Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

23.56 CHAPTER TWENTY-THREE
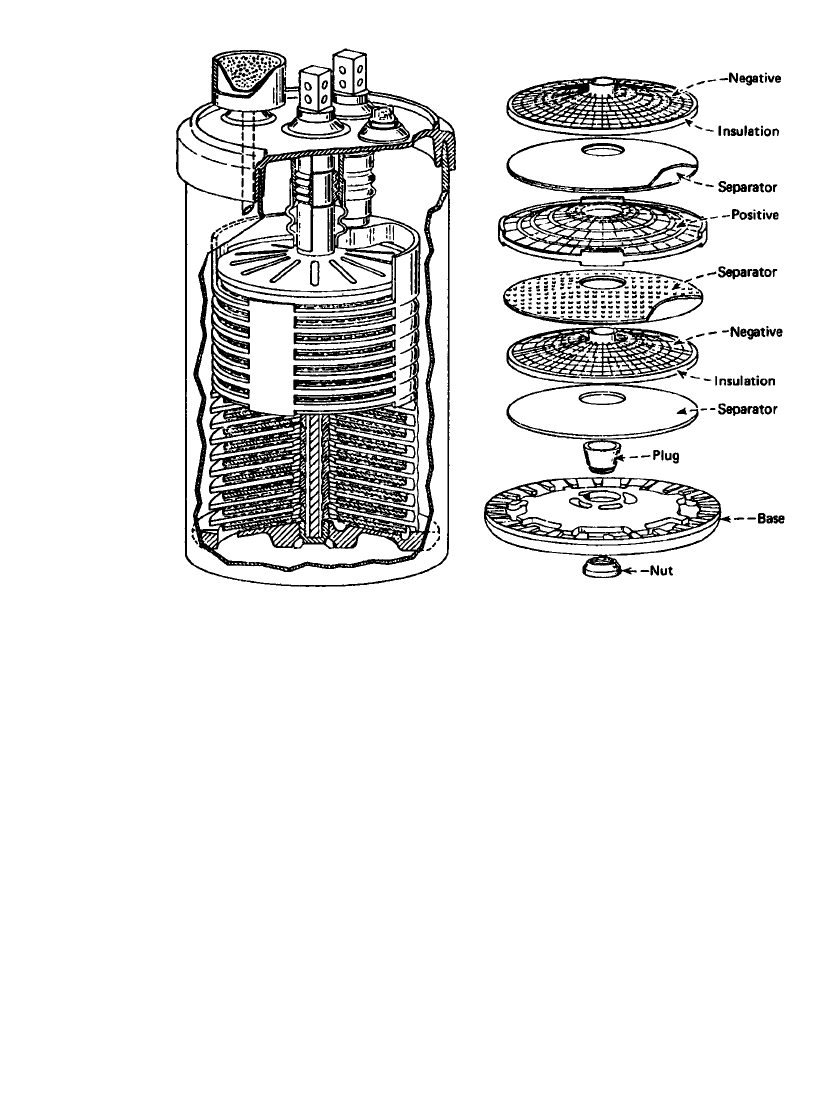
FIGURE 23.36 Cutaway and exploded views of Bell System lead-acid battery. (From Ref.
25.)
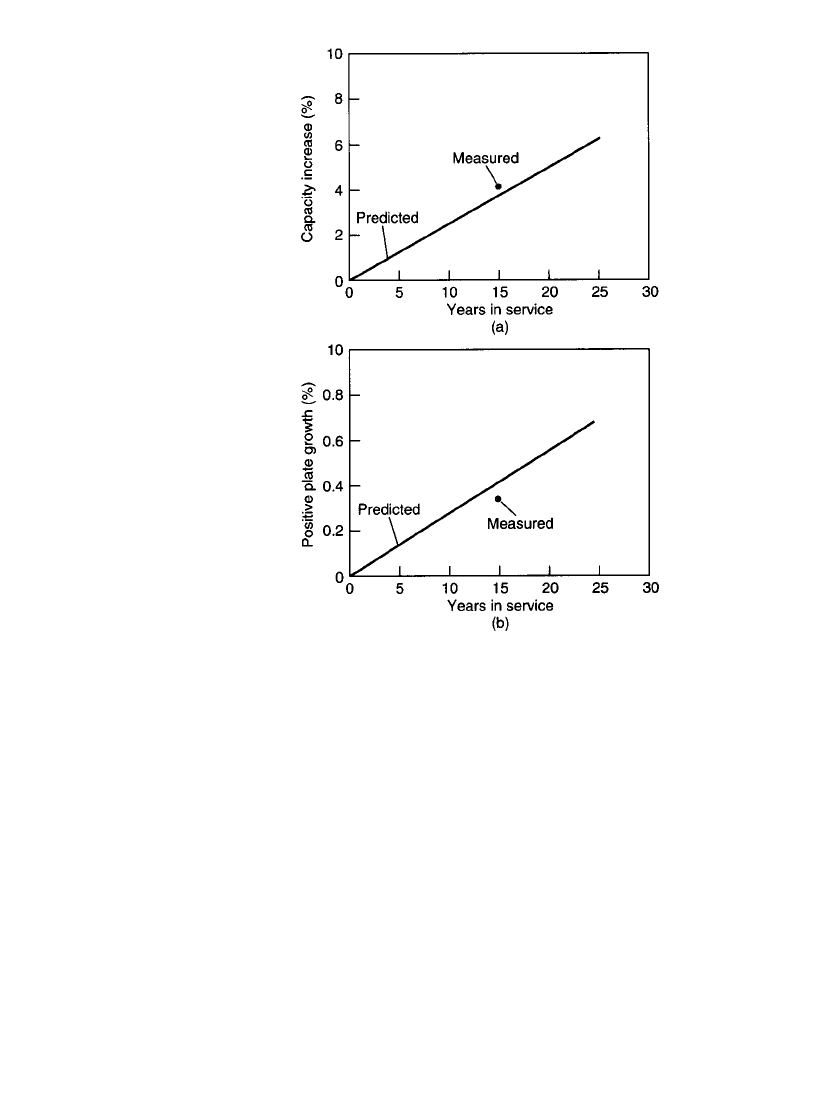
Accelerated tests projected a capacity increase to accompany the grid growth. In 1988
verification tests were conducted at a site which had cells manufactured in 1973, that is, 15
years earlier. A 24-V string, selected at random out of 11 strings, was discharged at the same
5-h rate used at the time they were manufactured. The capacity behavior on aging is com-
pared to the predicted capacity behavior at 22
⬚C, which was the average yearly temperature
of this particular location (Fig. 23.37a). The plate growth of the 54 plates measured is
summarized in Fig. 23.37b, where the findings were compared to that predicted from ac-
celerated testing. The expected capacity and plate growth increases at 22
⬚C and 15 years
were 0.25% per year or 3.8% total capacity and 0.027% per year or 0.4% plate growth,
respectively. More recent 23-year corrosion data projects a life of 68 to 69 years for round
cell batteries.
Some stationary batteries are designed to cycle rather than ‘‘float.’’ For these applications
the design criteria of the traction batteries are applicable (see Sec. 23.5.1). Applications of
cycling stationary batteries include load leveling, utility peak-power shaving, and photovol-
taic energy-storage systems.
LEAD-ACID BATTERIES 23.57
FIGURE 23.37 (a) Capacity after 15 years in service
vs. prediction from accelerated testing. (From Bi-
agetti.
28
)(b) Positive-plate growth after 15 years in ser-
vice vs. prediction from accelerated testing.
23.6.2 Performance Characteristics
Batteries for stationary applications may use cells with flat-pasted, tubular, Plante´, or Man-
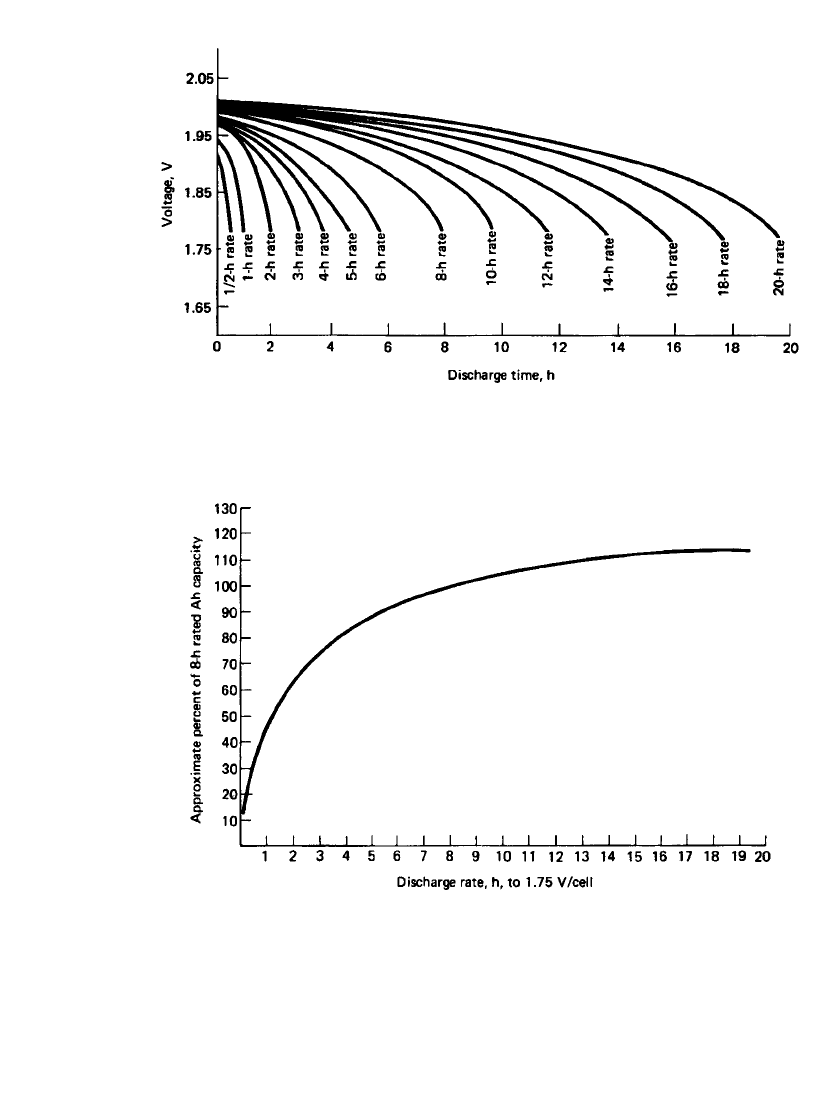
chester positive plates. Typical discharge curves for the flat-pasted-type stationary cell at
various discharge rates at 25
⬚C are shown in Fig. 23.38, and the effect of the discharge rate
on the capacity of the cell is summarized in Fig. 23.39. Generally, the discharge rate for
stationary cells is identified as the hourly rate (the current in Amperes that the battery will
deliver or the rate hours) rather than the C rate used for other types of batteries.
Figure 23.40(a)–(d) is a series of curves showing the specific performance characteristics
of the four types of stationary batteries at 25
⬚C based on positive-plate design. An electrolyte
with a specific gravity of 1.215 is used in all these batteries. The format used in these figures
consists of two sections. The lower log-log section shows the capacity (expressed in dis-
charge time) the particular positive plate will deliver at the specified current (expressed in
Amperes per positive plate) to various voltages including a final voltage. The upper semilog
section shows the cell voltage at various stages of the discharge at various discharge rates
(also expressed in Amperes per positive plate). The final voltage is the voltage at which the
cell can no longer supply useful energy.
23.58 CHAPTER TWENTY-THREE
FIGURE 23.38 Discharge curves of flat-pasted lead-acid stationary batteries (specific gravity
1.215) at various discharge rates at 25⬚C.
FIGURE 23.39 Effect of discharge rate on cell capacity at 25⬚C for flat-pasted lead-
acid stationary batteries (specific gravity 1.215) to 1.75-V end voltage.
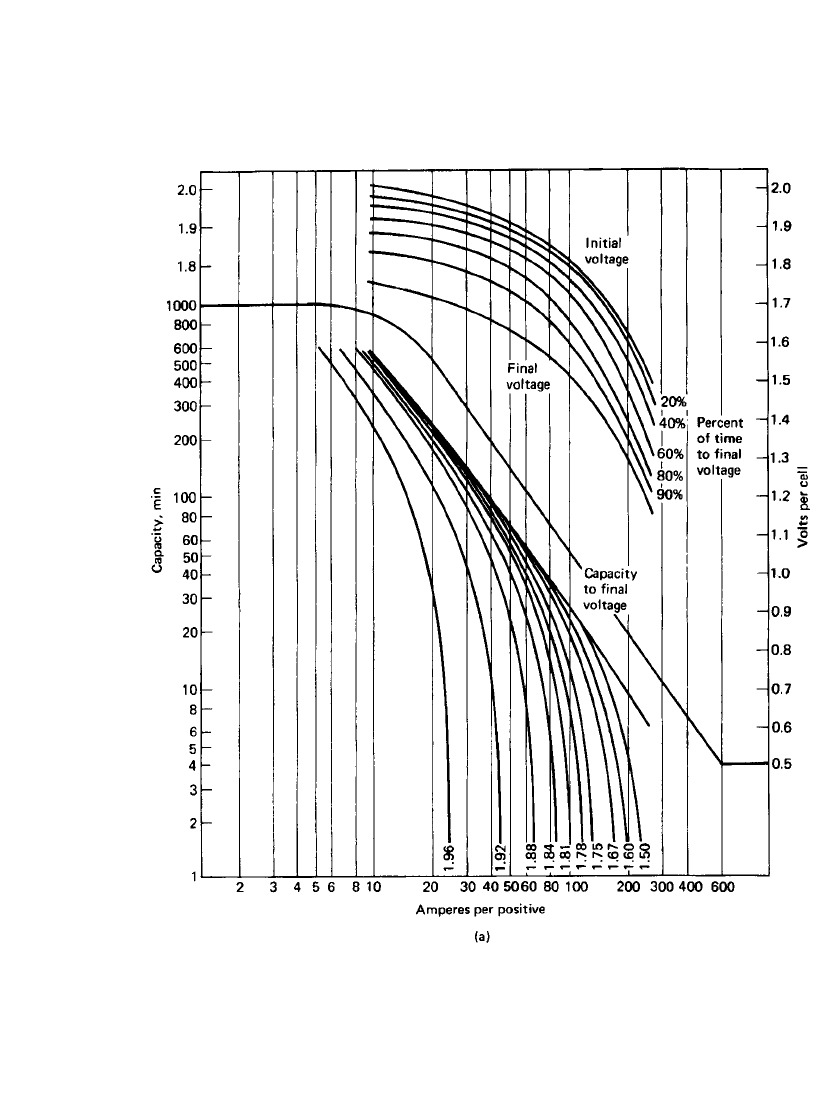
LEAD-ACID BATTERIES 23.59
FIGURE 23.40 Performance curves of lead-acid stationary batteries at 25⬚C (S-shaped curves,
based on positive-plate performance). (a) Antimony flat-pasted plate, 125 Ah at 8-h rate; 290-mm
height, 239-mm width, 8.6-mm thickness. (Courtesy of Enersys, Inc.)
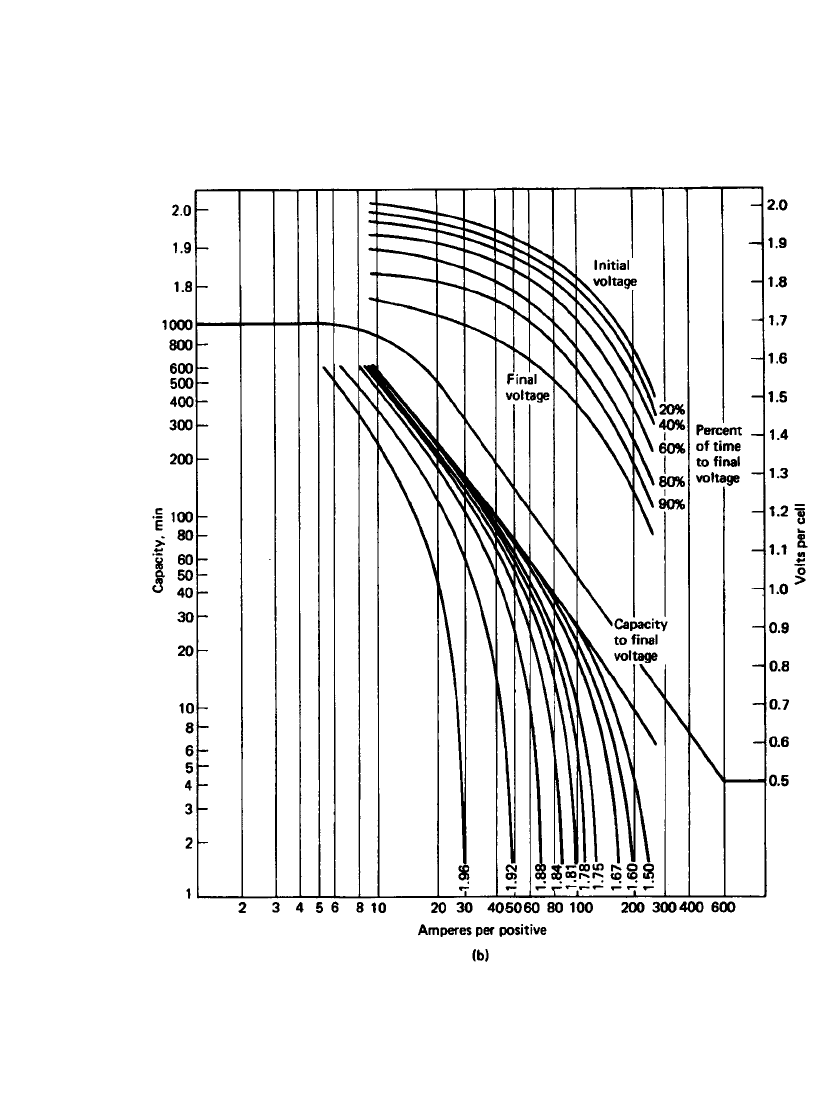
23.60 CHAPTER TWENTY-THREE
FIGURE 23.40 (b) Calcium flat-pasted plate, 125 Ah at 8-h rate; 290-mm height, 239-mm width,
8.6-mm thickness. (Courtesy of Enersys, Inc.)(Continued ).
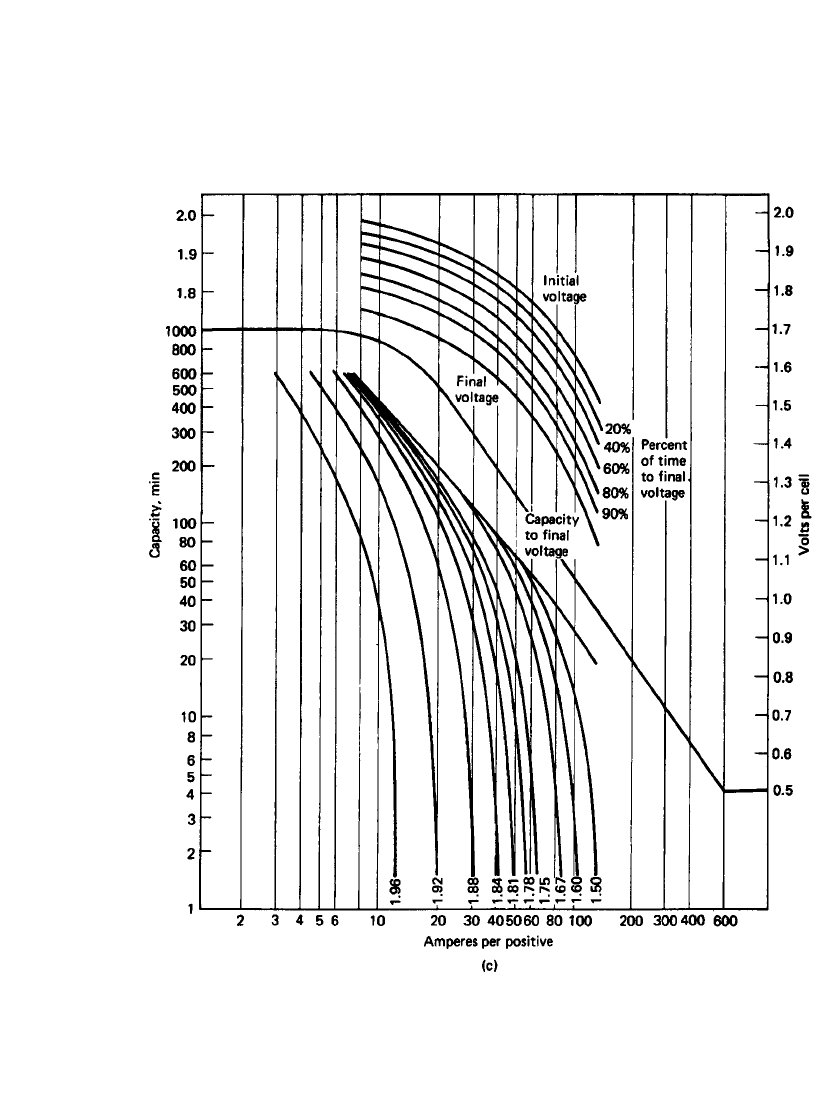
LEAD-ACID BATTERIES 23.61
FIGURE 23.40 (c) Ironclad tubular plate, 70 Ah at 8-h rate; 274-mm height, 203-mm width, 8.9-
mm thickness. (Courtesy of Enersys, Inc.)(Continued ).
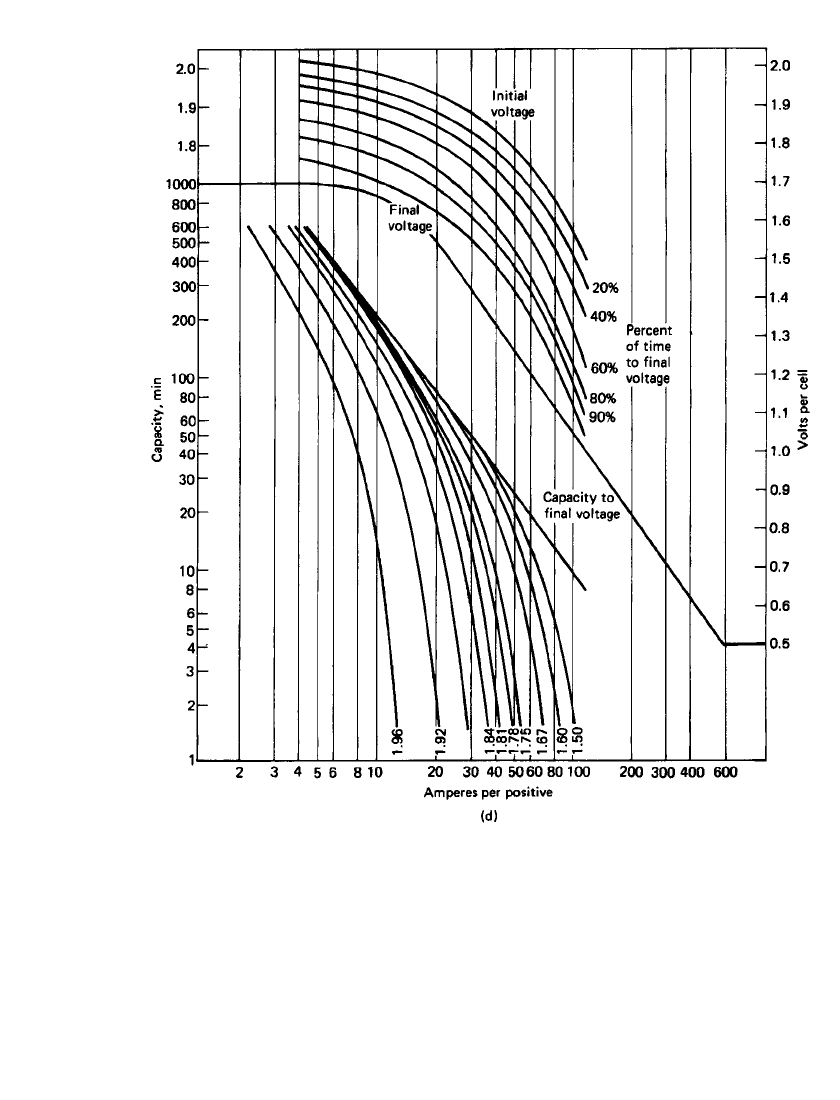
23.62 CHAPTER TWENTY-THREE
FIGURE 23.40 (d) Manchex plate, 40 Ah at 8-h rate; 197-mm height, 197-mm width, 11.2-mm
thickness. (Courtesy of Enersys, Inc.)(Continued ).
The energy density of the flat-pasted positive-plate and the tubular positive-plate batteries
is similar. It is lower for the Plante´ positive-plate batteries. The high-rate performance of the
flat-pasted positive cells is better because these plates can be made thinner than the tubular
or Plante´ plates.
The optimal temperature for the use of stationary batteries ranges from 20 to 30
⬚C, al-
though temperatures from
⫺40 to 50⬚C can be tolerated. The effect of temperature on the
capacity of stationary batteries at different discharge loads is shown in Fig. 23.41. High-
temperature operation, however, increases self-discharge, reduces cycle life, and causes other
adverse effects, as discussed in Sec. 23.8.1.
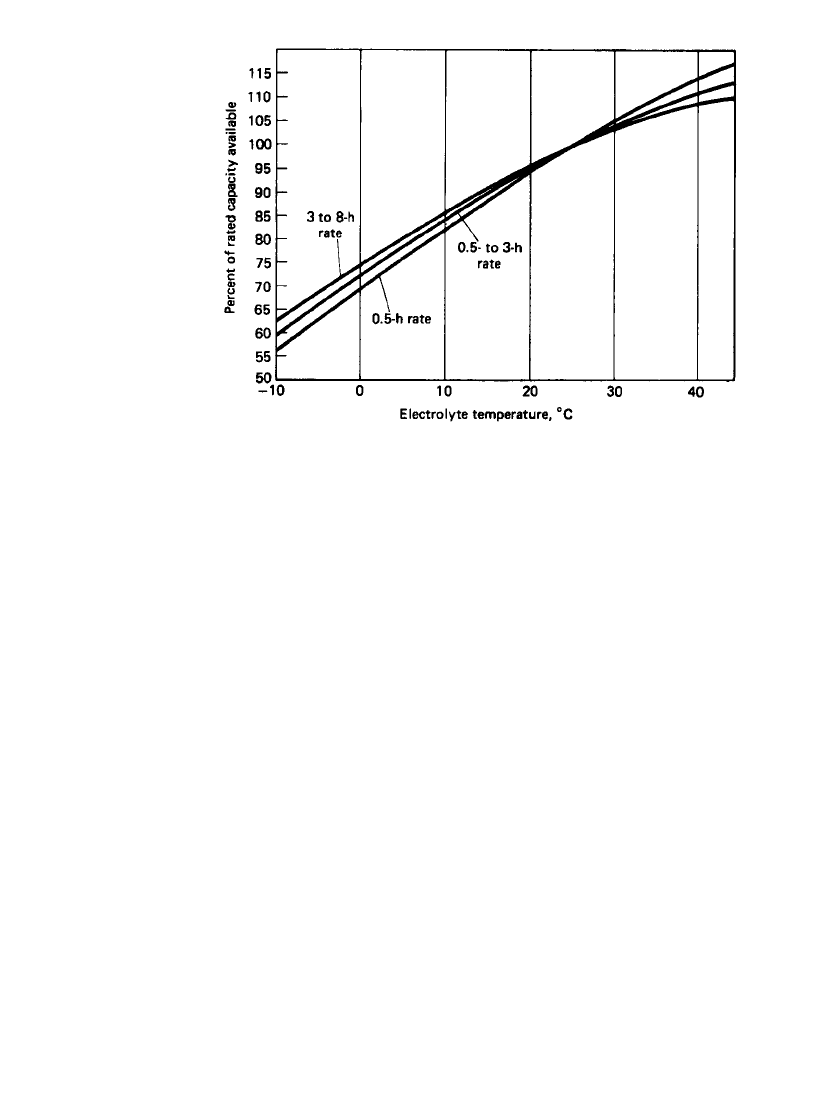
LEAD-ACID BATTERIES 23.63
FIGURE 23.41 Performance of flat-pasted lead-acid stationary batteries at
various temperatures and discharge rates. (Courtesy of C&D Technologies, Dy-
nasty Div.)
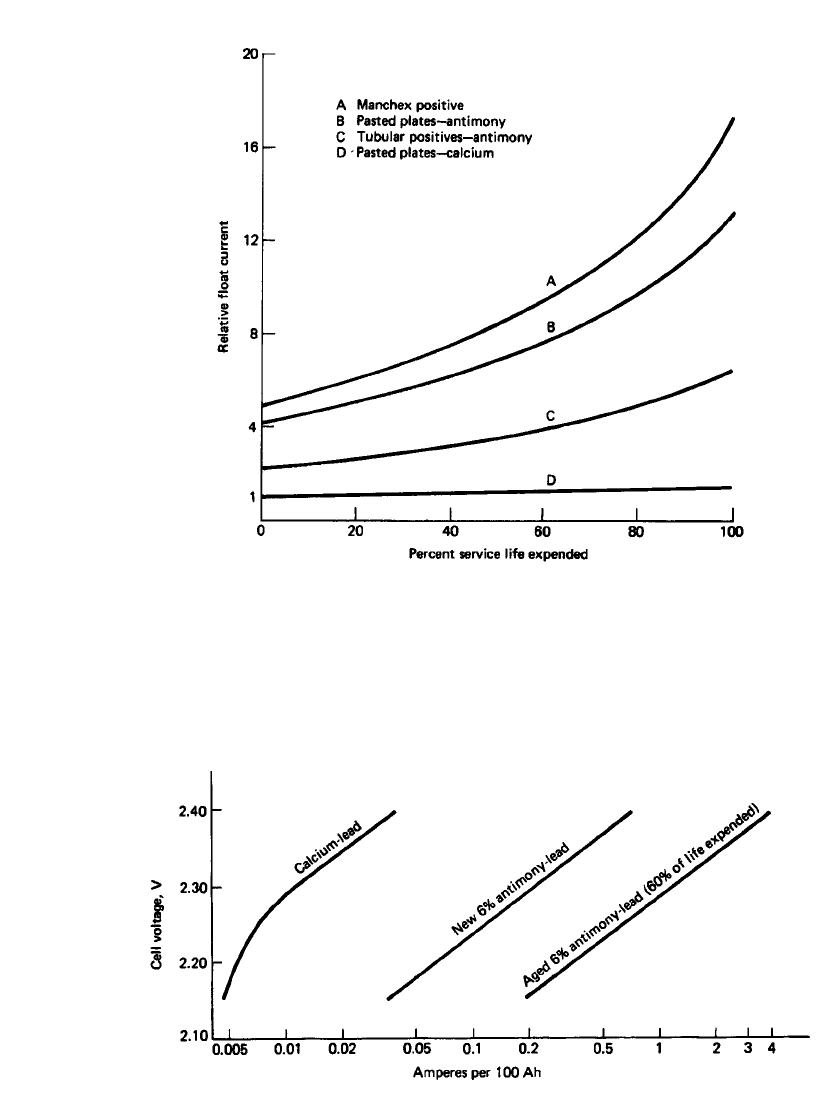
The rates of self-discharge of the various types of stationary batteries are compared in
Fig. 23.42, which shows the relative float current at a specified float voltage. The float current
under these conditions is a measure of self-discharge or local action. It is lowest for the
calcium-lead grid pasted positives and remains low throughout the life. The float current is
progressively higher for the tubular antimony-lead positives, the pasted antimony-lead pos-
itives, and the Manchester-type positives—at the beginning and throughout the battery’s life.
If the float current is not increased periodically, the antimonial cells will all become pro-
gressively self-discharged and sulfated.
For fully charged batteries, the self-discharge rate at 25
⬚C for the calcium-lead positive-
plate cells is about 1% per month, 3% for the Plante´, and about 7 to 15% for the antimonial
lead positive cells. At higher temperatures, the self-discharge rate increases significantly,
doubling for each 10
⬚C rise in temperature.
The float current for the calcium-lead and antimonial lead batteries is shown in Fig. 23.43
under float charge at voltages between 2.15 and 2.40 V per cell. It has been found that more
than 50 mV positive and negative overpotential is necessary to prevent self-discharge so that
0.005 A float current per 100 Ah of battery capacity is required for the lead-calcium batteries.
Antimonial lead batteries initially require at least 0.06 A per 100 Ah, but this increases to
0.6 A per 100 Ah as the battery ages. The higher float current also increases the rate of
water consumption and evolution of hydrogen gas.
Various, and at times conflicting, claims about the life of stationary battery designs are
made by the different manufacturers worldwide. Generally, the flat-pasted antimonial lead
batteries have the shortest life (5 to 18 years), followed by the flat-pasted calcium-lead
batteries (15 to 25 years), the tubular batteries (20 to 25 years), and the Plante´ batteries (25
years).
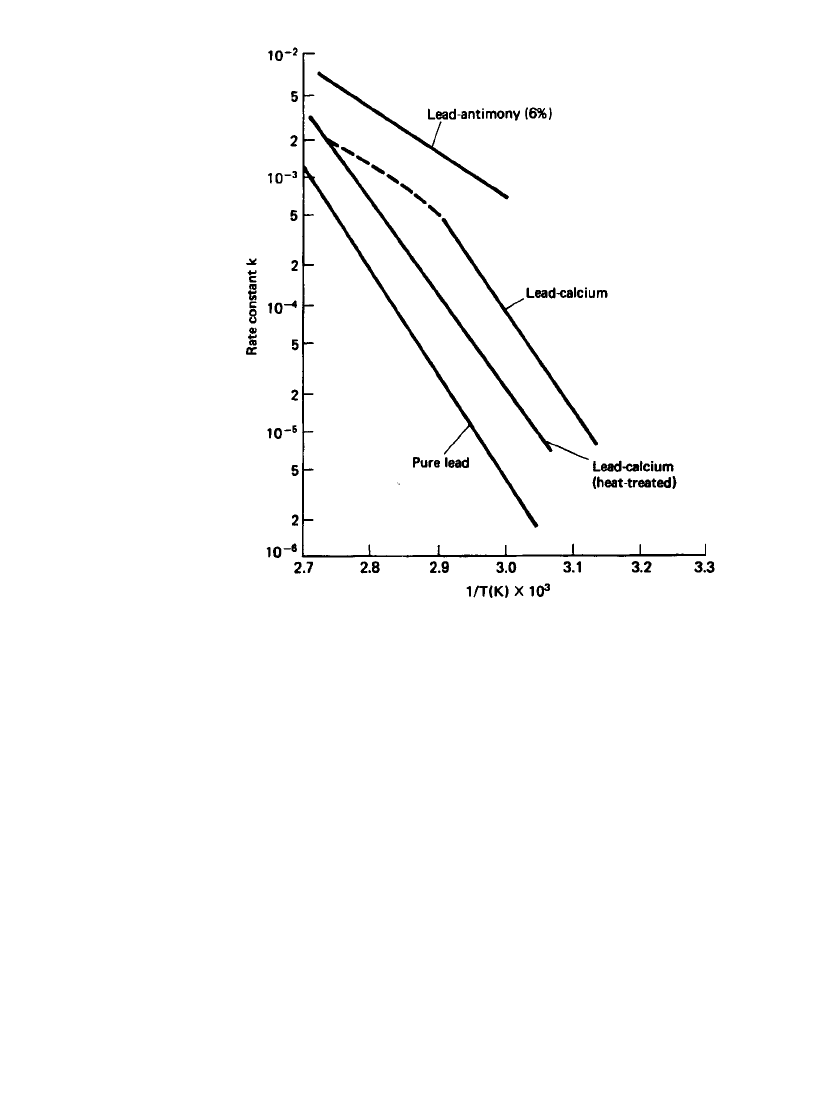
Life on float service has been found to be related to temperature (Arrhenius-type be-
havior), as plotted in Fig. 23.44. The growth rate constant k is plotted for several different
types of grid alloys used for the telephone system. At 25
⬚C the time to reach 4% growth,
an upper limit before the battery’s integrity is impaired, is calculated to be 13.8 years for
PbSb, 16.8 years for PbCa, and 82 years for pure lead.
25
23.64 CHAPTER TWENTY-THREE
FIGURE 23.42 Relative self-discharge of lead-acid stationary batteries of dif-
ferent construction.
FIGURE 23.43 Float current characteristics of stationary batteries at 25⬚C, 100-Ah cells, fully
charged, 1.210 specific gravity.
LEAD-ACID BATTERIES 23.65
FIGURE 23.44 Corrosion rate constant log k vs. 1 / T for different
lead-alloy grids. (From Ref. 25.)
23.6.3 Cell and Battery Types and Sizes
Stationary batteries, like traction batteries, are available in a variety of plate and cell sizes.
Stationary battery systems are assembled on insulated metal racks with groups of cells in
series, having nominal system voltages of between 12 and 160 V. Some battery installations
are made with several such series cell strings connected in parallel for greater storage ca-
pacity. Most stationary batteries in the United States are made with pasted positive plates.
In Europe, Plante´ and tubular positives are more popular.
Listings of stationary batteries with flat-pasted, tubular, and Plante´ plates are given in
Tables 23.15 to 23.17. The basic rating unit is the positive plate, given in Ampere-hours at
the 8-h rate unless specific otherwise, ratings of the cell are the product of the capacity of
a single positive plate multiplied by the number of positive plates. A popular stationary
battery size is the 1680-Ah cell (168-Ah positive, 10 positive or 21 plates per cell) for use
in telephone exchanges. The former Bell System has designed a special cylindrical cell in
this capacity rating (Lucent Technologies specification KS-20472, list 1).
