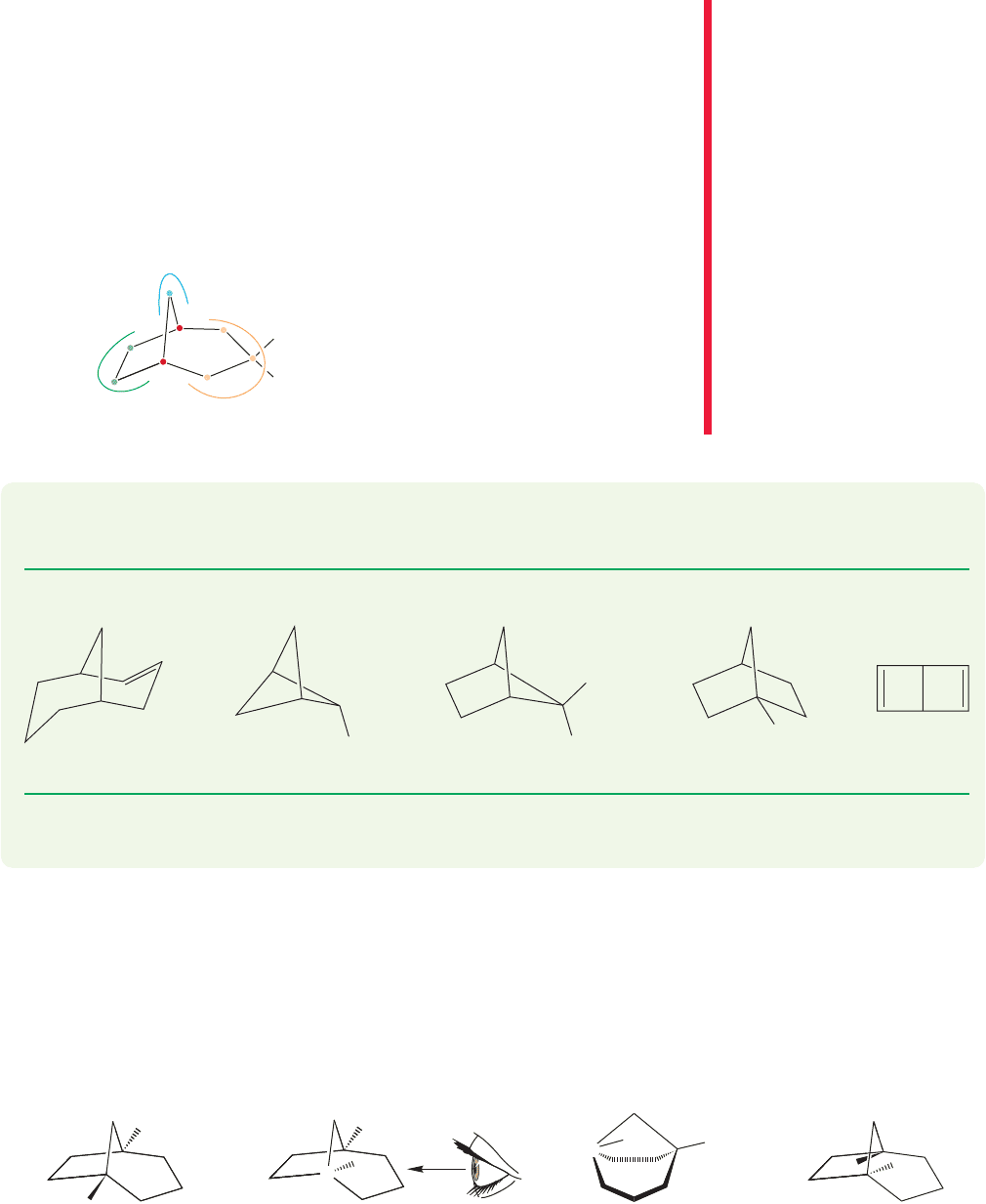
5.7 Bicyclic Compounds 215
Thus the base name is “octane.” The molecule is numbered by counting from the
bridgehead carbon [carbon number 1, C(1)] around the longest bridge first,proceed-
ing to the other bridgehead. One then continues counting around the second-longest
bridge,and finally numbers the shortest bridge. Any substituents can now be assigned
a number. So the compound in Figure 5.58 is a 3,3-dimethylbicyclooctane.
The bridges are counted from the bridgeheads and assigned numbers equal to the
number of atoms in the bridges, not counting the bridgehead atoms. These numbers are
enclosed in brackets,largest number first,between the designation “bicyclo”and the rest
of the name (Fig. 5.59). So this compound is called 3,3-dimethylbicyclo[3.2.1]octane.
(a) (b) (c) (d) (e)
CH
3
CH
3
CH
3
Cl
1-Atom
bridge
3-Atom
bridge
3,3-Dimethylbicyclo[3.2.1]octane
2-Atom
bridge
CH
3
CH
3
FIGURE 5.59 More of the naming
protocol for bridged bicyclic
compounds.This molecule is 3,3-
dimethylbicyclo[3.2.1]octane. The
red dots show the bridgehead atoms.
These are not counted in sizing the
bridges, but are counted in
numbering the compound.
PROBLEM 5.26 Make drawings of (a) cis-bicyclo[3.3.0]octane, (b) 1-fluorobicy-
clo[2.2.2]octane, (c) trans-9,9-dimethylbicyclo[6.1.0]nonane.
PROBLEM 5.27 Name the following compounds:
PROBLEM 5.28 How many signals would appear in the
13
C NMR spectrum for
each of the molecules in Problem 5.27?
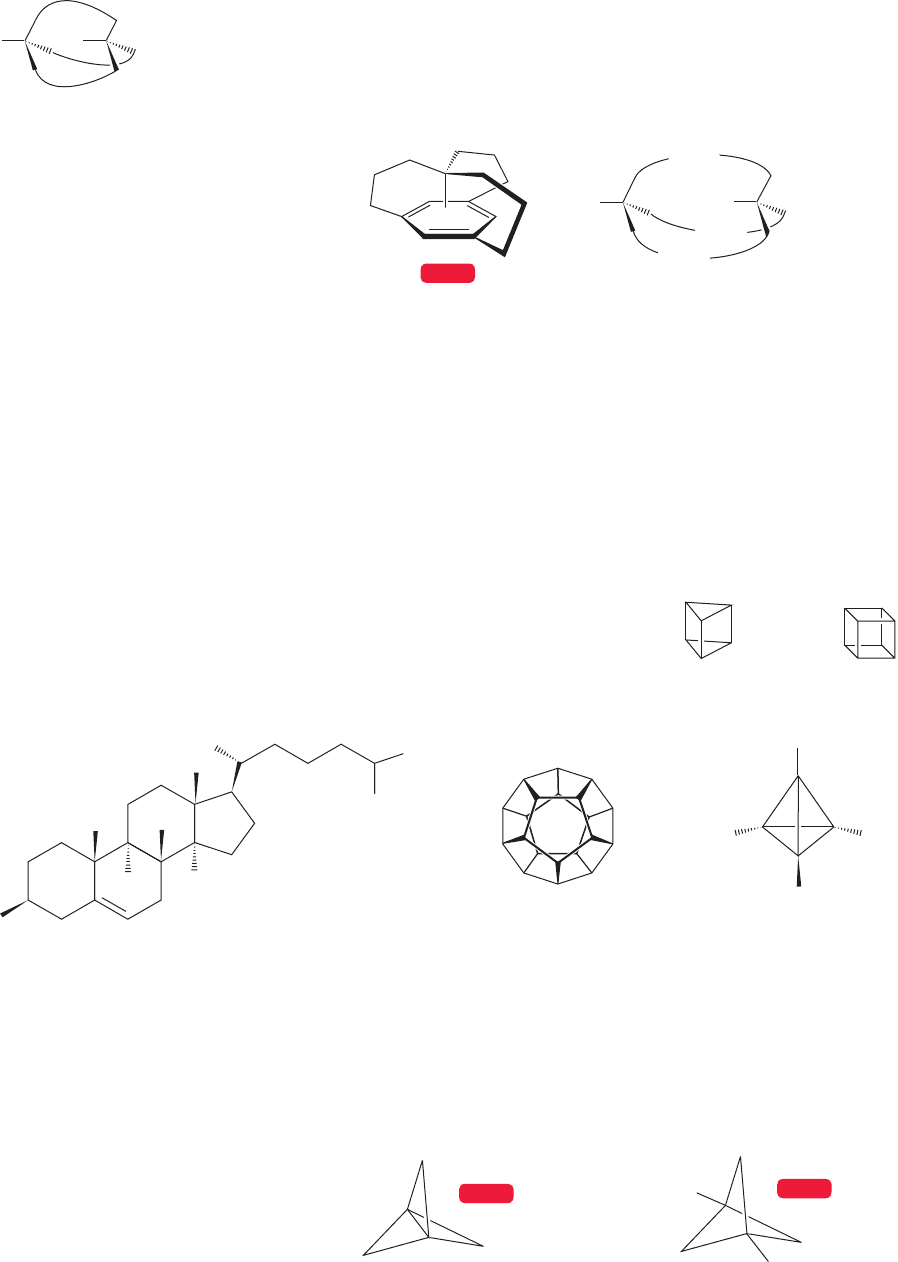
As with 1,2-fused systems, we must worry about the stereochemistry at the
bridgehead positions in bridged molecules. At first,this task may seem trivial—where
could these hydrogens be but where they are shown in the figures? The answer is,
“inside the cage!” In the compounds we have already examined, it is not easy for
hydrogens to occupy the inside position. As shown in Figure 5.60, all four bonds of
one or both of the bridgehead carbons of bicyclo[3.2.1]octane would be pointing
in the same direction if one or both bridgehead hydrogens were inside the cage.
“Out, out” bicyclo[3.2.1]octane
H
H
“In, out” bicyclo[3.2.1]octane
HH
H
C
Side view of “in, out”
bicyclo[3.2.1]octane
as seen by the eye
H
H
C
“In, in” bicyclo[3.2.1]octane
H
H
FIGURE 5.60 Three stereoisomers of bicyclo[3.2.1]octane.
Only the “out, out” isomer is known.
216 CHAPTER 5 Rings
But now imagine increasing the size of the bridges. In principle, it should be pos-
sible to make the bridge chains long enough so that a normal, nearly tetrahedral,
arrangement can be achieved with one or both bridgehead hydrogens inside the cage
(Fig. 5.61). And so it is.The molecules shown in Figure 5.62 are both known, as are
several other examples.
H
H
FIGURE 5.61 If the bridges are long
enough, there is no angle strain in an
“in, out” isomer.
WEB 3D
H
(CH
2
)
4
H
(CH
2
)
4
(CH
2
)
4
H
FIGURE 5.62 Two known “in” or “in, out” compounds.
5.8 Special Topic: Polycyclic Systems
We needn’t stop here. More rings can be attached in fused or bridged fashion to pro-
duce wondrously complex structures.The naming protocols are complicated,if ultimate-
ly logical, and won’t be covered here.Many of the compounds have common names that
are meant to be evocative of their shapes.Prismane and cubane are examples.The molec-
ular versions of the Platonic solids, tetrahedrane, cubane, and dodecahedrane are all
known,although the parent,unsubstituted tetrahedrane still evades synthesis (Fig.5.63).
Tetra-tert-butyltetrahedrane
Cholesterol
a polycyclic compound
a steroid
Cubane
Dodecahedrane
Prismane
C(CH
3
)
3
C(CH
3
)
3
C(CH
3
)
3
(CH
3
)
3
C
H
3
C
CH
3
CH
3
H
H
HO
H
CH
3
CH
3
FIGURE 5.63 Polycyclic natural
(cholesterol) and “unnatural” (the
rest) products.
WEB 3D
Tricyclo[1.1.1.0
1,3
]pentane
([1.1.1]propellane)
H
H
WEB 3D
Bicyclo[1.1.1]pentane
FIGURE 5.64 Two polycyclic small
ring compounds.
Spectacular new molecules containing rings are always appearing. In 1984,
Professor Kenneth B.Wiberg (b. 1927) of Yale University reported the construction
of the polycyclic molecule, tricyclo[1.1.1.0
1,3
]pentane (also known as [1.1.1]propel-
lane, Fig. 5.64), a marvelously exciting molecule.You are now only five chapters into
your study of organic chemistry, yet it is possible for you to appreciate why the chem-
ical world was knocked out by this compound.
5.9 Special Topic: Adamantanes in Materials and Biology 217
PROBLEM 5.29 Why is tricyclo[1.1.1.0
1,3
]pentane unusual (Fig. 5.64)? Would
you expect it to be especially stable or unstable with respect to its cousin bicy-
clo[1.1.1]pentane? Why?
Wiberg’s molecule was synthesized in a chemistry laboratory, and probably (?)
does not occur in Nature. Nature is by no means played out as a source of fasci-
nating polycyclic molecules, however. For example, in 2003 Sanae Furuya and
Shiro Terashima reported the synthesis of optically active tricycloillinone, a mol-
ecule isolated from the wood of Illicium tashiori (Fig. 5.65). This molecule
enhances the activity of choline acetyltransferase, an agent that catalyzes the syn-
thesis of acetylcholine. Why should we care about tricycloillinone? A form of
senile dementia (Alzheimer’s disease) is associated with reduced levels of acetyl-
choline, and a molecule that might be useful in increasing levels of acetylcholine
is of obvious importance to all of us.
H
2
C
O
O
O
CH
3
H
3
C
FIGURE 5.65 Tricycloillinone.
o-Carborane
The dots are carbons; every
other vertex is a boron. There is a
hydrogen atom at every vertex
CARBORANES: WEIRD BONDING
or six bonds, the dreaded red “X” is sure to follow. How does
Nature get away with it? If you did Problems 1.62 and 1.63,
you encountered triangular H
3
, and H
3
, molecules related
to the carboranes in that they, too, contain “too many”
bonds, in this case, two bonds to hydrogen. The answer to
this seeming impossibility is that those bonds are not the
simple two-electron bonds we are becoming used to, but
partial bonds containing fewer than two electrons.
Far from being “weird” and thus presumably exotic in
properties, the carboranes are almost unbelievably stable
compounds, sitting in bottles seemingly forever, and show-
ing a rich history and chemistry. Professor William
Lipscomb (b. 1919) won the Nobel Prize for chemistry in
1976 for explaining the bonding in carboranes. They are
now being used in both medicinal chemistry and materials
science. In Japan, for example, carboranes are used in
treating certain brain tumors in “Boron-neutron capture
therapy.” However, it must be admitted that the practical
development of these compounds was slow to happen.
Why? Perhaps we chemists were wary of all those
potential red X’s, sure to arrive if we drew too many bonds
to carbon!
As you’ve seen in this chapter, ring compounds can be
straightforward (cyclopentane, p. 190, is a nice example),
moderately complex (the mobile cyclohexanes, p. 197), or
exotic ([1.1.1]propellane, p. 216). Here is a compound that
surely qualifies as exotic, if not downright weird. It is com-
posed of two carbons (the dots) and ten borons (the other
10 vertices), and contains no fewer than 20 three-membered
rings of carbons and borons. Why “weird?” Count the bonds
to carbon. There are six bonds emanating from the carbon!
Six bonds? How can that be? If you draw a carbon with five
5.9 Special Topic: Adamantanes in
Materials and Biology
Consider constructing a polycyclic molecule by expanding a chair cyclohexane.
First, connect three of the axial bonds to a cap consisting of three methylene
(CH
2
) groups all connected to a single methine (CH) group. This process
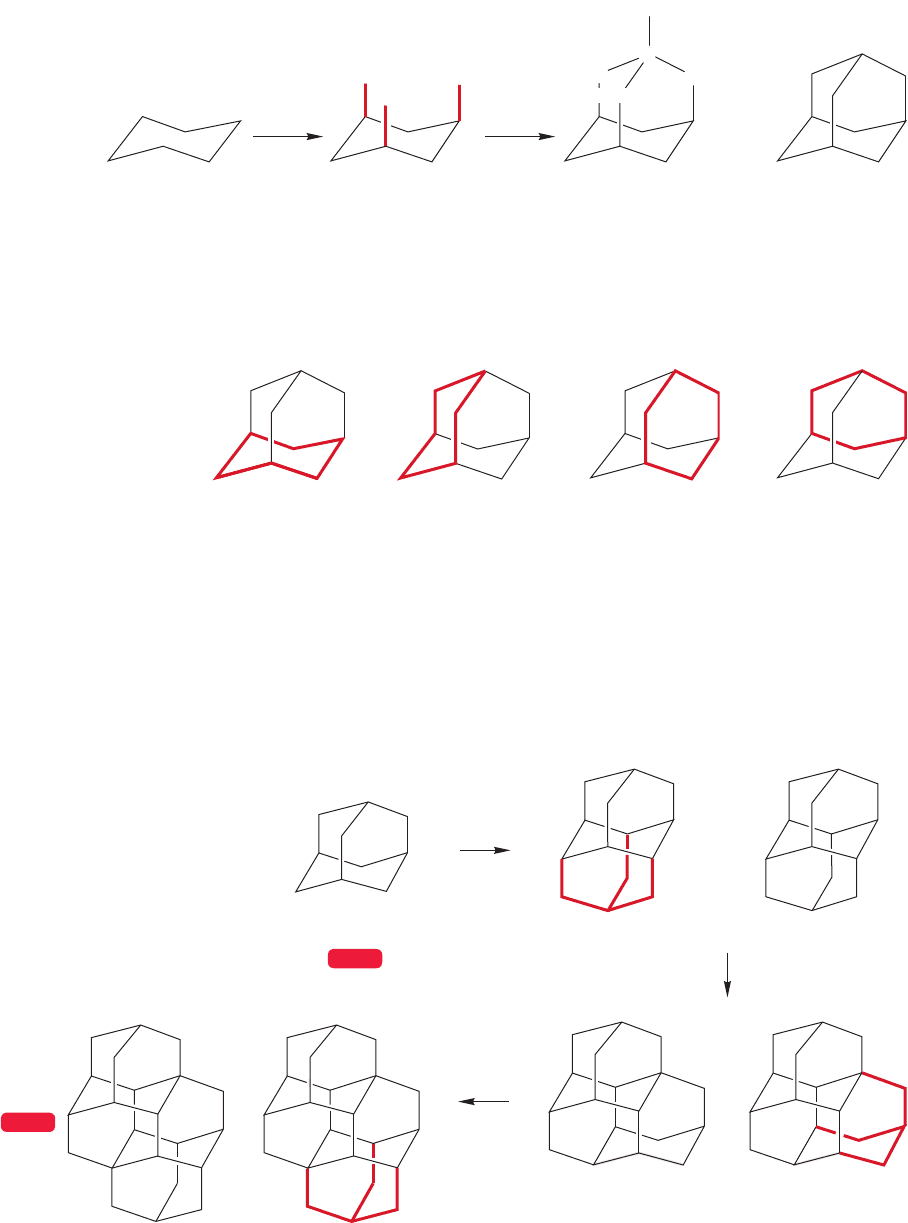
218 CHAPTER 5 Rings
produces the beautiful molecule tricyclo[3.3.1.1
3,7
]decane, better known as
“adamantane,” C
10
H
16
(Fig. 5.66).
=
Chair
cyclohexane
Add three methylene
groups
Cap with a CH Adamantane
(C
10
H
16
)
H
2
C
C
CH
2
H
2
C
H
CH
2
CH
2
CH
2
FIGURE 5.66 A schematic
construction of the polycyclic
molecule adamantane.
(a)
(b)
(c)
(d)
FIGURE 5.67 Adamantane is
composed entirely of chair
cyclohexanes. Those in (a) and (b) are
easy to see as chairs, but those in (c)
and (d) may require the use of
models.
As Figure 5.67 shows,adamantane is composed entirely of perfect chair six-mem-
bered rings. As you would expect, this molecule is nearly strain-free and constitutes
the thermodynamic minimum for all the C
10
H
16
isomers.
WEB 3D
WEB 3D
DiamantaneAdamantane
Triamantane
One isomer of tetramantane
cap
=
=
=
cap
cap
FIGURE 5.68 The continuation
of the capping process leads to
polyadamantanes and, ultimately,
to diamond.
Adamantane was first found in the 1930s in trace amounts in petroleum residues
by the Czech chemist Stanislav Landa (1898–1981), but it can now be made easi-
ly in quantity by a simple process discovered by the American chemist Paul von R.
Schleyer (b. 1930).
Consider what happens when we continue the capping process begun in our
transformation of chair cyclohexane into adamantane in Figure 5.66. Adamantane
itself is composed of only chair cyclohexanes, so we have a number of possible
places to start the process (Fig. 5.68). Addition of one more four-carbon cap
