Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

23.6 CHAPTER TWENTY-THREE
TABLE 23.4b World Battery Trends-SLI Shipment*
1990 1991
4
% of total
(1991) 1999
North America 84.9 81.4 35 120
Europe 67.3 69.6 29 79
Asia/ Pacific 53.7 58.8 24 —
Latin American 17.9 18.9 8 —
Africa/ Middle East 10.0
9.6 4 —
Total 233.8 238.3 100 320 est.
*In millions of units.
Source: From Amistadl.
4
An important aspect of lead is that it is a recoverable resource. It has been estimated that
more than 95% of the batteries sold in the United States are ultimately recycled, and it takes
considerably less energy to recycle lead, a low-melting metal (mp 327.4
⬚C), than to produce
the metals used in other storage battery systems (nickel, iron, zinc, silver, and cadmium) in
battery-grade quality.
23.2 CHEMISTRY
23.2.1 General Characteristics
The lead-acid battery uses lead dioxide as the active material of the positive electrode and
metallic lead, in a high-surface-area porous structure, as the negative active material. The
physical and chemical properties of these materials are listed in Table 23.5.
5
Typically, a
charged positive electrode contains both
␣
-PbO
2
(orthorhombic) and

-PbO
2
(tetragonal).
The equilibrium potential of the
␣
-PbO
2
is more positive than that of

-PbO
2
by 0.01 V.
The
␣ form also has a larger, more compact crystal morphology which is less active elec-
trochemically and slightly lower in capacity per unit weight; it does, however, promote longer
cycle life. Neither of the two forms is fully stoichiometric. Their composition can be rep-
resented by PbO
x
, with x varying between 1.85 and 2.05. The introduction of antimony, even
at low concentrations, in the preparation or cycling of these species leads to a considerable
increase in their performance. The preparation of the active material precursor consists of a
series of mixing and curing operations using leady lead oxide (PbO
⫹ Pb), sulfuric acid,
and water. The ratios of the reactants and curing conditions (temperature, humidity, and
time) affect the development of crystallinity and pore structure. The cured plate consists of
lead sulfate, lead oxide, and some residual lead (
⬍5%). The positive active material, which
is formed electrochemically from the cured plate, is a major factor influencing the perform-
ance and life of the lead-acid battery. In general the negative, or lead, electrode controls
cold-temperature performance (such as engine starting).
The electrolyte is a sulfuric acid solution, typically about 1.28 specific gravity or 37%
acid by weight in a fully charged condition.

LEAD-ACID BATTERIES 23.7
TABLE 23.5 Physical and Chemical Properties of Lead and Lead Oxides (PbO
2
)
Property Lead
␣
-PbO
2

-PbO
2
Molecular weight, g/ mol 207.2 239.19 239.19
Composition PbO:
1.94–2.03
PbO:
1.87–2.03
Crystalline form Face-centered
cubic
Rhombic (columbite) Tetragonal (rutile)
Lattice parameters, nm a
⫽ 0.4949 a ⫽ 0.4977
b
⫽ 0.5948
c
⫽ 0.5444
a
⫽ 0.491–0.497
c
⫽ 0.337–0.340
X-ray density, g /cm
3
11.34 9.80 ⬃9.80
Practical density at 20
⬚C
(depends on purity), g /cm
3
11.34 9.1–9.4 9.1–9.4
Heat capacity, cal /deg
mol 6.80 14.87 14.87
Specific heat, cal/ g 0.0306 0.062 0.062
Electrical resistivity, at 20
⬚C,
⍀/cm
20
⬃100 ⫻ 10
3
Electrochemical potential in
4.4M H
2
SO
4
at 31.8⬚C, V
0.356
⬃1.709 ⬃1.692
Melting point,
⬚C 327.4
Source: Ref. 5 and others.
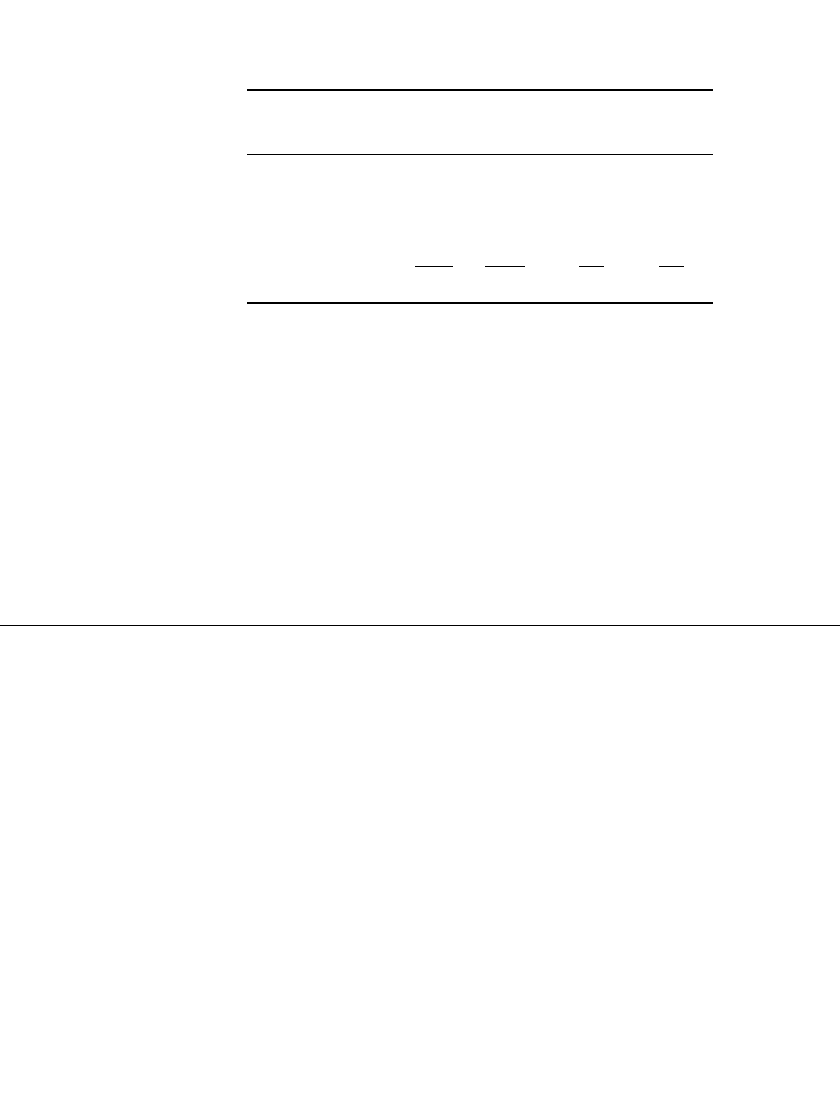
As the cell discharges, both electrodes are converted to lead sulfate. The process reverses
on charge:
discharge
2
⫹
—— —
Pb Pb ⫹ 2eNegative electrode
———
charge
discharge
2
⫹
2
⫺
—— —
Pb ⫹ SO PbSO
———
4
4
charge
discharge
⫹
2
⫹
—— —
PbO ⫹ 4H ⫹ 2e Pb ⫹ 2H OPositive electrode
———
2
2
charge
discharge
2
⫹
2
⫺
—— —
Pb ⫹ SO PbSO
———
4
4
charge
discharge
—— —
Pb ⫹ PbO ⫹ 2H SO 2PbSO ⫹ 2H OOverall reaction
———
224
42
charge
As shown, the basic electrode processes in the positive and negative electrodes involve a
dissolution-precipitation mechanism and not a solid-state ion transport or film formation
mechanism.
5
The discharge-charge mechanism, known as double-sulfate reaction, is also
shown graphically in Fig. 23.1.
6
As the sulfuric acid in the electrolyte is consumed during
discharge, producing water, the electrolyte is an ‘‘active’’ material and in certain battery
designs can be the capacity-limiting material.
As the cell approaches full charge and the majority of the PbSO
4
has been converted to
Pb or PbO
2
, the cell voltage on charge becomes greater than the gassing voltage (about 2.39
V per cell) and the overcharge reactions begin, resulting in the production of hydrogen and
oxygen (gassing) and the resultant loss of water.
23.8 CHAPTER TWENTY-THREE
FIGURE 23.1 Discharge and charge reactions of lead-acid cell. (a) Discharge reac-
tions. (b) Charge reactions. (From Mantell.
6
)
⫹
2H ⫹ 2e → HNegative electrode
2
⫹
1
HO⫺ 2e → ⁄
2
O ⫹ 2HPositive electrode
22
1
HO→ H ⫹ ⁄
2
OOverall reaction
222
In sealed lead-acid cells this reaction is controlled to minimize hydrogen evolution and the
loss of water by recombination of the evolved oxygen with the negative plate (see Sec. 24.2).
The general performance characteristics of the lead-acid cell, during charge and discharge,
are shown in Fig. 23.2. As the cell is discharged, the voltage decreases due to depletion of
material, internal resistance losses, and polarization. If the discharge current is constant, the
voltage under load decreases smoothly to the cutoff voltage and the specific gravity decreases
in proportion to the Ampere-hours discharged.
An analysis of the behavior of the positive and negative plates can be done by measuring
the voltage between each electrode and a reference electrode, the ‘‘half-cell’’ voltage. Figure
23.3 illustrates this analysis, using a cadmium reference electrode. The industry is, however,
shifting away from cadmium reference electrodes to more stable materials as discussed later
(Sec. 23.2.3).
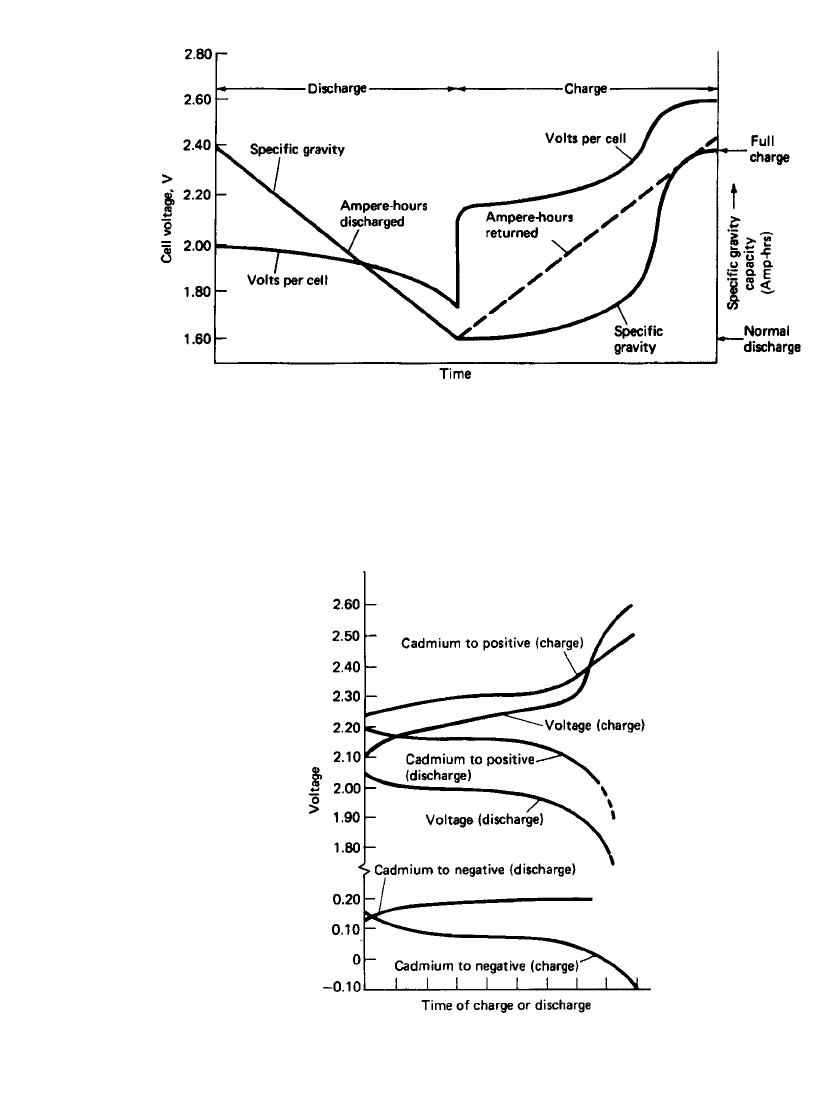
LEAD-ACID BATTERIES 23.9
FIGURE 23.2 Typical voltage and specific gravity characteristics of lead-acid cell at constant-
rate discharge and charge.
FIGURE 23.3 Typical charge-discharge curves of
lead-acid cell. (From Mantell.
6
)
23.10 CHAPTER TWENTY-THREE
Voltage. The nominal voltage of the lead-acid cell is 2 V; the voltage on open circuit is a
direct function of the electrolyte concentration, ranging from 2.125 V for a cell with 1.28
specific gravity electrolyte to 2.05 V with 1.21 specific gravity (see Sec. 23.2.2). The end
or cutoff voltage on moderate-rate discharges is 1.75 V per cell, but may range to as low as
1.0 V per cell at extremely high discharge rates at low temperatures.
Specific Gravity. The selection of specific gravity used for the electrolyte depends on the
application and service requirements (see Table 23.12). The electrolyte concentration must
be high enough for good ionic conductivity and to fulfill electrochemical requirements, but
not so high as to cause separator deterioration or corrosion of other parts of the cell, which
would shorten life and increase self-discharge. The electrolyte concentration is deliberately
reduced in high-temperature climates. During discharge, the specific gravity decreases in
proportion to the Ampere-hours discharged (Table 23.6). The specific gravity is thus a means
for checking the state of charge of the battery. On charge, the change in specific gravity
should similarly be proportional to the Ampere-hour charge accepted by the cell. However,
there is a lag because complete mixing of the electrolyte does not occur until gassing com-
mences near the end of the charge.
TABLE 23.6 Specific Gravity of Lead-Acid Battery
Electrolytes at Different States of Charge for Various
Designs.*
State of charge
Specific gravity
ABCD
100% (full charge) 1.330 1.280 1.265 1.225
75% 1.300 1.250 1.225 1.185
50% 1.270 1.220 1.190 1.150
25% 1.240 1.190 1.155 1.115
Discharged 1.210 1.160 1.120 1.0
Assumes flooded cell design.
*Specific gravity may range from 100 to 150 points between full
charge and discharge depending on cell design: A—electric vehicle
battery; B—traction battery; C—SLI battery; D—stationary battery.
23.2.2 Open-Circuit Voltage Characteristics
The open-circuit voltage for a battery system is a function of temperature and electrolyte
concentration as expressed in the Nernst equation for the lead-acid cell (see also Chap. 2).
RT
␣
HSO
24
E ⫽ 2.047 ⫹ ln
冉冊
F
␣
HO
2
Since the concentration of the electrolyte varies, the relative activities of H
2
SO
4
and H
2
O
in the Nernst equation change. A graph of the open-circuit voltage versus electrolyte con-
centration at 25
⬚C is given in Fig. 23.4. The plot is fairly linear above 1.10 specific gravity,
but shows strong deviations at lower concentrations. The open-circuit voltage is also affected
by temperature. The temperature coefficient of the open-circuit voltage of the lead-acid bat-
tery is shown in Fig. 23.5. Where dE/dT is positive, such as above 0.5 Molar H
2
SO
4
, the
reversible potential of the system increases with increasing temperature. Below 0.5 M, the
temperature coefficient is negative. Most lead-acid batteries operate above 2 Molar H
2
SO
4
(1.120 specific gravity) and have a thermal coefficient of about ⫹0.2 mV/⬚C.
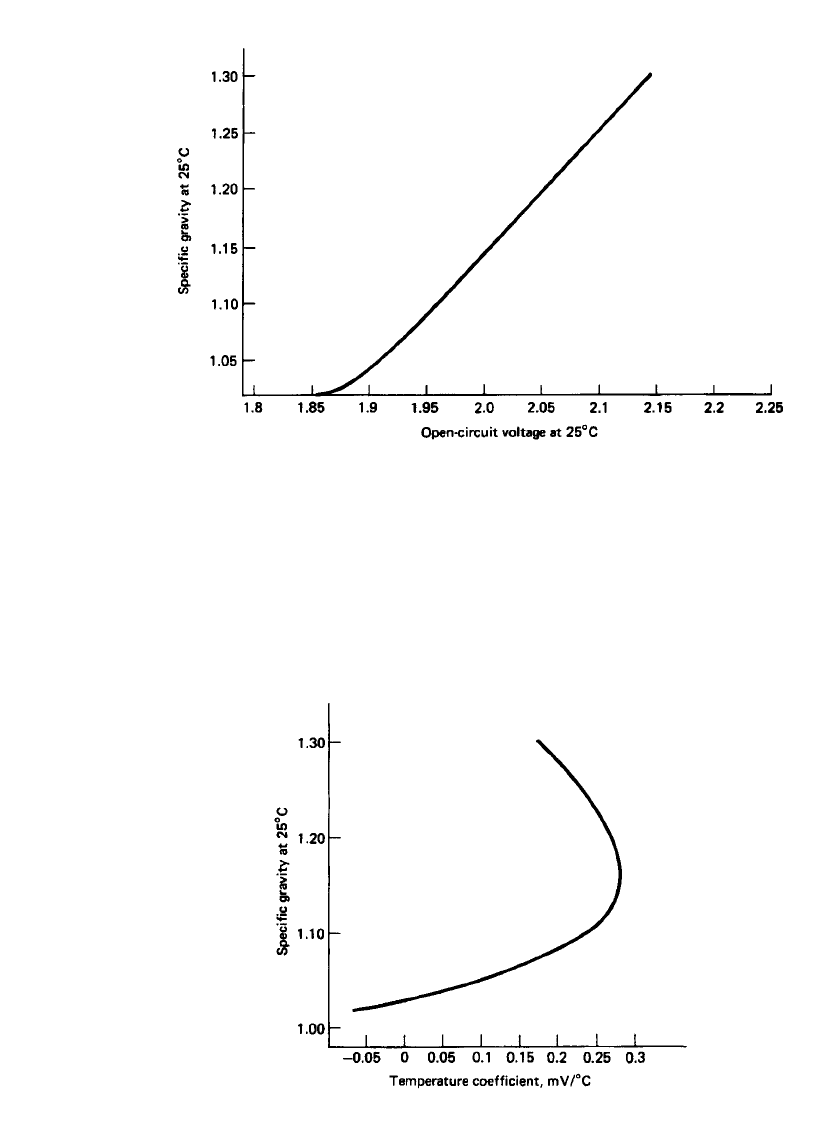
LEAD-ACID BATTERIES 23.11
FIGURE 23.4 Open-circuit voltage of lead-acid cell as a function of electrolyte specific
gravity.
FIGURE 23.5 Temperature coefficient of open-circuit voltage
of lead-acid cell as a function of electrolyte specific gravity.

23.12 CHAPTER TWENTY-THREE
23.2.3 Polarization and Resistive Losses
When a battery is being discharged, the voltage under load is lower than the open-circuit
voltage at the same concentrations of H
2
SO
4
and H
2
O in the electrolyte and Pb or PbO
2
and
PbSO
4
in the plates. The thermodynamically stable state for batteries is the discharged state.
Work (charging) must be done to cause the equilibria of the electrochemical reactions to go
toward PbO
2
in the positive and Pb in the negative. Thus the voltage of the power source
for recharging the lead-acid battery must be higher than the Nernst voltage of the battery on
open circuit.
These deviations from the open-circuit voltage during charge or discharge are due, in
part, to resistive losses in the battery and, in part, to polarization. These losses can be
measured by use of an interrupted discharge, where the IR losses can be estimated by Ohm’s
law (
⌬E/ ⌬I ⫽ R) within a few seconds to a few minutes after the discharge is stopped. The
effect of polarization can take several hours to measure in order for diffusion to allow the
plate interiors to reequilibrate. AC impedance spectroscopy techniques are also of value.
Polarization is more easily measured by use of a reference electrode. The standard reference
of hydrogen on platinum is not practical for most measurements on lead-acid batteries, and
several other sulfate-based reference electrode systems are used. A review of reference elec-
trodes
7
neglects several very practical sulfate electrodes. Still, a commonly used electrode
for battery maintenance is the cadmium ‘‘stick,’’ but it is not especially stable (
20 mV/
day). Mercury-mercurous sulfate reference electrodes are stable and are available from sev-
eral vendors. A novel Pb/ H
2
SO
4
/PbO
2
reference electrode has been patented.
8
This electrode
measures the polarization on charge or discharge directly, without need for a correction of
different thermal coefficients of EMF. The change in polarization between the start and the
end of discharge is typically 50 to several hundred mV, and the cell capacity is limited by
the plate group (positive or negative) that has the largest change in polarization during
discharge. When both groups in a cell change about equally, the capacity limitation is more
likely depletion of H
2
SO
4
in the electrolyte than depletion of Pb or PbO
2
in the plates. On
charge, the polarization is a good measure that both positives and negatives have been re-
charged: the plate polarizations change by more than 60 mV between start and end of
recharge. Polarization voltages stabilize at some value when plates are recharged and are
gassing freely.
23.2.4 Self-Discharge
The equilibria of the electrode reactions are normally in the discharge direction since, ther-
modynamically, the discharged state is most stable. The rate of self-discharge [loss of ca-
pacity (charge) when no external load is applied] of the lead-acid cell is fairly rapid, but it
can be reduced significantly by incorporating certain design features.
The rate of self-discharge depends on several factors. Lead and lead dioxide are ther-
modynamically unstable in sulfuric acid solutions, and on open circuit, they react with the
electrolyte. Oxygen is evolved at the positive electrode and hydrogen at the negative, at a
rate dependent on temperature and acid concentration (the gassing rate increases with in-
creasing acid concentration) as follows:
1
PbO ⫹ HSO → PbSO ⫹ HO⫹ ⁄
2
O
224 42 2
Pb ⫹ HSO → PbSO ⫹ H
24 4 2
For most positives, the formation of PbSO
4
by self-discharge is slow, typically much less
than 0.5%/day at 25
⬚C. (Some positives which have been made with nonantimonial grids
can fail by a different mechanism on open circuit, namely, the development of a grid-active
material barrier layer.) The self-discharge of the negative is generally more rapid, especially
if the cell is contaminated with various catalytic metallic ions. For example, antimony lost
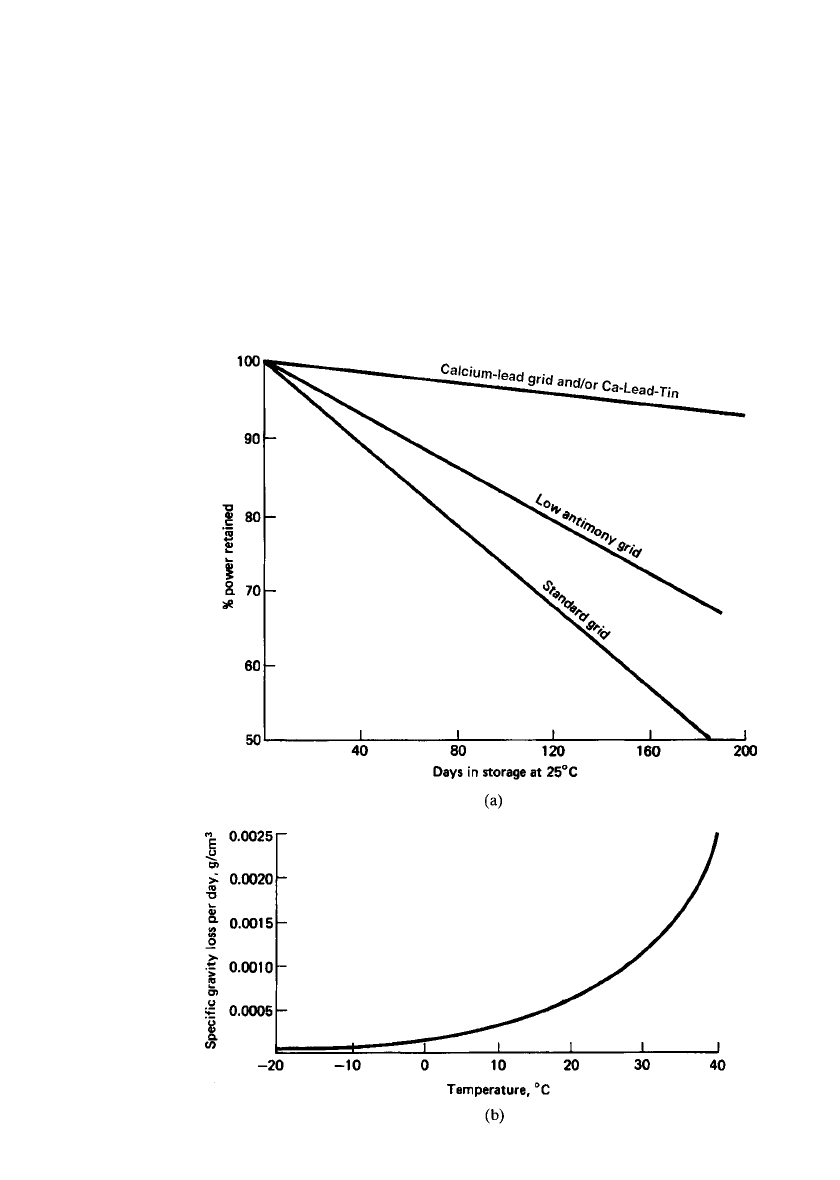
LEAD-ACID BATTERIES 23.13
FIGURE 23.6 (a) Capacity retention during stand or storage at 25⬚C. (From Sa-
batino.
9
)(b) Loss of specific gravity per day with temperature of a new, fully charged
lead-acid battery with 6% antimonial lead grids. (From Ref. 10.)
from the positive grids by corrosion can diffuse to the negative, where it is deposited, re-
sulting in a ‘‘local action’’ discharge cell which converts some lead active material to PbSO
4
.
New batteries with lead-antimony grids lose about 1% of charge per day at 25
⬚C, but the
charge loss increases by a factor of 2 to 5 as the battery ages. Batteries with nonantimonial
lead grids lose less than 0.5% of charge per day regardless of age. This is illustrated in Fig.
23.6a.
9
Maintenance-free and charge-retention-type batteries, where the self-discharge rate
must be minimized, use low-antimony or antimony-free alloy (such as calcium-lead) grids.
However, because of other beneficial effects of antimony, its complete elimination may not
be desirable, and low-antimony–lead alloys are a useful compromise.
Self-discharge is temperature-dependent, as shown in Fig. 23.6b.
10
The graph shows the
fall in specific gravity per day of a new fully charged battery with 6% antimonial lead grids.
Self-discharge can thus be minimized by storing batteries at temperatures between 5 and
15
⬚C.
23.14 CHAPTER TWENTY-THREE
TABLE 23.7 Properties of Sulfuric Acid Solutions*
Specific
gravity
At
15
⬚C
At
25⬚C
Temperature
coeff.
␣
H
2
SO
4
Wt., % Vol., % Mol /L
Freezing
point,
⬚C
Elecro-
chemical
equivalent
(per liter of
acid), Ah
1.00 1.000 — 0 0 0 0 0
1.05 1.049 33 7.3 4.2 0.82
⫺3.3 22
1.10 1.097 48 14.3 8.5 1.65
⫺7.7 44
1.15 1.146 60 20.9 13.0 2.51
⫺15 67
1.20 1.196 68 27.2 17.7 3.39
⫺27 90
1.25 1.245 72 33.2 22.6 4.31
⫺52 115
1.30 1.295 75 39.1 27.6 5.26
⫺70 141
1.35 1.345 77 44.7 32.8 6.23
⫺49 167
1.40 1.395 79 50.0 38.0 7.21
⫺36
1.45 1.445 82 55.0 43.3 8.2
⫺29
1.50 1.495 85 59.7 48.7 9.2
⫺29
*To calculate the specific gravity for any temperature, ⬚C, SG (t) ⫽ SG (15⬚C) ⫹
␣
⫻ 10
⫺
5
(15 ⫺ t).
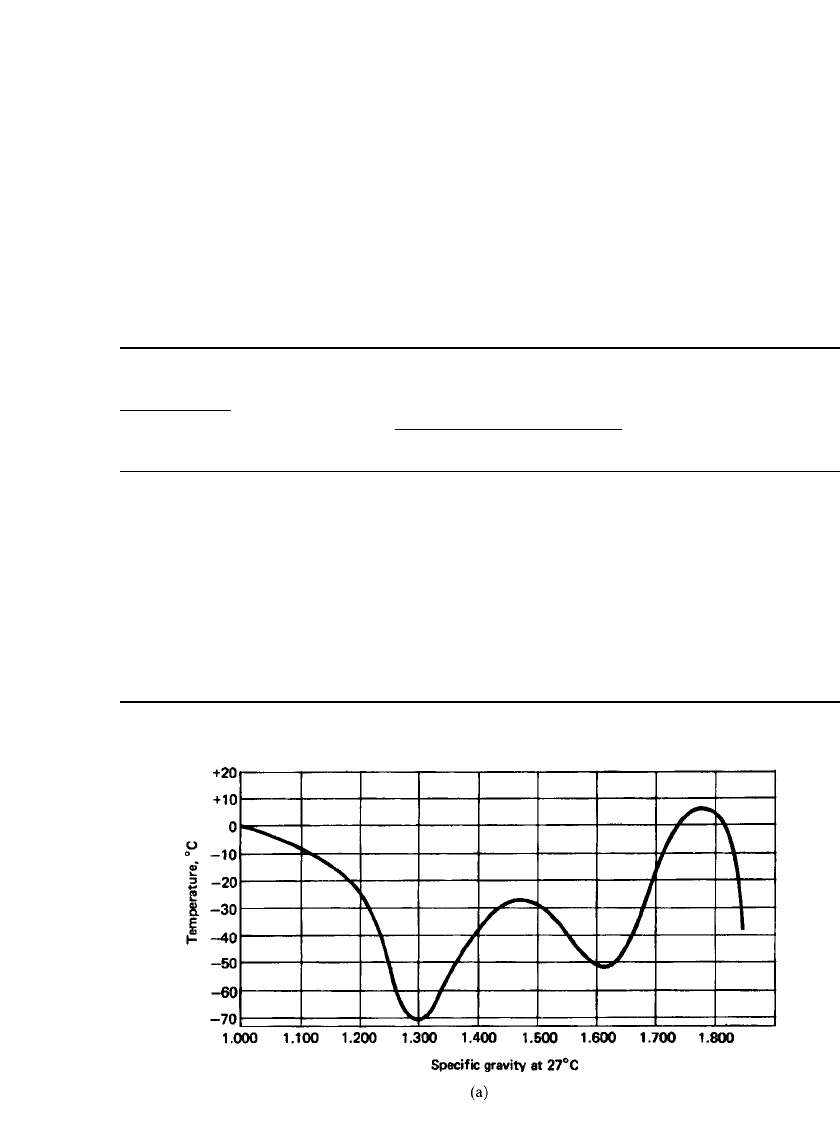
FIGURE 23.7a Freezing points of sulfuric acid solutions at various specific gravities.
The inflection points result from the different water to SO
3
hydration ratios.
23.2.5 Characteristics and Properties of Sulfuric Acid
The major characteristics and properties of the sulfuric acid electrolyte, as they apply to the
operation of the lead-acid battery, are listed in Table 23.7. The freezing points of sulfuric
acid solutions at various concentrations are also plotted in Fig. 23.7a. The freezing point of
aqueous sulfuric acid solutions varies significantly with concentration. Batteries must there-
fore be designed so that the electrolyte concentration is above the value at which the elec-
trolyte would freeze when exposed to the anticipated cold. Alternatively, the battery can be
insulated or heated so that it remains above the electrolyte freezing temperature.
Figure 23.7b shows the specific resistivity of sulfuric acid solutions at various specific
gravities as a function of temperature from
⫺40 to 40⬚C.
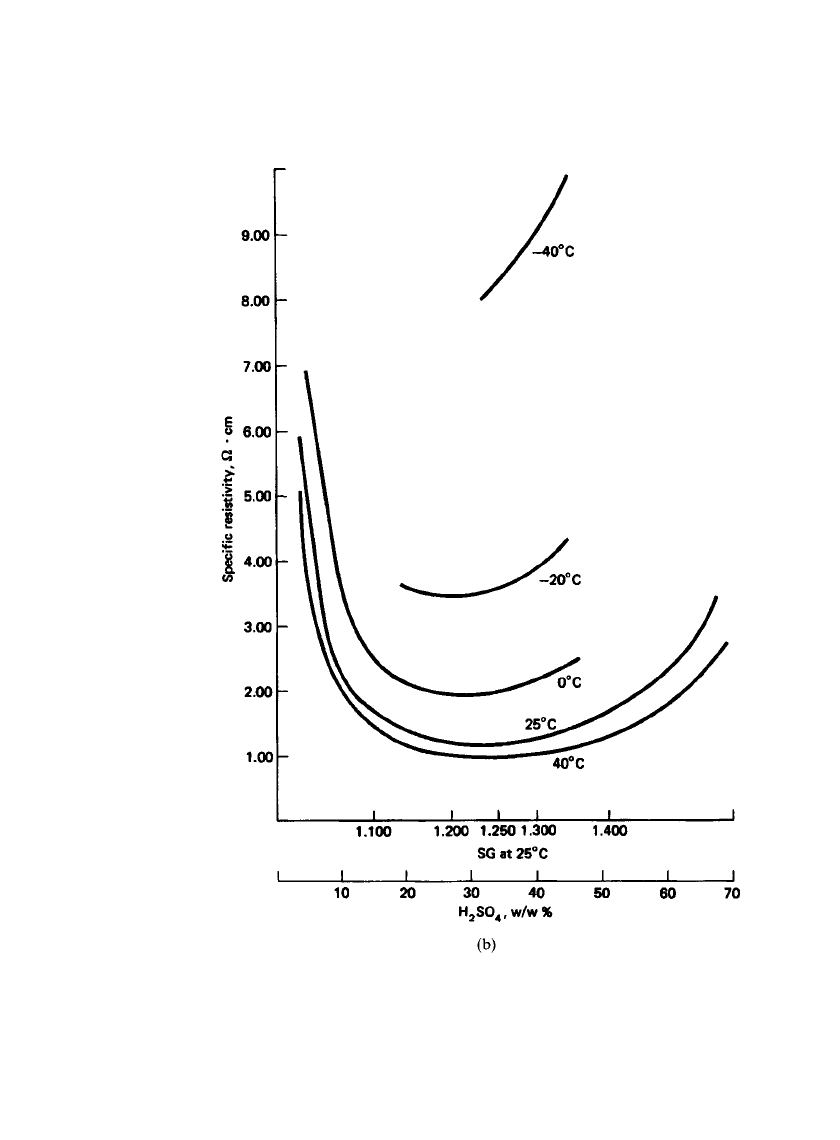
LEAD-ACID BATTERIES 23.15
FIGURE 23.7b Specific resistivity of sulfuric acid solutions at various specific
gravities and temperatures.
