Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


22.20 CHAPTER TWENTY-TWO
The charge retention of the lead-acid batteries is dependent on the design, electrolyte
concentration, and formulation of the grid alloy as well as other factors. The charge retention
of the standard automotive SLI batteries, using the standard antimonial-lead grid, is poor,
and these batteries have little capacity remaining after six-months’ storage at room temper-
ature. The low antimonial-lead designs and the maintenance-free batteries have much better
charge retention with losses on the order of 20 to 40% per year.
One of the potential advantages of the lithium metal rechargeable batteries is their good
charge retention which, in many cases, should be similar to the charge retention character-
istics of the lithium primary batteries.
22.3.6 Life
The cycle life and calendar life of the different secondary battery systems are also listed in
Table 22.3. Again, these data are approximate because specific performance is dependent on
the particular design and the conditions under which the battery is used. The depth of dis-
charge (DOD), for example, as illustrated in Fig. 22.7, and the charging regime strongly
influences the battery’s life.
4
Of the conventional secondary systems, the nickel-iron and the vented pocket-type nickel-
cadmium batteries are best with regard to cycle life and total lifetime. The nickel-hydrogen
battery developed mainly for aerospace applications, has demonstrated very long cycle life
under shallow depth of discharge. The lead-acid batteries do not match the performance of
the best alkaline batteries. The pasted cells have the shortest life of the lead-acid cells; the
best cycle life is obtained with the tubular design, and the Plante´ design has the best lifetime.
One of the disadvantages of using zinc, lithium, and other metals with high negative
standard potentials in rechargeable batteries is the difficulty of successful recharging and
obtaining good cycle and calendar lives. The nickel-zinc battery has recently been improved
to provide extended cycle life as seen in Fig. 22.7. The lithium-ion system, however, has
also been shown to have good cycle life.
Nickel
zinc
Akaline-
MnO
2
10
0
50
100
Depth of discharge, %
100 1000
Cycles to failure
10,000 100,000
Lead-acid
Nickel-cadmium
FIGURE 22.7 Effect of depth of discharge on cycle life of secondary battery systems.
SECONDARY BATTERIES—INTRODUCTION 22.21
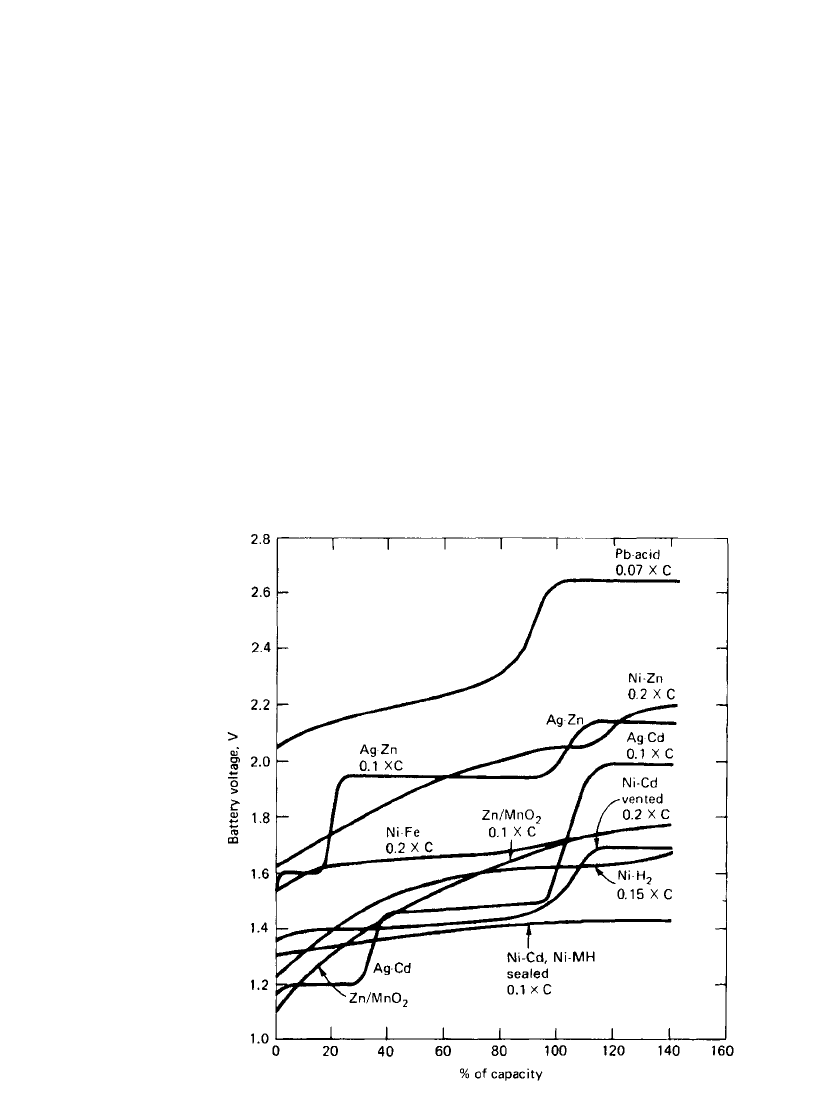
FIGURE 22.8 Typical charge characteristics of secondary battery systems,
constant-current charge at 20⬚C. (Adapted from Falk and Salkind. Ref. 5.)
22.3.7 Charge Characteristics
The typical charge curves of the various secondary aqueous-systems at normal constant-
current charge rates are shown in Fig. 22.8. Most of the batteries can be charged under
constant-current conditions, which is usually the preferred method of charging, although, in
practice, constant-voltage or modified constant-voltage methods are used. Some of the sealed
batteries, however, may not be charged by constant-voltage methods because of the possi-
bility of thermal runaway. Generally the vented nickel-cadmium battery has the most favor-
able charge properties and can be charged by a number of methods and in a short time.
These batteries can be charged over a wide temperature range and can be overcharged to
some degree without damage. Nickel-iron batteries, sealed nickel-cadmium batteries, and
sealed nickel/ metal hydride batteries have good charge characteristics, but the temperature
range is narrower for these systems. The nickel/ metal hydride battery is more sensitive to
overcharge, and charge control to prevent overheating is advisable. The lead-acid battery
also has good charge characteristics, but care must be taken to prevent excessive overcharg-
ing. The zinc/ manganese dioxide and zinc/ silver oxide batteries are most sensitive with
regard to charging; overcharging is very detrimental to battery life. Figure 22.9 shows typical
constant current–constant voltage charging characteristics of an 18650 lithium ion battery.
Table 22.5 summarizes the typical conditions for charging the different systems. However,
the chapters on individual battery systems and manufacturers’ recommendations should be
consulted because of the different procedures used.
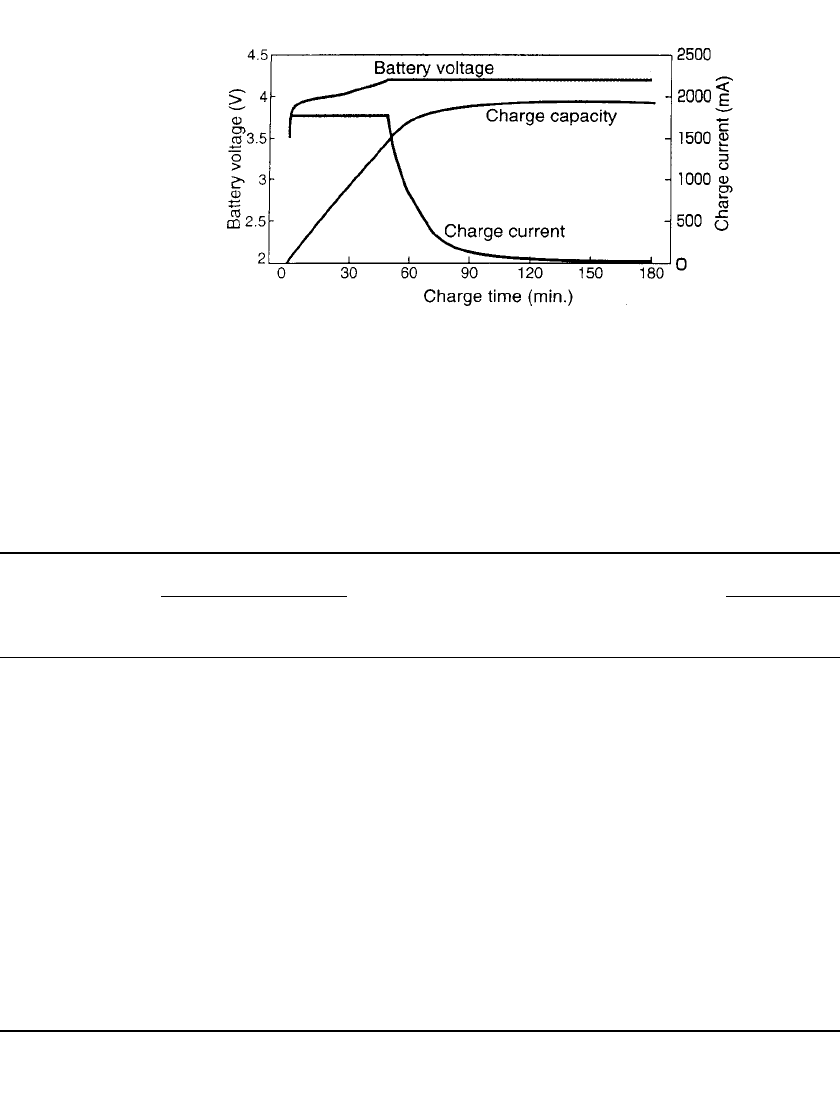
22.22 CHAPTER TWENTY-TWO
FIGURE 22.9 Charging characteristics of a typical cylindrical 18650 lith-
ium ion battery at 20⬚C. Battery is charged at constant current of 1.8 Amps
(nominal C-rate) to 4.2 Volts followed by a taper charge at this voltage for a
total time of 2 hours.
TABLE 22.5 Charging Characteristics of Secondary Batteries
System
Charged methods*
Preferred
Not
recommended
Recommended
constant-current
charge
rate, C (A)
Over-
charge-
ability
Temperature
range for
charging,
⬚C
Efficiencies†
Ah,
%
Wh,
%
Lithium ion cc, cv 0.20 None ⫺20 to 50 99 95
Lead-acid
Pasted, Plante´ cc, cv 0.07 Fair
⫺40 to 50 90 75
Tubular cc, cv 0.07 Fair
⫺40 to 50 80 70
Nickel-cadmium:
Industrial vented cc, cv 0.2 Very good
⫺50 to 40 70 60
Sintered vented cc, cv 0.2 Very good
⫺55 to 75 70–80 60–70
Sealed cc cv 0.1–0.3‡ Very good 0 to 40 65–70 55–65
Nickel/ metal
hydride cc cv 0.1‡ Fair 0 to 40 65–70 55–65
Nickel-iron cc cv 0.2 Very good 0 to 45 80 60
Nickel-zinc cc, cv 0.1–0.4 Fair
⫺20 to 40 85 70
Silver-zinc cc 0.05–0.1 Poor 0 to 50 90 75
Silver-cadmium cc 0.01–0.2 Fair
⫺40 to 50 90 70
Zn/ MnO
2
cv cc w /o
v. limit
Fair 10–30 55–65
* Constant current (cc) includes two-rate charging, and constant voltage (cv) includes modified constant-voltage charging.
† All data are related to normal rates of charge and discharge and room-temperature operation.
‡ Fast charge procedures can be used with charge control.
Source: Based on Falk and Salkind Ref. 5.

SECONDARY BATTERIES—INTRODUCTION 22.23
Many manufacturers are now recommending ‘‘fast’’ charge methods to meet consumer
and application demand for recharging in less than 2 to 3 h. These methods require control
to cut off the charge before an excessive rise in gassing, pressure, or temperature occurs.
These could cause venting or a more serious safety hazard, or they could result in a dele-
terious effect on the battery’s performance or life. Pulse charging is also being employed
with some systems to provide higher charge rates.
In general, control techniques are useful for recharging most secondary batteries. They
can be employed in several ways: to prevent overcharging, to facilitate ‘‘fast’’ charging, to
sense when a potentially deleterious or unsafe condition may arise and cut off the charge or
reduce the charging rate to safe levels. Similarly, discharge controls are also being used to
maintain cell balance and to prevent overdischarge.
Another approach is the ‘‘smart’’ battery. These batteries incorporate features:
1. To control the charge so that the battery can be charged optimally and safely
2. For fuel gauging to indicate the remaining charge left in the battery
3. Safety devices to alert the user to unsafe or undesirable operation or to cut off the battery
from the circuit when these occur.
See Sec. 5.6
22.3.8 Cost
The cost of a secondary battery may be evaluated on several bases, depending on the mode
of operation. The initial cost is one of the bases for consideration. Other factors are the
number of charge-discharge cycles that are available, or the number delivered in an appli-
cation, during a battery’s lifetime, or the cost determined on a dollar-per-cycle or dollar-per-
total-kilowatt-hour basis. The cost of charging, maintenance, and associated equipment may
also have to be considered in this evaluation. See Sec. 6.4.3. In an emergency standby service
or SLI-type application, the important factors may be the calendar life of the battery (rather
than as cycle life) and the cost is evaluated on a dollar-per-operating-year basis.
The lead-acid battery system is by far the least costly of the secondary batteries, partic-
ularly the SLI type. The lead-acid traction and stationary batteries, having more expensive
constructional features and not as broad a production base, are several times more costly,
but are still less expensive than the other secondary batteries. The nickel-cadmium and the
rechargeable zinc/manganese dioxide batteries are next lowest in cost, followed by the
nickel/metal hydride battery. The cost is very dependent on the cell size or capacity, the
smaller button cells being considerably more expensive than the larger cylindrical and pris-
matic cells. The nickel-iron battery is more expensive and, for this reason among others, lost
out to the less expensive battery system.
The most expensive of the conventional-type secondary batteries are the silver batteries.
Their higher cost and low cycle life have limited their use to special applications, mostly in
the military and space applications, which require their high energy density. The nickel-
hydrogen system is more expensive due to its pressurized design and a relatively limited
production. However, their excellent cycle life under conditions of shallow discharge make
them attractive for aerospace applications. The cost of cylindrical lithium ion batteries has
been decreasing rapidly as production rates have increased and has recently been stated to
be $1.22 /Wh.
6
An important objective of the program for the development of secondary batteries for
electric vehicles and energy storage is to reduce the cost of these battery systems. Cost
factors are discussed in Chap. 37.

22.24 CHAPTER TWENTY-TWO
ACKNOWLEDGMENT
The contributions of Prof. Alvin J. Salkind and Mr. Anthony G. Cannone to this chapter are
gratefully acknowledged.
REFERENCES
1. A. J. Salkind, D. T. Ferrell, and A. J. Hedges, ‘‘Secondary Batteries 1952–1977,’’ J. Electrochem.
Soc. 125(8), Aug. 1978.
2. A. J. Salkind, Director, Battery Laboratory, Rutgers University, NJ, 2001.
3. H. Takeshita, Proc. 18th Int. Seminar on Primary & Secondary Batteries, Fort Lauderdale, FL (March
5–8, 2001).
4. L. H. Thaller, ‘‘Expected Cycle Life vs. Depth of Discharge Relationships of Well-Behaved Single
Cells and Cell Strings,’’ J. Electrochem. Soc. 130(5), May 1983.
5. S. U. Falk and A. J. Salkind, Alkaline Storage Batteries, Wiley, New York, 1969.
6. D. MacArthur, G. Blomgren, and R. Powers, Lithium and Lithium Ion Batteries, 2000, Powers As-
sociates, Sept. 30, 2000.

23.1
CHAPTER 23
LEAD-ACID BATTERIES
Alvin J. Salkind, Anthony G. Cannone, and Forrest A. Trumbure
23.1 GENERAL CHARACTERISTICS
The lead-acid battery has been a successful article of commerce for over a century. Its
production and use continue to grow because of new applications for battery power in energy
storage, emergency power, and electric and hybrid vehicles (including off-road vehicles) and
because of the increased number of vehicles for which it provides the energy for engine
starting, vehicle lighting, and engine ignition (SLI). Its sales represent approximately 40 to
45% of the sales value of all batteries in the world, or a market value, in 1999, of about
$15 billion at manufacturers’ levels and 2 to 3 times this value at retail levels. (These values
do not include some countries such as Russia and China, for which complete market data
are not available.) This battery system is also used extensively in telephone systems, power
tools, communication devices, emergency lighting systems, and as the power source for
mining and material-handling equipment. The wide use of the lead-acid battery in many
designs, sizes, and system voltages is accounted for by the low price and the ease of man-
ufacture on a local geographic basis of this battery system. The lead-acid battery is almost
always the least expensive storage battery for any application, while still providing good
performance and life characteristics.
New uses, designs, and fabrication processes are still being introduced at significant rates.
Some of the new designs are for modern electric-vehicle, energy-storage, and electronics
applications. There have been many improvements in lead-acid battery design and charger
system logic to make high-voltage batteries more uniform in performance. Electric-vehicle
batteries are typically 100 to 300 V systems. The lead-acid battery has a high electrical
turnaround efficiency, 75 to 80%, which makes the system attractive for electric-vehicle and
energy-storage use. Traditional vertical-plate batteries are capable of energy densities greater
than 40 Wh/kg, and a horizontal-plate design with higher energy and power densities have
found use in traction and fork lift applications. Modified lead-acid batteries are being inves-
tigated for both electric and hybrid-drive vehicles. The world’s largest energy-storage battery
system was finished in late 1988. This 40-MWh battery, located in Chino, Calif., uses in-
dividual industrial-size lead-acid cells in series and parallel connection to make a 10-MW
system delivering energy into the utility grid at 2000 V and 8000 A for 4 hours. AC to DC
conversion is built into the system. This battery operated for more than a decade as a
demonstration project. At the other extreme, small individual lead-acid cells and batteries
are now available with quick connects for use in small electric appliances and electronics
applications. Many of these newer applications require low-maintenance or maintenance-free
designs. Thin film capacitor-like lead-acid batteries have become commercially available in
the past few years, for consumer and electronic applications. These are discussed in detail

23.2 CHAPTER TWENTY-THREE
in Chap. 24. Some larger industrial cells are often virtually maintenance-free using the
oxygen-recombination principle and a resealable Bunsen vent. An approach to high-energy-
density, high-power-density, high-cycle-life lead-acid battery design is the bipolar design, a
design which is still being pursued. The problems which prevent this design from larger
scale commercial use relate to the availability of a bipolar material which is electronically
conductive, nonporous to ions, low cost, and stable against both positive and negative active
materials. Conductive plastics, which are used in some battery systems, have not been suc-
cessful in lead batteries. Experiments have been carried out with a bipole made from tin
oxide coated glass encapsulated in a plastic matrix, and with multilayers of different lead
alloys to slow the penetration of the bipole by corrosion.
The overall advantages and disadvantages of the lead-acid battery, compared with other
systems, are listed in Table 23.1.
TABLE 23.1 Major Advantages and Disadvantages of Lead-Acid Batteries
Advantages Disadvantages
Popular low-cost secondary battery—capable of
manufacture on a local basis, worldwide,
from low to high rates of production
Available in large quantities and in a variety of
sizes and designs—manufactured in sizes
from smaller than 1 Ah to several thousand
Ampere-hours
Good high-rate performance—suitable for
engine starting (but outperformed by some
nickel-cadmium and nickel metal-hydride
batteries)
Moderately good low- and high-temperature
performance
Electrically efficient—turnaround efficiency of
over 70%, comparing discharge energy out
with charge energy in
High cell voltage—open-circuit voltage of
⬎2.0 V is the highest of all aqueous-
electrolyte battery systems
Good float service
Easy state-of-charge indication
Good charge retention for intermittent charge
applications (if grids are made with high-
overvoltage alloys)
Available in maintenance-free designs
Low cost compared with other secondary
batteries
Cell components are easily recycled.
Relatively low cycle life (50–500 cycles)*
Limited energy density—typically 30–40 Wh / kg
Long-term storage in a discharged condition can
lead to irreversible polarization of electrodes
(sulfation)
Difficult to manufacture in very small sizes (it is
easier to make nickel-cadmium button cells in
the smaller than 500-mAh size)
Hydrogen evolution in some designs can be an
explosion hazard (flame arrestors are installed
to prevent this hazard)
Stibene and arsine evolution in designs with
antimony and arsenic in grid alloys can be a
health hazard
Thermal runaway in improperly designed batteries
or charging equipment
Positive post blister corrosion with some designs
*Up to 2000 cycles can be attained with special designs.

LEAD-ACID BATTERIES 23.3
The lead-acid battery is manufactured in a variety of sizes and designs, ranging from less
than 1 to over 10,000 Ah. Table 23.2 lists many of the various types of lead-acid batteries
that are available.
TABLE 23.2 Types and Characteristics of Lead-Acid Batteries
Type Construction Typical applications
SLI (starting, lighting, ignition) Flat-pasted plates (option:
maintenance-free construction)
Automotive, marine, aircraft,
diesel engines in vehicles and
for stationary power
Traction Flat-pasted plates; tubular and
gauntlet plates
Industrial trucks (material
handling)
Vehicular propulsion Flat-pasted plates; tubular and
gauntlet plates; also composite
construction
Electric vehicles, golf carts,
hybrid vehicles, mine cars,
personnel carriers
Submarine Tubular plates; flat-pasted plates Submarines
Stationary (including energy-
storage types such as charge
retention, solar photovoltaic,
load leveling)
Plante´;* Manchester;* tubular
and gauntlet plates; flat-pasted
plates; circular conical plates
Standby emergency power:
telephone exchange,
uninterruptible power
systems (UPS), load leveling,
signaling
Portable (see chap. 24) Flat-pasted plates (gelled
electrolyte, electrolyte
absorbed in separator);
spirally wound electrodes;
tubular plates
Consumer and instrument
applications: portable tools,
appliances, lighting,
emergency lighting, radio,
TV, alarm systems
*Now rarely used.
23.1.1 History
Practical lead-acid batteries began with the research and inventions of Raymond Gaston
Plante´ in 1860, although batteries containing sulfuric acid or lead components were discussed
earlier.
1
Table 23.3 lists the events in the technical development of the lead-acid battery. In
Plante´’s fabrication method, two long strips of lead foil and intermediate layers of coarse
cloth were spirally wound and immersed in a solution of about 10% sulfuric acid. The early
Plante´ cells had little capacity, since the amount of stored energy depended on the corrosion
of a lead foil to lead dioxide to form the positive active material, and similarly the negative
electrode was formed by roughening of another foil (on cycling) to form an extended surface.
Primary cells were used as the power sources for this formation. The capacity of Plante´ cells
was increased on repeated cycling as corrosion of the substrate foils created more active
material and increased the surface area. In the 1870s magnetoelectric generators became
available to Plante´, and about this time the Siemens dynamo began to be installed in central
electric plants. Lead-acid batteries found an early market to provide load leveling and to
average out the demand peaks. They were charged at night, similar to the procedure now
planned for modern load-leveling energy-storage systems.
Subsequent to Plante´’s first developments, numerous experiments were done on acceler-
ating the formation process and coating lead foil with lead oxides on a lead plate pretreated
by the Plante´ method. Attention then turned to other methods for retaining active material,
and two main technological paths evolved.
1. Coating a lead oxide paste on cast or expanded grids, rather than foil, in which the active
material developed structural strength and retention properties by a ‘‘cementation’’ process
(interlocked crystalline lattice) through the grid and active mass. This is generally referred
to as flat-plate design.
2. The tubular electrode design, in which a central conducting wire or rod is surrounded by
active material and the assembly encased in an electrolyte porous insulated tube, which
can be either square, round, or oval.

23.4 CHAPTER TWENTY-THREE
TABLE 23.3 Events in Technical Development of Lead-Acid Battery
Precursor systems
1836 Daniell Two-fluid cell; copper /copper sulfate/ sulfuric acid/ zinc
1840 Grove Two-fluid cell; carbon /fuming nitric acid / sulfuric acid/ zinc
1854 Sindesten Polarized lead electrodes with external source
Lead-acid battery developments
1860 Plante´ First practical lead-acid battery, corroded lead foils to form active
material
1881 Faure Pasted lead foils with lead oxide-sulfuric acid pastes for positive
electrode, to increase capacity
1881 Sellon Lead-antimony alloy grid
1881 Volckmar Perforated lead plates to provide pockets for support of oxide
1882 Brush Mechanically bonded lead oxide to lead plates
1882 Gladstone and
Tribs
Double sulfate theory of reaction in lead-acid battery:
PbO
2
⫹ Pb ⫹ 2H
2
SO
4
2PbSO
4
⫹ 2H
2
O
1883 Tudor Pasted mixture of lead oxides on grid pretreated by Plante´
method
1886 Lucas Formed lead plates in solutions of chlorates and perchlorates
1890 Phillipart Early tubular construction—individual rings
1890 Woodward Early tubular construction
1910 Smith Slotted rubber tube, Exide tubular construction
1920 to
present
Materials and equipment research, especially expanders, oxides,
and fabrication techniques
1935 Haring and
Thomas
Lead-calcium alloy grid
1935 Hamer and
Harned
Experimental proof of double sulfate theory of reaction
1956–
1960
Bode and Voss
Ruetschi and
Cahan
Burbank
Feitknecht
Clarification of properties of two crystalline forms of PbO
2
(alpha and beta)
1970s McClelland
and Devit
Commercial spiral-wound sealed lead acid battery
Expanded metal grid technology; composite plastic /metal grids;
sealed and maintenance-free lead-acid batteries; glass fiber and
improved separators; through-the-partition intercell connectors;
heat-sealed plastic case-to-cover assemblies; high-energy-
density batteries (above 40 Wh/ kg); conical grid (round) cell
for long-life float service in telecommunications facilities
1980s Sealed valve-regulated batteries; quasi-bipolar engine starter
batteries; improved low-temp. performance; world’s largest
battery installed (Chino, Calif.); 40-MWh lead-acid load
leveling
1990s Electric-vehicle interest reemerges; bipolar battery designs for
highpower use in uninterruptible power supplies, power tool
market and electronic back-up. Thin foil cells, small cells for
consumer and current road applications.
2000s Planned introduction of 36 Volt SLI battery system for
automobiles

LEAD-ACID BATTERIES 23.5
TABLE 23.4a Market Growth of Lead-Acid Battery in United States*
1960 1969 1980 1991 1999** 2003 est
MV registration 74 105 158 190 250 260
SLI units (original equipment
and replacement)
34 47 62 76 100 110
Car-battery ratio‡ 2.2 2.2 2.5 2.5 2.5 2.4
SLI sales, $ 330 510 1675 2100 2700 2800
Industrial, $ 70 105 380 550 1015 1500
Consumer, $ 1
3 55 100 150 160
Total, $ 400 620 2110 2750 3965 4460
*All units in millions, except car-battery ratio, values are at manufacturers’ pricing.
Battery prices are affected by the price of lead.
Lead prices varied from $0.40 to $1.40 / kg between 1978 and 1999, (lead price Dec. 1999
⫽ $0.91/
kg.)
‡A considerable number of SLI-type batteries are used in boats and other vehicles, therefore this ratio
is not an exact representation of the useful life of an automotive battery.
NOTE: The Canadian market is approximately 10% of the U.S. market.
Year 2003 estimates from ‘‘Battery Man’’ of Nov. 1999.
**A large growth in industrial battery sales began in 1995.
Simultaneous with the advances in developing and retaining active material was work in
strengthening the grid by casting it from lead alloys such as lead-antimony (e.g., Sellon,
1881) or lead-calcium (e.g., Haring and Thomas, 1935).
2
The technical knowledge for an
economical manufacture of reliable lead-acid batteries was in place by the end of the nine-
teenth century, and subsequent growth of the industry was rapid. Improvements in design,
manufacturing equipment and methods, recovery methods, active material utilization and
production, supporting structures and components, and nonactive components such as sep-
arators, cases, and seals continue to improve the economic and performance characteristics
of lead-acid batteries. Intense development work continues on applications for energy stor-
age, electric bicycles and electric vehicles.
23.1.2 Manufacturing Statistics and Lead Use
The major present-day use of lead-acid storage batteries is in vehicle starting (SLI) appli-
cations. Most vehicles use a 12-V battery with a capacity in the range of 40 to 60 Ah. This
battery, weighing about 14.5 kg, has sufficient high-rate capacity to deliver the 450 to 800 A
necessary to start an automobile engine. The power demand for the automobiles in the year
2003 is expected to be greater than that supplied by the present 12-V battery systems. With
all of the new and planned onboard systems, the battery is expected to be a nominal 36
Volts which corresponds to a 42 V charged voltage.
Approximately 60% of the battery weight is lead or lead components. Batteries represent
the most important use of lead in the world. The yearly consumption of lead for the man-
ufacture of batteries was about 3 million metric tonnes in 1992, approximately half of the
total world output of primary and secondary lead. The percentage of lead used in batteries
is increasing as other uses disappear because of environmental and health concerns. There
is a direct correlation between the number of motor vehicles registered and the number of
SLI battery units sold annually, as shown in Table 23.4a.
3
Although SLI units represent over
70% of the monetary value of the total lead battery market at present, this percentage is
declining as the other applications for storage batteries continue to grow. A world perspective
on the manufacture and use of SLI lead-acid batteries is presented in Table 23.4b.
