Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

23.16 CHAPTER TWENTY-THREE
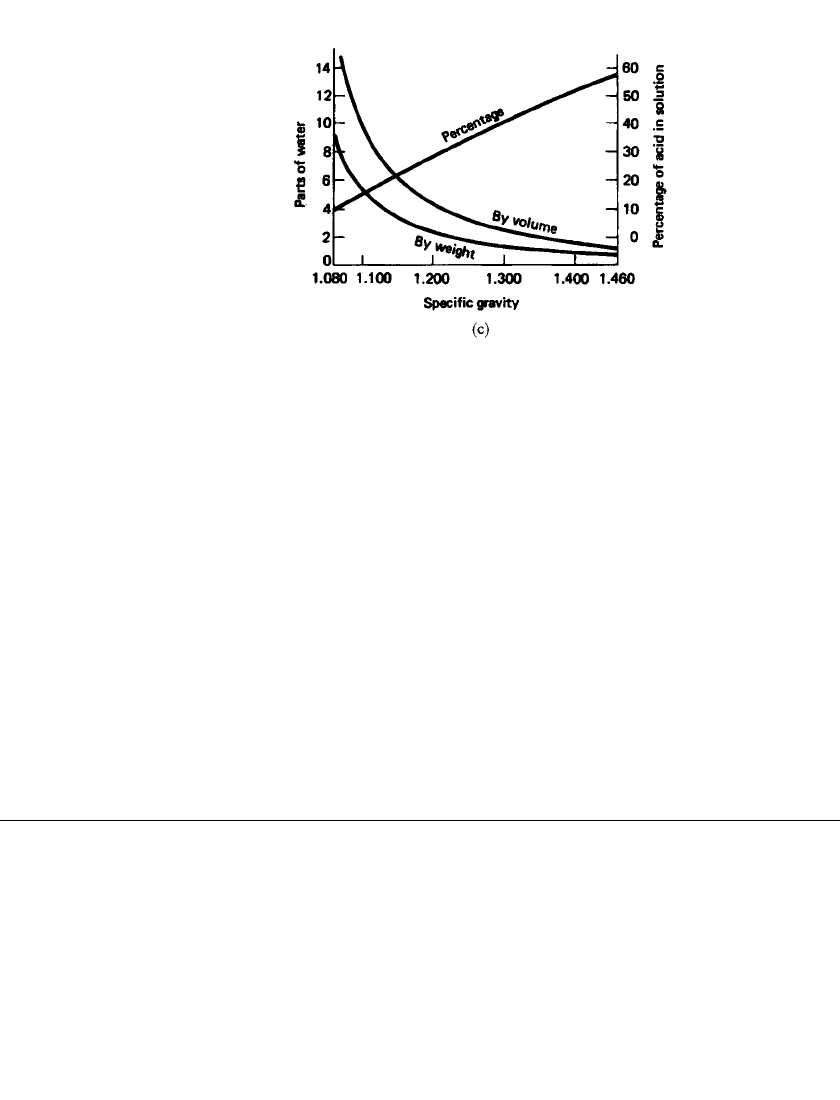
FIGURE 23.7c Preparation of sulfuric acid solutions of
any specific gravity from concentrated sulfuric acid.
(From G. W. Vinal, Storage Batteries, Wiley, New York,
1955, p. 129.)
The specific gravities for several types of lead-acid battery designs are given in Table
23.12; the change in specific gravity at different states of charge is shown in Table 23.6. A
comparison with freezing-point data will show that battery type A will freeze at
⫺30⬚C when
fully discharged while battery type D will freeze at about
⫺5⬚C, a factor which must be
considered in the design of the battery and the battery housing. The acid concentration for
most lead-acid batteries for use in temperate climates is usually between 1.26 and 1.28
specific gravity. Higher-concentration electrolytes tend to attack some separators and other
components; lower concentrations tend to be insufficiently conductive in a partially charged
cell and freeze at low temperatures. In high-temperature climates, a lower concentration is
used, and in stationary cells with larger proportional electrolyte volumes and no high-rate
demands, electrolytes with specific gravity as low as 1.21 are used (see Table 23.12).
Figure 23.7c indicates the method of preparing sulfuric acid solutions of any specific
gravity from concentrated sulfuric acid.
23.3 CONSTRUCTIONAL FEATURES, MATERIALS, AND
MANUFACTURING METHODS
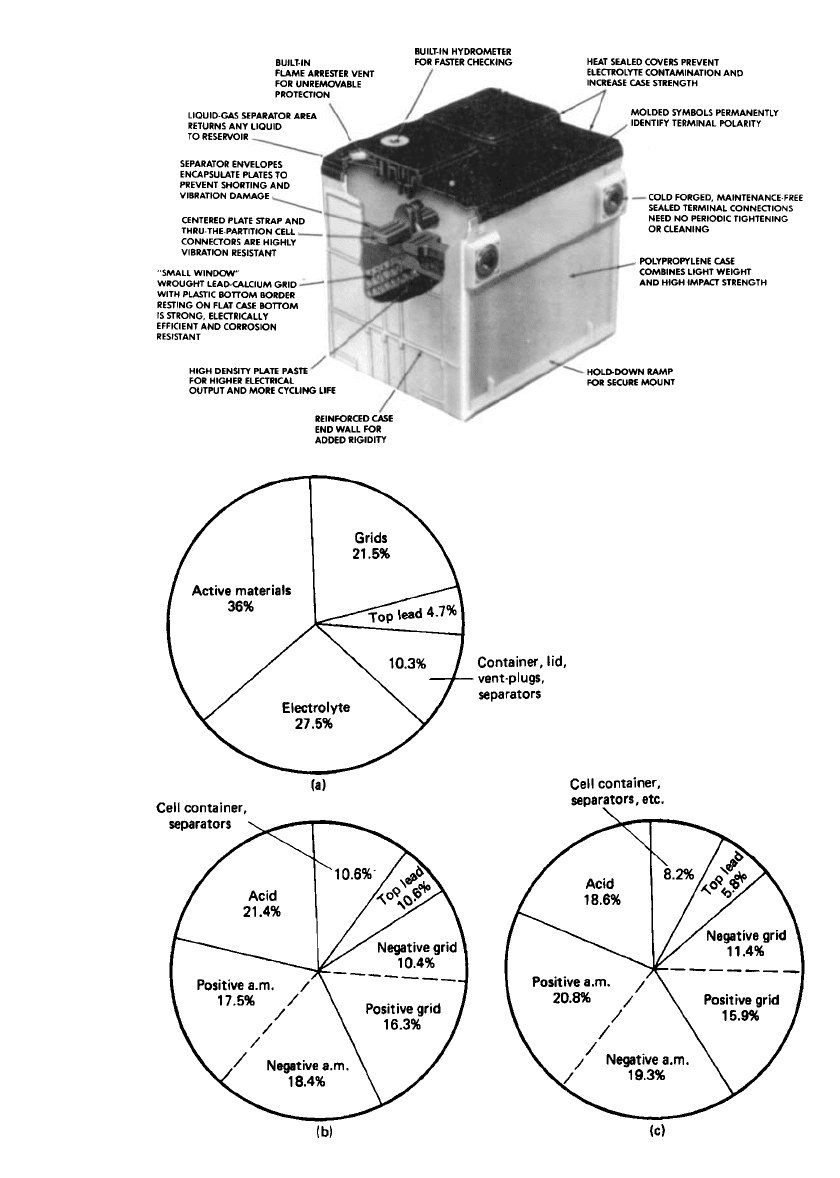
Lead-acid batteries consist of several major components, as shown in a cutaway view of Fig.
23.8. This figure shows the construction of an automotive SLI battery. Batteries for other
applications have analogous components, as illustrated and described in Secs. 23.4–23.6.
The applications of the various cells and batteries dictate the design, size, quantities, and
types of materials that are used.
The active components of a typical lead-acid battery constitute less than one-half of its
total weight. A breakout of the weights of the components of several types of lead-acid
batteries is shown in Fig. 23.9.
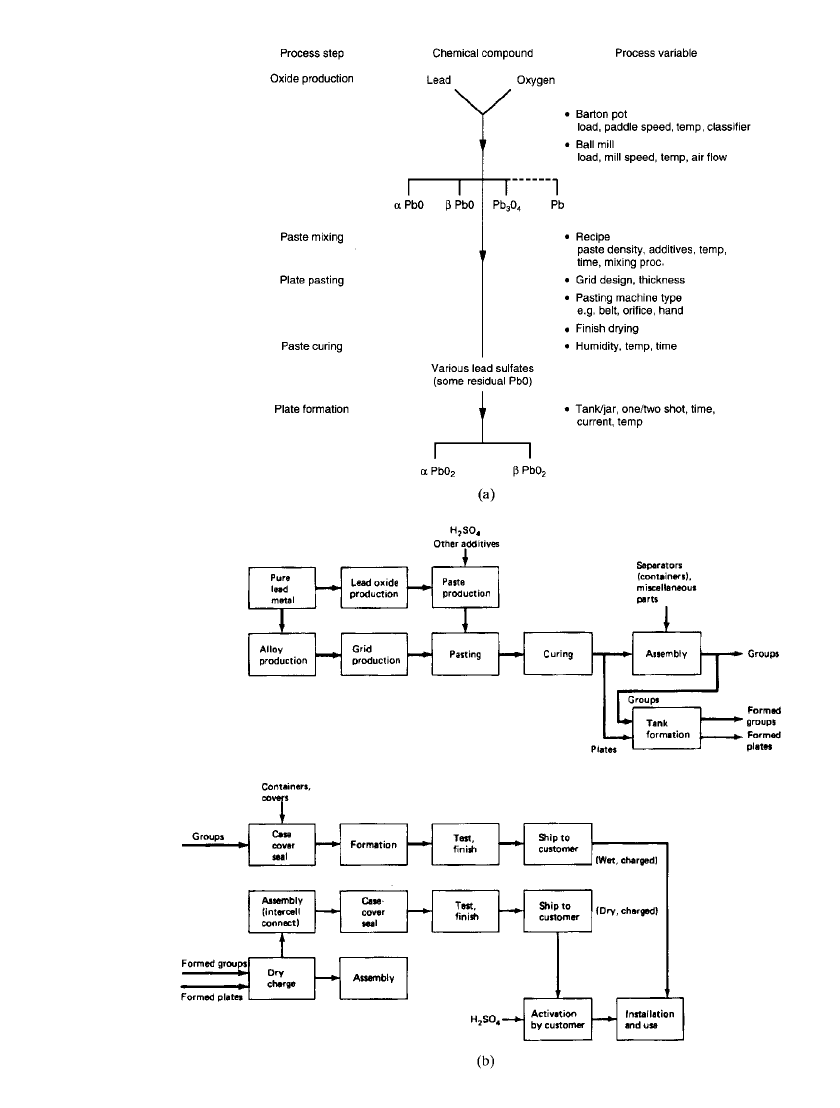
The battery components are fabricated and processed as shown in the flowsheets of Fig.
23.10. The major starting material is highly purified lead.
11
The lead is used for the produc-
tion of alloys (for subsequent conversion to grids) and for the production of lead oxides [for
subsequent conversion first to paste and ultimately to the lead dioxide positive active material
(Fig. 23.10a) and the sponge lead negative active material].
LEAD-ACID BATTERIES 23.17
FIGURE 23.8 Maintenance-free lead-acid SLI battery. (Courtesy of Delphi Energy Systems.)
FIGURE 23.9 Weight analysis of typical lead-acid batteries. (a) SLI battery. (b) Tubular industrial
battery. (c) Flat-plate traction battery. (From Ref. 10.)
23.18 CHAPTER TWENTY-THREE
FIGURE 23.10 (a) Chemical compounds and process parameters in production of SLI
batteries. (b) Production flow sheet for lead-acid batteries.

LEAD-ACID BATTERIES 23.19
Automotive lead-acid batteries (SLI) are produced mainly in high-volume plants with a
great deal of automation. Many modern factories are capable of producing quantities on the
order of 20,000 to 30,000 batteries per day. On average, an automated facility might require
less than 500 employees, including all staffing levels. The automation has been prompted
by environmental, reliability, and cost considerations.
23.3.1 Alloy Production
Pure lead is generally too soft to be used as a grid material. Exceptions that use pure lead
plates are some special, very thick plate Plante´ or pasted-plate batteries, some small spiral
wound batteries, some valve regulated cells and batteries (see Fig. 23.12c) and a cylindrical
cell. The latter were developed by Bell Laboratories (now part of Lucent Technologies) (see
Fig. 23.36).
12
The pure lead has been hardened, traditionally, by the addition of antimony metal. The
amount of antimony has varied between 5 and 12% by weight, generally dependent on the
availability and cost of antimony. Typical modern alloys, especially for deep-cycling appli-
cations, contain 4 to 6% antimony. The trend in grid alloys is to go to even lower antimony
contents, in the range of 1.5 to 2% antimony, in order to reduce the maintenance (water
addition) that the battery will require. As the antimony content goes below 4%, the addition
of small amounts of other elements is necessary to prevent grid fabrication defects and grid
brittleness. These elements, such as sulfur, copper, arsenic, selenium, tellurium and various
combinations of these elements act as grain refiners to decrease the lead grain size.
13–15
Some of the alloying elements, not previously described as grain refiners, fall into two
broad classes of elements that are beneficial or detrimental to grid production or battery
performance. Beneficial elements include tin, which operates synergistically with antimony
and arsenic to improve metal fluidity and castability. Silver and cobalt are also thought to
improve corrosion resistance. Detrimental elements include iron, which increases drossing;
1
nickel, which affects battery operation; and manganese, which attacks paper separators. Cad-
mium has been used in grid alloys to enhance processability in antimonial alloys to minimize
the detrimental effects of antimony. Cadmium, however, is not popular because of its toxicity
and difficulty of removal during lead recovery (recycling) operations. Bismuth exists in many
lead ore feedstocks and has been reported to both increase and decrease grid corrosion rates.
A second class of lead alloys has been developed which uses calcium or other alkaline
earth elements for stiffening. These alloys were developed originally for telephone service
applications.
2,16
Antimony from the grids is dissolved during battery operation and migrates
to the negative plates where it redeposits, which results in increased hydrogen evolution and
water loss. For telephone applications, more stable battery operation and less frequent wa-
tering were desired. The composition of the alloy depends somewhat on the grid manufac-
turing process. Calcium is used in the range of 0.03 to 0.20% but for corrosion resistance
the preferred range is 0.03 to 0.05%. A variation has been to substitute strontium for calcium.
Barium has been investigated but is generally felt to be detrimental to performance. Tin has
been used to enhance the mechanical and corrosion-resistant properties of the Pb-Ca alloys
and is usually used in the range of 0.25 to 2.0% by weight. The trend in nonantimonial
alloy development is toward ternary alloys (Pb-Ca-Sn) containing a minimal amount of tin
because of the expense of this element. Some batteries are produced with a quaternary
alloy—the fourth element being aluminum—to stabilize the drossing loss of the alkaline
earth element (calcium or strontium) from the molten alloy. Grain refining is done by the
alkaline earth metal, and no other elements (impurities) are desired. The properties of the
alloys are summarized in Table 23.8.
13
23.20
TABLE 23.8 Properties of Lead Alloys
Alloys of the 1970s
Property
Conventional
antimony
Low
antimony
Cast
lead-calcium-tin
0.1Ca
0.3Sn
0.1Ca
0.7Sn
Lead-
strontium
tin-
aluminum
Lead-
cadmium-
antimony
Wrought-lead-
calcium-tin
0.065Ca
0.7Sn
Ultimate tensile
strength, Pa
⫻ 10
⫺
6
38–46 33–40 40–43 47–50 53 33–40 60
Percent elongation 20–25 10–15 25–35 20–30 15 25 10–15
Property
Cast
conventional
antimony
Cast
low-
antimony
Cast lead-
calcium
Cast lead-
strontium
Cast
lead-cadmium
antimony
Wrought
lead-calcium-tin
(1st generation)
Ease of grid manufacture Good Fair Fair Fair to good Fair Good
Mechanical Good Fair Fair to good Fair to good Fair Good
Corrosion Fair Fair Good Good Fair Good
Battery performance Poor Fair Good Good Good Fair to good
Economics Good Good Fair Poor Fair Fair to good
23.21
Alloys of the 1980s and 1990s
Property
Cast alloys
Lead-
calcium-tin
0.1Ca
0.3Sn
Lead-
calcium
0.1Ca
Lead-
calcium-tin
with
aluminum
Lead-
calcium
with
aluminum
Wrought alloys
Lead-
calcium-tin
0.065Ca
0.3Sn
Lead-
calcium-tin
0.065Ca
0.5Sn
Lead-
calcium
0.075Ca
Low
antimony
2.5–3.0%
Sb
Cast and
wrought
Lead
0.01–1.5Sn
Ultimate tensile
strength, Pa
⫻ 10
⫺
6
40–43 37–39 40–43 37–39 43–47 47 43 37–40
Percent
elongation
25–35 30–45 25–35 30–45 15 15 25 25–40
Property
Cast alloys
Low
antimony Lead-calcium
Wrought alloys
Wrought
lead-calcium-tin
(2d generation)
Wrought
low antimony
Ease of grid manufacture Fair to good Good (aluminum) Good Good Conductivity
Mechanical Fair Fair to good Good Good and corrosion-
Corrosion Fair Good Good Fair to good equivalent to
Battery performance Fair to good Good Fair to good Fair pure lead
Economics Good Good (lower tin) Good Good
NOTE: Alloy constituents given in weight percent.

23.22 CHAPTER TWENTY-THREE
23.3.2 Grid Production
Two general classes of grid production methods virtually describe all modern production,
but two other classes of production techniques might become widespread in the future. These
are listed in Table 23.9.
TABLE 23.9 Grid Production Methods
Book mold cast
Gravity cast
Injection molded (die cast)
Mechanically worked (Plante´, Manchester)
Continuous cast, drum cast
Continuous cast, wrought-expanded, cast-expended
Casting
Working
Expansion
Progressive die expansion
Precision expanded
Rotary expanded
Rotary expansion
Diagonal/ slit expansion
Punching
Composite
The purposes of the grid are to hold the active material mechanically and conduct elec-
tricity between the active material and the cell terminals. The mechanical support can be
provided by nonmetallic materials (polymer, ceramic, rubber, etc.) inside the plate, but these
are not electrically conductive. Additional mechanical support is sometimes gained by the
construction method or by various wrappings on the outside of the plate. Metals other than
lead alloys have been investigated to provide electrical conductivity, and some (copper, alu-
minum, silver) are more conductive than lead. These alternate conductors are not corrosion-
resistant in the sulfuric acid electrolyte and are often more expensive than lead alloys. Ti-
tanium has been evaluated as a grid material; it is not corroded after special surface
treatments but is very expensive. Copper grids are used in the negatives of some submarine
batteries.
The grid design is generally a rectangular framework with a tab or lug for connection to
the post strap. For cast grids, the framework features a heavy external frame and a lighter
internal structure of horizontal and vertical bars. In some grid designs the frame tapers with
the greater width near the lug; the internal bars may also be tapered. A recent advance in
grid design is the ‘‘radial’’ grid, with the vertical wires displaced along the frame, pointing
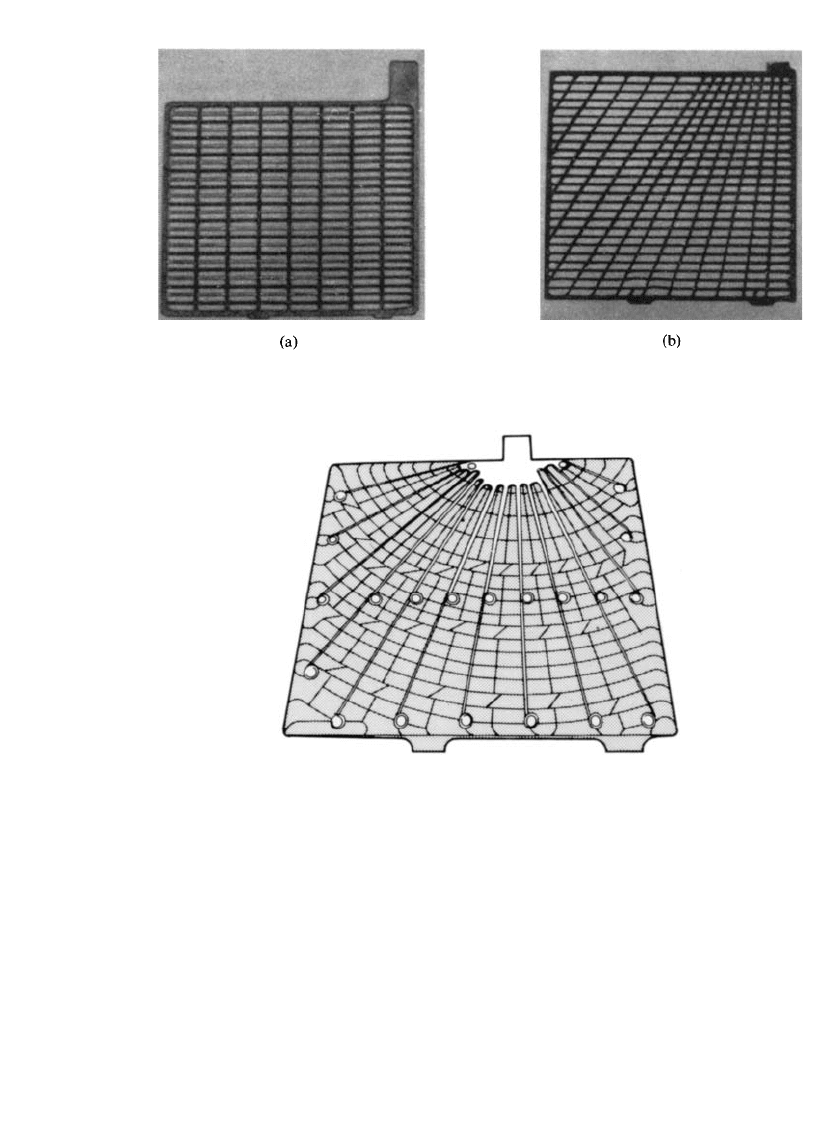
directly toward the tab area in order to increase grid conductivity (Fig. 23.11). The radial
design has been further refined to a composite of lead alloy radial conductor arrangement
cast into a rectangular plastic frame. An example of this composite grid is shown in Fig.
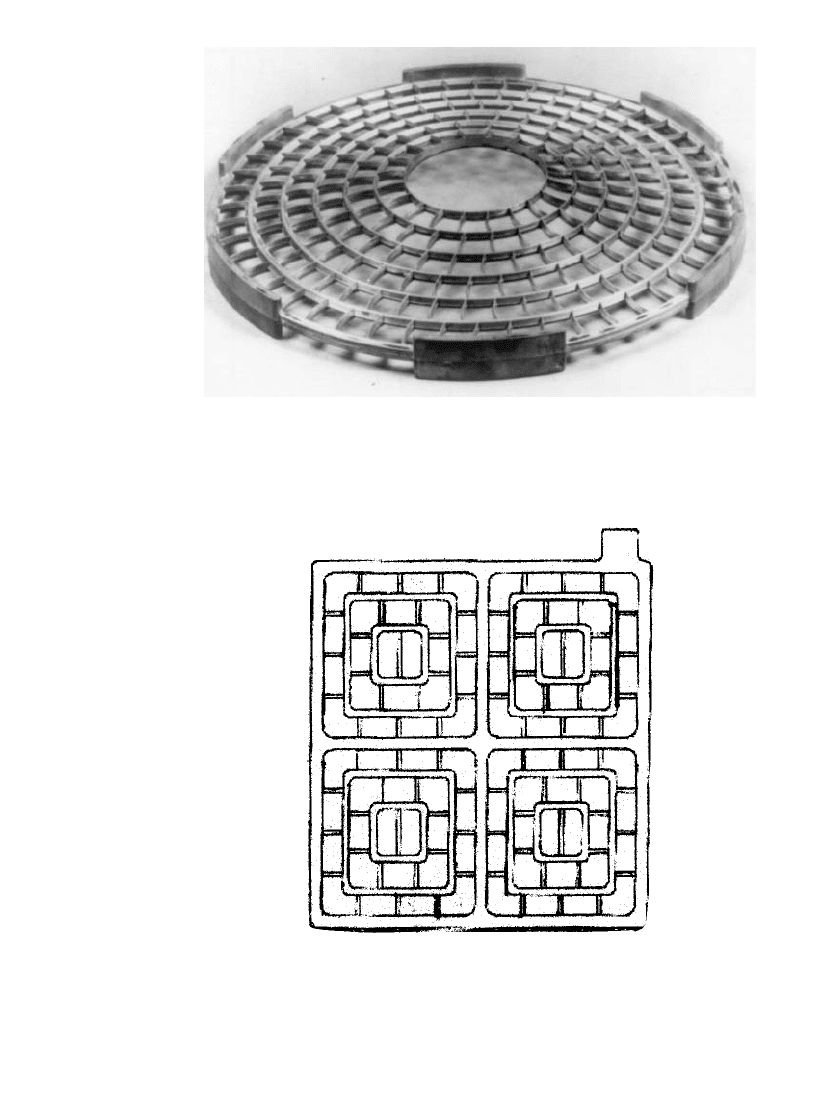
23.12a. The grids used in the cylindrical-cell design (Fig. 23.12b) incorporate both concentric
and radial members. This system has been in commercial production since 1970, with most
of the original cells still in use. An example of a balanced positive grid design is shown in
Fig. 23.12c.
LEAD-ACID BATTERIES 23.23
FIGURE 23.11 Examples of lead-acid battery cast grids. (a) Conventional cast flat grid. (b)
Radial-design grid.
FIGURE 23.12a Composite grid, radial conductor. Grid
combines diagonal conducting members with light robust
plastic frame.
‘‘Book-mold’’ casting historically accounted for most grid production. Permanent molds
are made from steel (Meehanite) blocks by machining grooves to form the grid frames and
internal lattice structure. The molds are filled when closed with an amount of lead sufficient
to form the grid and leave an excess gate or sprue which is subsequently trimmed off by a
cutting or stamping operation. The grid alloy is put into the mold from a ladle in a recir-
culation lead alloy stream, from a metering valve in a nonrecirculation lead stream, or from
a hand-filled ladle. A variation on book-mold casting is injection molding or die casting of
battery grids. Here the lead alloy is forced into a clamped mold by high injection pressure.
Depending on the alloy characteristics, injection molding can be capable of very high pro-
duction rates.
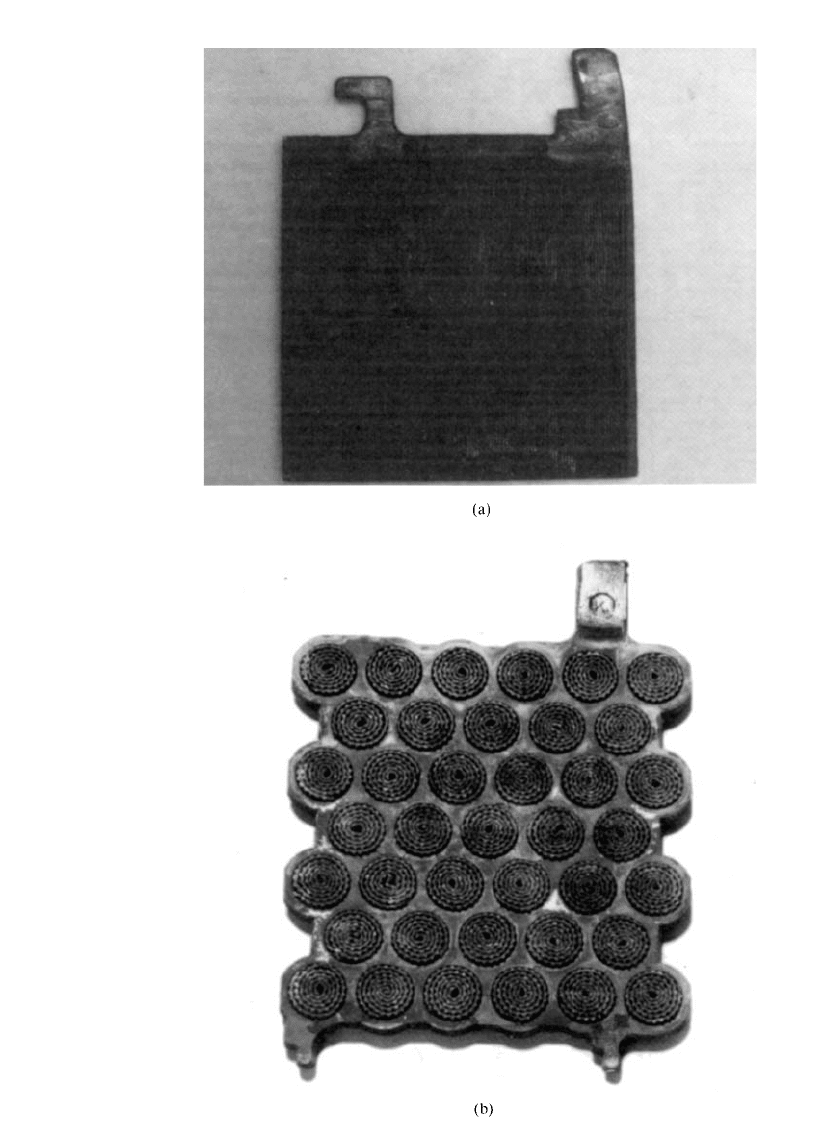
Another method of grid manufacture is via mechanical treatment of a strip or slab of lead
alloy. The traditional procedures (Plante´-type plate) have been either to cut grooves into a
thick lead plate, thereby increasing its surface area, or to crimp and roll up lead strips into
rosettes which are inserted into round holes in a cast plate. The resultant plates are formed
electrolytically into positives in the classic Plante´ and Manchester designs (Fig. 23.13).
23.24 CHAPTER TWENTY-THREE
FIGURE 23.12b Balanced positive design
27
takes into account grid corrosion and
growth and promotes the maintenance of contact of the grid with the active material,
while maintaining the shape of the plate and its angle with the horizontal. This
concept has also been carried into the prismatic grid structure. (Courtesy of AT&T.)
FIGURE 23.12c Balanced rectangular positive grid
design. This design promotes active material contact
and accounts for grid corrosion and growth in a pris-
matic cell. (From Ref. 36.)
LEAD-ACID BATTERIES 23.25
FIGURE 23.13 Plante´ and Manchester plates. (a) Plante´. (b) Manchester.
