Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


23.26 CHAPTER TWENTY-THREE
The third major grid production method is circumferential continuous casting onto a mold
cut into the surface of a drum. Successful high-speed production of up to 150 grids per
minute has been reported. Continuous-cast grids are not symmetrical about a central planar
axis and need to be overpasted to hold the active material in place.
A fourth major grid production method, expansion from wrought or cast lead alloy strip,
is rapidly supplanting book-mold casting as the preferred method for the manufacture of SLI
battery grids. The advantages of this method are lower grid weight per unit of battery elec-
trical performance, the capability to manufacture a wide variety of sizes with a minimum
investment in tooling, a very high-rate production capability (up to 600 plates per minute),
and very uniform grid and plate sizes. Most development and commercialization have been
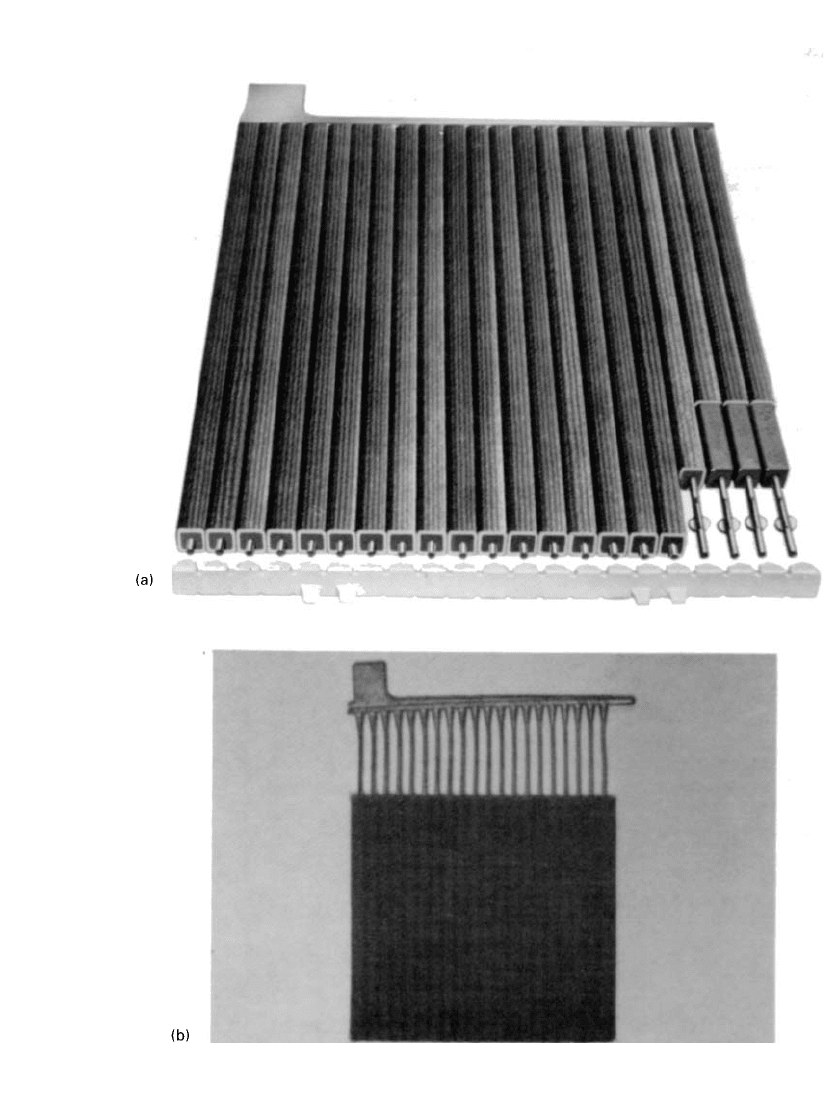
done on nonantimonial lead-calcium tin alloys (Fig. 23.14). Strip is produced from cast slabs
by a variety of proprietary metal-working processes, and the thin strip is slit to the width
specified by the battery manufacturer. The worked metal increases in strength as it decreases
in thickness during this processing.
FIGURE 23.14 Expanded wrought grid
for lead-acid batteries.
Machinery to produce grids from wrought strip has been developed and put into produc-
tion by several manufacturers. Four types of machinery are involved: progressive die expan-
sion, precision expansion, rotary expansion, and diagonal slit expansion. Progressive die
expansion has been the most extensively utilized of the four methods, but rotary expansion
is of increasing importance. Continuous drum casting of automotive grids is also challenging
the expansion processes as the dominant manufacturing method for calcium alloy grids used
in negative plates in automotive batteries. Positive-plate grids are most often produced of
low-antimony lead cast in book molds.
Whatever grid production method is used, there is often the need for small cast parts for
plate and cell interconnections and connection to external equipment. These parts have tra-
ditionally been cast in fixed molds, sometimes with mold inserts to allow a variety of similar
parts to be made in each mold. Newer battery production methods often produce these
various interconnections automatically in the course of battery assembly.

LEAD-ACID BATTERIES 23.27
23.3.3 Lead Oxide Production
Lead is used to make the active materials as well as the grids. The lead must be highly
refined (usually virgin or primary lead) to preclude contamination of the battery. It is de-
scribed as corroding-grade lead in ASTM specification B29.
11
Lead is oxidized by either of
two processes—the Barton pot or the ball mill.
17
In the Barton pot process, a fine stream of
molten lead is swept around inside a heated pot-shaped vessel, and oxygen from the air
reacts with fine droplets or particles to produce an oxide coating around each droplet. Typical
Barton pot oxides contain 15 to 30% free lead, which usually exists as the core of each fine
leady oxide spherically shaped particle. Barton pots are available in a variety of sizes up to
1000 kg /h output.
Ball milling describes a larger variety of processes. Lead pieces are put into a rotary
mechanical mill, and the attrition of the pieces causes fine metallic flakes to form. These are
oxidized by an airflow, and the airflow also serves to remove the leady oxide particles to
collection in a baghouse. The feedstock for ball mills can range from small cast slugs weigh-
ing less than 30 g to full pigs of lead weighing approximately 30 kg. Typical ball mill oxides
also contain 15 to 30% free lead in the shape of a flattened platelet core surrounded by an
oxide coating.
Some battery positive plates use an additive of red lead (Pb
3
O
4
), which is more conductive
than PbO, to facilitate the electrochemical formation of PbO
2
, Red lead is produced from
leady oxide by roasting this material in an airflow until the desired conversion is complete.
Such processing reduces the free lead content and generally increases the oxide particle size.
A variety of other oxides and lead-containing materials have been used to produce battery
plates but are of only historical interest.
17
Positive plates for the Lucent Technologies bat-
teries (formerly Bell Laboratories) were initially made with tetrabasic lead sulfate
(4PbO
PbSO
4
), which is a precursor for
␣
-PbO
2
. These plates now contain up to 25% red
lead (Pb
3
O
4
) in order to facilitate the electrochemical formation process.
23.3.4 Paste Production
Lead oxide is converted to a plastic doughlike material so that it can be affixed to the grids.
Leady oxide is mixed with water and sulfuric acid in a mechanical mixer. Three types of
mixers are commonly used—the change can or pony mixer, the muller, and a vertical muller.
The pony mixer is the traditional unit. A preweighed amount of leady oxide is placed
into the mixing tub, and this is wetted first with water and then with sulfuric acid solution.
Dry paste additives, if any, are premixed into the leady oxide before water addition. These
additives can be plastic fibers to enhance the mechanical strength of the dried paste, ex-
panders to maintain negative-plate porosity in operation, and various other proprietary ad-
ditives which ease processing or are believed to improve battery performance. Muller mixers
are usually filled first with the water component, then the oxide, then the acid.
As mixing proceeds, the paste viscosity increases, then decreases, as measured by the
amount of power consumed by the mixer motor. The paste becomes hot from the mechanical
mixing and from the reaction of H
2
SO
4
with the leady oxide. Paste temperature is controlled
by cooling jackets on the mixer or by evaporation of water from the paste. The amounts of
water and acid for a given amount of oxide will be different for the two mixer types and
will also depend on the intended use of the plates: SLI plates are generally made at a low
PbO:H
2
SO
4
ratio and deep-cycling plates at a high PbO:H
2
SO
4
ratio. Sulfuric acid acts as a
bulking agent—the more acid used, the lower the plate density will be. The total amount of
liquids and the type of mixer used will affect final paste consistency (viscosity). Paste mixing
is controlled by the measurement of paste density using a cup with a hemispherical cavity
and by the measurement of paste consistency with a penetrometer.

23.28 CHAPTER TWENTY-THREE
23.3.5 Pasting
Pasting is the process by which the paste is actually integrated with the grid to produce a
battery plate. This process is a form of extrusion, and the paste is pressed by hand trowel
or by machine into the grid interstices. Two types of pasting machines are used: a fixed-
orifice paster that pushes paste into both sides of the plate simultaneously and a belt paster
in which paste is pressed into the open side of a grid that is being conveyed past a paste
hopper on a porous belt. The amount of paste applied to a plate by a belt paster is regulated
by the spacing of the hopper above the grid on the belt and the type of troweling (roller or
rubber squeegee) used at the hopper exit. Using identical paste and grids, a trowel roller
machine packs the paste both thicker and more densely than a rubber squeegee machine. As
plates are pasted on either belt pasting machine, water is forced out of the paste, into the
belt, and ultimately to a sump on or near the machine. The sump material can be used in
place of some of the liquids for subsequent batches of negative paste.
Grids are automatically or manually placed onto the belt before being moved under the
paste hopper. Most smaller-sized plates are made as ‘‘doubles’’ joined at the feet (cast) or
at the tab edge (wrought expanded), or as panels of varying number of plates. Typically the
belt is 35 to 50 cm wide and can handle such doubles. Industrial stationary or traction plates
(being larger) are pasted by lengthwise feed into the machine or are hand-pasted.
After pasting, plates are racked or stacked for curing. Stacked plates contain enough
moisture to stick together, and so before stacking, the plate surfaces are dried somewhat by
a rapid passage through a high-temperature drier or over heated platens. Some carbon dioxide
from the combustion process might be absorbed on the surface such that the surface is made
‘‘harder.’’ The flash drying process may also help start the curing reactions. Thicker plates
are usually placed the long edge upward in racks rather than being stacked horizontally on
pallets after flash drying.
Wrought expanded plates and some cast plates are cut into discrete plate portions by a
slitter machine in the pasting line. Some manufacturers also have the plate lugs brushed
clean of paste and surface oxidation on the same machinery.
In Europe, and less commonly in the United States, many of the heavy-duty battery
positive plates are made in porous tubular sheaths. The grid is cast or injection-molded of
lead, with long-finned spines attached to a header bar and a connection lug. Individual woven
fiberglass plastic sheaths or a multitube gauntlet are placed on the spines. These plates are
filled with powder or with a slurried paste until the tubes are full. A plastic cap plugs the
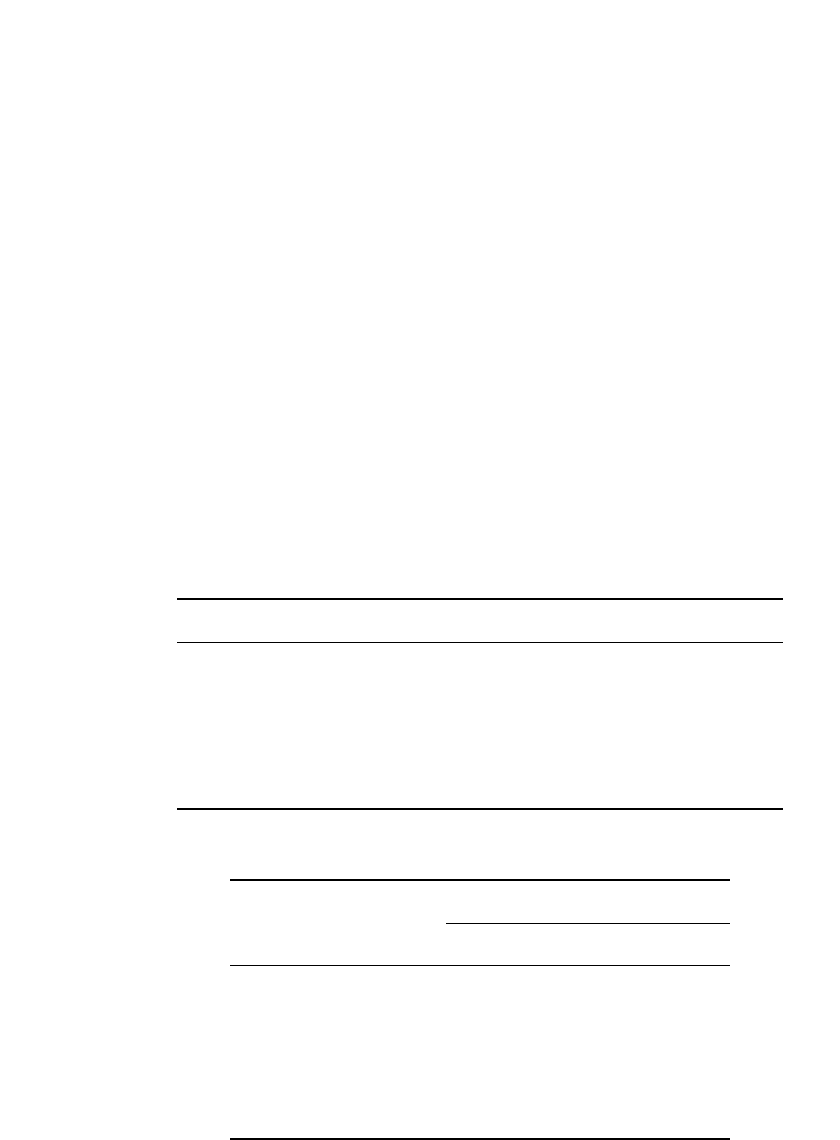
open sheath ends and becomes the bottom of the plate (Fig. 23.15).
LEAD-ACID BATTERIES 23.29
FIGURE 23.15 Tubular and gauntlet plates. (a) Tubular. (b) Gauntlet.

23.30 CHAPTER TWENTY-THREE
23.3.6 Curing
The curing process is used to make the paste into a cohesive, porous mass and to help
produce a bond between the paste and the grid. Several different curing processes are used
for lead oxides, depending on paste formulation and the intended use of the battery.
15
Typical cure for SLI plates is ‘‘hydroset,’’ at low temperature and low humidity for 24 to
72 h. The temperature is preferably between 25 and 40⬚C; the humidity is that contained in
the flash-dried plates, typically 8 to 20% H
2
O by weight. The plates are usually covered by
canvas, plastic, or other materials to help retain both temperature and moisture. Some man-
ufacturers use enclosed rooms for the hydroset, and these rooms may be heated where
required by climatic conditions. As the plates cure, they reach a peak temperature, and
temperature and humidity decrease. Hydroset typically produces tribasic lead sulfate, which
gives high energy density.
More and more manufacturers are using curing ovens, where temperature and humidity
can be precisely controlled. This controls the peak temperature and makes sure that sufficient
moisture is available to oxidize the remaining free lead in the paste. Peak temperatures in
the range of 65 to 90
⬚C are used; at higher temperatures or longer times, significant amounts
of tetrabasic lead sulfate are produced in the plate, and the plates generally have lower energy
density. At the end of curing, the free lead content of the paste should be below 5%, pref-
erably as low as possible. (Insufficient paste cure is observed as ‘‘soft,’’ easily broken pasted
plates, usually pale in color.) If the plates have not cured, they can be rewetted and reheated
to force the cure. Another process to force completion of curing is to dip the partially cured
plates into dilute sulfuric acid. This latter process (‘‘pickling’’) is also used for cure of
powder-filled tubular positive plates.
Cured plates are stored until use. Shelf life is not critical, but the high cost of inventory
usually makes storage time minimal.
23.3.7 Assembly
The simplest cell consists of one negative, one positive, and one separator between them.
Most practical cells contain about 3 to 30 plates with separators in between. Individual or
leaf separators are generally used. The use of ‘‘envelope’’ separators, which surround either
the positive or negative plate, or both, is becoming more popular in small, sealed cells, SLI,
motive power, and standby batteries to facilitate production and to control lead contamination
during manufacture.
Separators are used to electrically insulate each plate from its nearest counterelectrode
neighbors but must be porous enough to allow acid transport into or out of the plates. The
properties of typical separators are given in Table 23.10.
SLI batteries use either phenolic /cellulosic separators, sintered PVC separators or more
recently a trend toward microporous polyethylene in either ‘‘leaf’’ or ‘‘envelope’’ form. The
average pore diameters are in the 5 to 30-
m range. Heavy-duty batteries usually have
microporous rubber or polyolefin (mainly polyethylene) separators which have smaller pore
sizes and give longer life at a higher cost. Glass fiber scrim is added to some separator
materials to improve acid retention.
Industrial traction-pasted positive plates are usually wrapped with several layers of fibrous
glass matting to help retain the active material during use. The inner layer consists of usually
very fine parallel strands of ‘‘slyver’’ glass, and subsequent layers are randomly oriented
glass fibers held together by a plastic (styrene or acrylic) binder. The matting is held in place
by a heat-sealed, perforated rubber retainer. In lieu of plate wrapping, many heavy-duty
batteries use a layer of glass matting on the ribs of the separator to help retain the positive
active material.

LEAD-ACID BATTERIES 23.31
TABLE 23.10 Comparison of Various Types of Battery Separator Materials
Type Rubber Cellulose PVC PE
Glass
fiber Microglass
Year available 1930 1945 1950 1970 1980 1985
Backweb, mils 20
⫹ 17–30 12–20 7–30 22–26 10–150
Porosity, % 60
565 540⫹ 60 585 590⫹
Maximum pore ⬎535 25 ⬍1 45 15–30
size,
m
Average pore 3 25 15
⬍0.1 80 10–15
size,
m
Electrical 30–50 25–30 15–30 8–40 10
⬍5
resistance, (in acid)
m
⍀in
2
Purity Good Fair Good Good Good Excellent
Corrosion Good Fair Excellent Excellent Excellent Excellent
resistance
Flexibility Brittle Brittle Brittle Excellent Good Fair to good
Source: Battery Man. (Mar. 1993).
Plates and separators are stacked manually or by a stacking machine. Stacked elements
are staged on roller conveyors or carts as input to the interplate welding operations. Welding
is done by two general methods: melting of the lugs in a mold with the lugs facing upward,
or immersion of lugs facing downward into pools of molten lead alloy contained in a pre-
heated mold. The first method is the traditional assembly method for lead-acid batteries. In
this method the plate lugs fit up through slots in a mold ‘‘comb’’; the shape and the size of
the group strap are delineated by the ‘‘dam’’ and ‘‘back iron’’ portions of the tooling. Some
battery manufacturers use slotted ‘‘crowfoot’’ posts to fit over the plate lugs to speed the
welding process. The second welding method is called the ‘‘cast-on strap’’ process and is
typically used for SLI cells. Stacked elements are loaded into slots of the cast-on machine.
A mold which has cutouts corresponding to the desired straps and posts is preheated and
filled with the appropriate molten lead alloy, making sure not to join lead or lead-calcium
alloys with antimony alloys. The mold and the stacked elements are moved until the plate
lugs are immersed in the strap cutouts. External cooling solidifies the strap onto and around
each lug, and the elements are moved to a point where they can be dropped into a battery
case. Visual examination can differentiate between the two welding methods: fixture-welded
plate straps are usually thicker and smoother than cast-on straps; cast-on straps also will
usually show a convex meniscus of metal between adjacent plate lugs on the underside of
the strap if the lug is properly cleaned of paste. A good weld is required between each plate
lug and the strap so that high-rate discharge performance is maximized. The resultant as-
semblage of plates and separators is known as an element, and the welded subelements are
known as groups. Electrical testing for short circuits is usually done on elements before
further assembly.
Cast-on battery elements are either continuously connected or made in discrete one-cell
modules. The first method requires that long intercell connections be used, which travel over
the intercell partition and are seated in a slot in this partition; this is known as the loop-
over-partition design. In the second cast-on method, tabs on the ends of the plate straps are
positioned over holes that have been prepunched into the intercell partitions of a battery
case. These tabs are welded together manually with a very small torch or automatically by
a resistance welding machine. The latter also squeezes the tabs and the intercell partition to
provide a leak-proof seal.

23.32 CHAPTER TWENTY-THREE
Industrial traction cells and old-style SLI cells have been connected into batteries after
the cell cases and covers are sealed together. Traction batteries are needed in thousands of
different sizes for various applications, and the standard unit of construction is a cell, not a
quantity of plates and separators. A heavy steel tray is fabricated and coated with an acid-
resistant coating (urethane, epoxy, etc.). Traction cells are placed into the tray and shimmed
as necessary, and intercell connections are welded on. Heavy flexible wires (made from
welding cable) are welded to the end cells for connection to the external circuit.
23.3.8 Case-to-Cover Seal
Four different processes have been used to seal battery cases and covers together. Enclosed
cells are necessary to minimize safety hazards related to the acidic electrolyte, to the poten-
tially explosive gases produced on overcharge, and to electrical shock. Most SLI batteries
and many modern traction cells are sealed with fusion of the case and cover. The fusion
comes from preheating each on a platen, then forcing the two together mechanically, or from
ultrasonic welding of the case and cover. Fusion-sealed batteries are virtually impossible to
repair. At best the elements can be salvaged, but the cover and usually the case are discarded
and replaced. A few SLI batteries are sealed using an epoxy cement which fills a groove in
the cover; the battery is inserted and positioned so that the case and intercell partition lips
fit into the epoxy-filled groove. Heat is used to activate the catalyst to set the epoxy.
Some small deep-cycling batteries feature tar (asphalt)-sealed cases and individual cell
covers. Here the tar seal allows easy repair to the battery. Traditionally all batteries were
made this way before about 1960, but heat seals are typical for SLI batteries today. Molten
tar is dispensed from a heated kettle to fill a groove between the cover and the case. The
tar must be hot enough to flow easily but cool and viscous enough to solidify before running
down into the cell.
Stationary batteries in plastic cases are sealed with epoxy glues, with solvent cement, or
(for PVC copolymer cases and covers) with a thermal seal. Terminals are cast or welded on.
Some very large stationary and traction cells are made so that coolant can be circulated
through the terminals, and others are made with terminals with copper inserts for increased
conductivity and mechanical strength.
23.3.9 Tank Formation
Plates or assembled groups can be electrically formed or charged before assembly into the
case. When SLI plates are formed, these are usually formed as ‘‘doubles,’’ with two to five
panels stacked together in a slotted plastic formation tank, spaced an inch or less from stacks
of counterelectrode-pasted panels in adjacent slots. The stacks are arranged so that all positive
lugs protrude out of one side of the tank top and all negative lugs protrude out of the other
side of the tank top. All lugs with the same polarity are connected by welding to a heavy
lead bar, and the two bars are connected to a low-voltage, constant-current power supply.
The tank is filled with electrolyte, and current is passed until the plates have been formed:
the positives are converted to a deep brownish black and the negatives to a soft gray which
shows a bright metallic streak when scratched. Industrial plates are usually formed singly.
Sometimes these are also formed against dummy plates or grids. A variety of tank materials
have been used, but the most common are PVC, polyethylene, or lead. The tanks are arranged
so that the acid can be drained and refilled because formation increases the electrolyte con-
centration.
A variety of formation conditions are used, with variations in electrolyte density, charging
rate (current), and temperature. Electrolyte is typically dilute, in the range of 1.050–1.150
specific gravity. The charging rate is usually fixed, but some manufacturers use a sequence
of two or three different charging rates for different periods of time.
Tank-formed groups or plates are somewhat unstable (negatives will spontaneously oxi-
dize in air) and therefore are ‘‘dry charged’’ before use (see Sec. 23.3.11).
LEAD-ACID BATTERIES 23.33
TABLE 23.11 Formation Processes
One-shot Two-shot
Typical application SLI All others, some SLI
Electrolyte concentrations, sp gr.:
Initial
Final
1.200
1.280
1.005–1.150
1.150–1.230
Subsequent processing None Dump and refill with 1.280–1.330
sp gr electrolyte; continue charge
for several hours
TABLE 23.12 Specific Gravity of Electrolytes at Full Charge at 25⬚C
Type of battery
Specific gravity
Temperate climates Tropical climates
SLI 1.260–1.290 1.210–1.230
Heavy duty 1.260–1.290 1.210–1.240
Golf cart 1.260–1.290 1.240–1.260
Golf cart (electric vehicle) 1.275–1.325 1.240–1.275
Traction 1.275–1.325 1.240–1.275
Stationary 1.210–1.225 1.200–1.220
Diesel starting (raiload) 1.250
Aircraft 1.260–1.285 1.260–1.285
Modern electronic chargers for formation are made to operate in a constant-current mode,
either by a saturable reactor control or by use of silicon controlled rectifiers (SCRs). The
most recent development in formation chargers is to control the current and time by use of
a microcomputer. Some formation schedules include charge at three or more different cur-
rents which start at low currents, go to higher currents, and then revert to lower currents.
Current adjustment during formation minimizes damage to the cells by high temperatures
and the need for cell cooling by water spray or forced air.
23.3.10 Case Formation
The more usual method of formation is to completely assemble the battery, fill it with
electrolyte, and then apply the formation charge. This method is used for SLI and most
stationary and traction batteries. A variety of formation conditions are used, similar to those
for tank formation. The two major formation processes are the two-shot formation process
(used for stationary and traction batteries) and the one-shot formation process (used for most
SLI batteries). In the two-shot formation, the electrolyte is dumped to remove the low-density
initial electrolyte and refilled with more concentrated electrolyte, chosen so that when this
is mixed with the dilute initial acid residue which is absorbed in the elements or trapped in
the case, the cell electrolyte will equilibrate at the desired density (Table 23.11). Typical
values of the electrolyte specific gravity at full charge after formation are given in Table
23.12.

23.34 CHAPTER TWENTY-THREE
23.3.11 Dry Charge
The performance of wet batteries degrades with long periods of inactivity, especially when
stored at warm temperatures. A loss of 1 to 3% of capacity each day is possible with SLI
batteries that contain antimonial lead grids. The loss on stand can be much lower for main-
tenance-free batteries (0.1 to 3%/day). When lead-acid batteries must be stored for a long
time, especially in high ambient temperatures, or when batteries are shipped for export, their
performance can be stabilized by removal of the electrolyte by one of several methods.
When the electrolyte is removed, the battery is termed ‘‘dry-charged’’ (that is, charged
and dry) or ‘‘charged and moist.’’ The first process is done before the battery elements are
assembled inside the case and cover. The plates can be tank-formed, water-washed, then
dried in an inert gas before the element-welding portion of assembly. Alternately the welded
element can be tank-formed, washed, and then dried in an inert gas. The latter process is
simpler to carry out, but it is necessary that the separators can be rewetted easily after being
washed and dried. The assembly (case, elements, cover) is completed and the battery is
sealed. The battery can be stored in this dry-charged state for up to several years before
reactivation and use.
Several processing innovations have been commercialized in the past 10 years to convert
wet-charged batteries into moist or semidry batteries. In one process, most of the electrolyte
is removed by centrifugation. Another process uses an inorganic salt (sodium sulfate) in the
electrolyte, which minimizes degradation during storage and assists in an eventual reacti-
vation. A battery is formed, dumped, refilled with electrolyte which contains the additive,
high-rate-discharge tested, and then finally dumped. The high-rate electrical discharge (to
simulate engine cranking) allows testing of an assembled ‘‘damp-dry’’ battery, but this also
probably blocks the plate surfaces with a thin layer of small lead sulfate crystals. These
crystals then minimize plate or separator degradation during storage when the battery is
sealed.
23.3.12 Testing and Finishing
Electrical tests are used to check the performance of batteries before they are sold and often
before they are put into use. The type of test employed depends on the intended use for the
battery. SLI batteries are tested by brief discharges at very high currents (200 to 1500 A) to
simulate engine-cranking performance. Stationary and traction cells are discharged at a rate
specified by the user, usually in the range of 1 to 10 h if stationary and 4 to 8 h for traction.
The discharge for SLI batteries is usually done by dissipation through a fixed low-value
resistor or by a brief, high-rate electrical discharge driven by a power supply. Heavy-duty
batteries are discharged through a resistor, a transistorized load, or an inverter.
The final manufacturing steps consist of improving the battery appearance by washing,
drying, painting, installing vent plugs, and labeling as desired. Rubber-case batteries are
usually painted; plastic-case batteries are not. A large variety of plaques and labels are
available which can describe the battery, its performance, and use. Product liability require-
ments in many countries mandate that the user be warned of the hazardous nature of the
battery, especially that the electrolyte is corrosive and that gases are formed which can be
explosive.
Traction batteries are physically sized to fit a myriad of different forklift trucks, and so
the final assembly for a traction battery consists of inserting preformed and pretested cells
into a sturdy metal box (tray), making intercell connections, making cable connections, and
sometimes adding a tar or plastic (urethane) material in the spaces between cell covers.

LEAD-ACID BATTERIES 23.35
23.3.13 Shipping
Small batteries (SLI and golf-cart types) are palletized several layers high for long-distance
shipment. The batteries are cushioned by five-sided (slipover) or six-sided cardboard boxes
with cardboard or wood sheet between layers. The batteries are held laterally by banding or
by plastic sheet which is shrink- or stretch-wrapped around a full pallet. Pallets need to be
very sturdy to withstand the battery weight and handling abuse. Large batteries are palletized,
banded, and cushioned as appropriate.
Batteries have traditionally been shipped only minimal distances because of their fragile
nature, their weight, and their corrosive contents. The latter cause common carriers to charge
a significant premium for battery shipment and usually preclude shipment by air. As the
number of small, localized-sales battery manufacturers continues to decrease in the United
States, the remaining large manufacturers now usually ship to their distribution chain on
their own trucks.
23.3.14 Activation of Dry-Charged Batteries
When batteries have been dry-charged, they have to be reactivated before use. Activation
consists of unpacking the battery, filling the cells with electrolyte (which sometimes is
shipped with the battery in a separate package), charging the battery (if time is available),
and testing the battery performance. When dry-charged batteries are activated, the materials
which had been used to seal the vent holes must be removed and discarded.
23.4 SLI (AUTOMOTIVE) BATTERIES: CONSTRUCTION AND
PERFORMANCE
23.4.1 General Characteristics
The design of lead-acid batteries is varied in order to maximize the desired type of perform-
ance. Tradeoffs exist for optimization among such parameters as power density, energy den-
sity, cycle life, ‘‘float-service’’ life, and cost.
High power density requires that the internal resistance of the battery be minimal. This
affects grid design, the porosity, thickness, and type of separator, and the method of intercell
connection. High power and energy densities also require that plates and separators be thin
and very porous and, usually, that paste density be very low. High cycle life requires premium
separators, high paste density, the presence of
␣
-PbO
2
or another bonding agent, modest
depth of discharge, good maintenance, and, usually, the use of high-antimony (5 to 7% Sb)
grid alloy. Low cost requires both minimum fixed and variable costs, high-speed automated
processing, and no premium materials for the grid, paste, separator, and other cell and battery
components.
1,14,18–20
The automotive industry is planning to introduce a 36 /42 volt battery system in the first
decade of the new millennium (see Sec. 23.9.1).
23.4.2 Construction
An SLI battery whose main function is to start an internal combustion engine, discharges
briefly but at a high current. Once the engine is running, a generator or alternator system
recharges the battery and then maintains it on ‘‘float’’ at full charge or slight overcharge. In
recent automobile designs the parasitic electrical load of lights, motors, and electronics
causes a gradual discharge of the battery when the engine is not in operation. This factor,
coupled with normal self-discharge, introduces a significant cycling component into the nor-
mal cranking /floating duty cycles. Studies of SLI battery life and failure modes are presented
in Secs. 23.4.3 and 23.8.4.
