Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

information is invaluable for the development of a working model of the passivation
process. The following surface layers have been attributed to the passivation process:
barrier oxide layer, salt deposit layer, and alloy surface layer.
Alloying of transition elements, not surprisingly, often results in the formation
of several different oxidation states. Some oxidation states are apparent at only a trace
level and may be excluded from the general discussion. The main parameters that
determine the oxidation state of an element in a passive film are:
The passivation potential or oxidizing power of the solution
The pH of the test electrolyte
Local pH within highly hydrated passive films
The age of the passive film
The location of the ion relative to the external surface
For the purpose of a general introduction to the nature of the passive films formed on
stainless steels, we shall consider one of the media most corrosive to stainless steels,
i.e., acidic chloride solutions. In this discussion we shall consider several studies that
include a range of stainless steel compositions. Trends in the nature of the passive
film formed on these alloys will be emphasized.
It is common to find that the passive film is duplex, consisting of an inner oxide
barrier film that is considerably less hydrated than the often thinner, outer deposit
film. The outer film often contains salts or hydroxides of the alloy consituent metals.
Although the inner oxide is the primary diffusion barrier against egressing cations
and ingressing anions such as Cl
–
, there is some evidence that in some cases the
deposit film may serve to control ion transport in the passive film by the influence of
fixed charges. In other cases it has been considered to be a relatively insoluble film
that serves to protect the metal surface from aggressive ions while a barrier film
develops underneath.
It will also be shown that the thickness of the passive films, while varying with
the potential of passivation, is commonly only a few nanometers. Finally, we shall see
that in austenitic stainless steels the active stage of repassivation often results in
considerable change in composition at the alloy surface. The possible role of this
modified alloy layer in the overall passivation process will be discussed.
BARRIER AND DEPOSIT LAYERS
Surface analysis has provided the basis of our knowledge of the duplex nature of the
passive film formed on austenitic stainless steels. Destructive depth profiling using
inert gas ion sputter etching and nondestructive variable-angle electron spectroscopy
are commonly used to probe the composition of these layers. Both types of analysis
will be illustrated.
The first example involves the use of XPS with inert gas ion sputter depth
profiling. This study [1] involved the active dissolution and passivation of the
stainless steel, AVESTA 832SL (Table 1).
The test electrolyte was a deaerated solution of 0.1 M HCl + 0.4 M NaCl. The
polarization behavior of the alloy is compared in Figure 1 with the alloy constituent
metals. The corrosion potential is seen to be more noble than that of Cr and Fe but
close to that of Ni and Mo. This is quite typical for the corrosion potential of
218 Clayton and Olefjord
Copyright © 2002 Marcel Dekker, Inc.
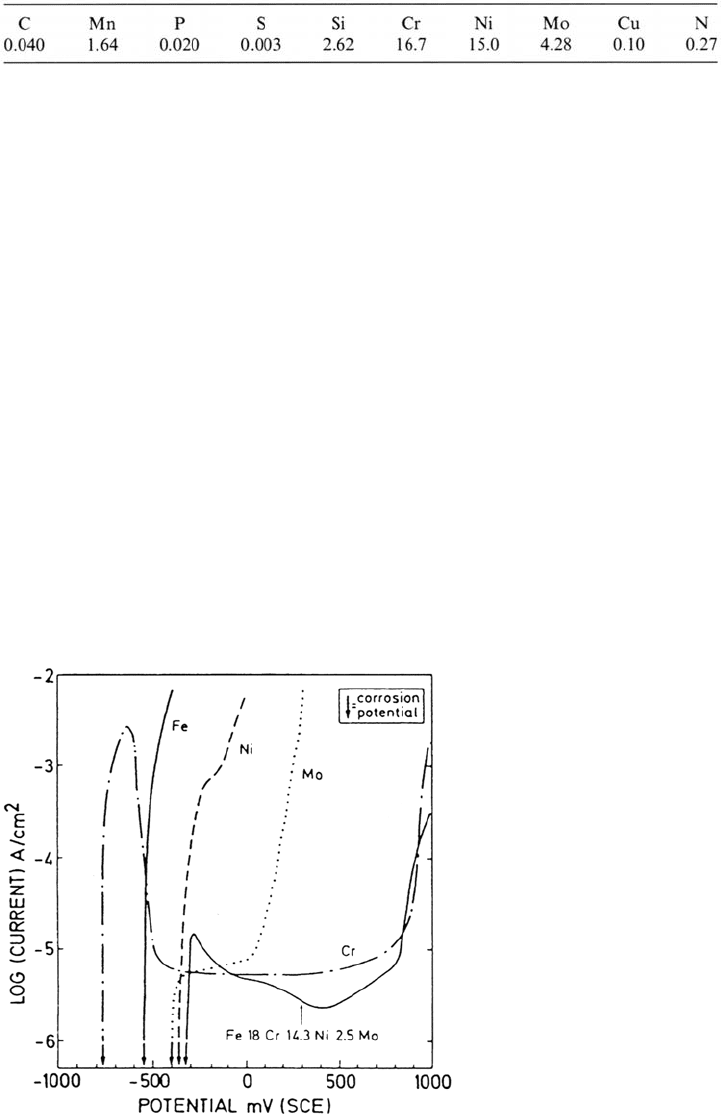
austenitic alloys. It will be shown that Ni and Mo are enriched on the surface in the
metallic state during anodic dissolution. As a consequence, the corrosion potential
becomes close to the corrosion potential of these elements. From the polarization data
it is suggested that both Cr and Mo are more likely to contribute to passivity,
especially the barrier layer, than Fe or Ni and that Mo will contribute only in a
narrow range of potential before it undergoes transpassive dissolution. These simple
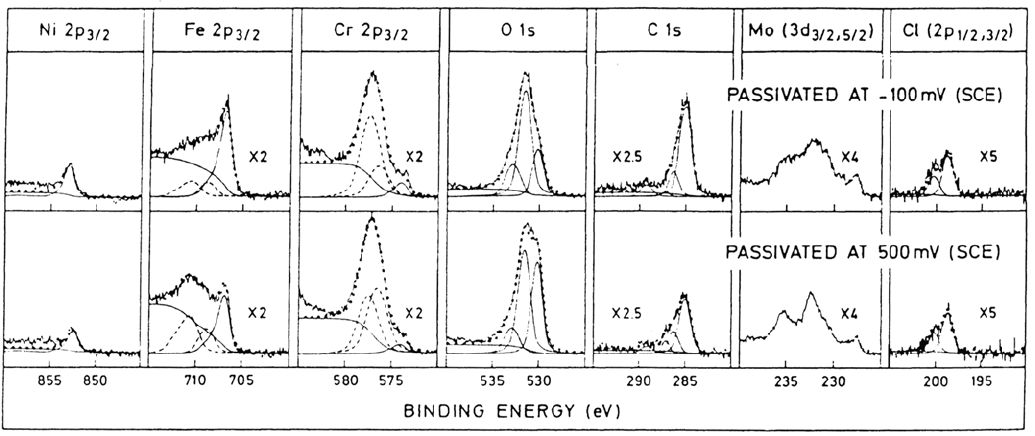
indications will be shown to be only partially correct. In Figure 2 are typical XPS
spectra of the outer region of the surface films obtained in the same study for the alloy
polarized at passive potentials (–100 and 500 mV vs. SCE).
The thicknesses of the films formed at the passive potentials –100 and 500 mV
(SCE) were calculated to be 1.0 and 1.5 nm, respectively. It can be seen from Figure
2 that the Ni spectra contain almost no detectable oxidized Ni. Therefore, Ni does not
contribute directly to the structure of the barrier or deposit films. This is surprising
because Ni is well known to contribute to pitting resistance [2]. In addition, it can be
seen that the film formed at 500 mV (SCE), which is several hundred millivolts above
the transpassive potential of Mo, contains contributions from Mo
4+
(as MoO
2
),
which has been shown to be the main cation in the passive film formed on Mo in 0.1
M HCl [3]. Therefore, it would appear that Mo contributes directly to the passive
Passivity of Austenitic Stainless Steels 219
Figure 1 Anodic polarization curves of the pure metals Fe, Cr, Mo, and Ni and of an
austenitic stainless steel, Fe18Cr14.3Ni2.5Mo (at %) (Fe16.7Cr15.0Ni4.28Mo wt%)
exposed to 0.1 M HCl + 0.4 M NaCl at 25°C. Sweep rate, 3 mV/s. (From Ref. 1.)
Table 1 Composition of AVESTA 832L (wt %)
Copyright © 2002 Marcel Dekker, Inc.
220 Clayton and Olefjord
Figure 2 XPS spectra recorded from Fel8Crl4.3Ni2.5Mo (at %) after passivation at –100 mV (SCE) and 500 mV (SCE).
(From Ref. 1.)
Copyright © 2002 Marcel Dekker, Inc.
film formed on austenitic stainless steel. The general spectra of Figure 2 provide no
direct information concerning the duplex nature of the passive film. However, in
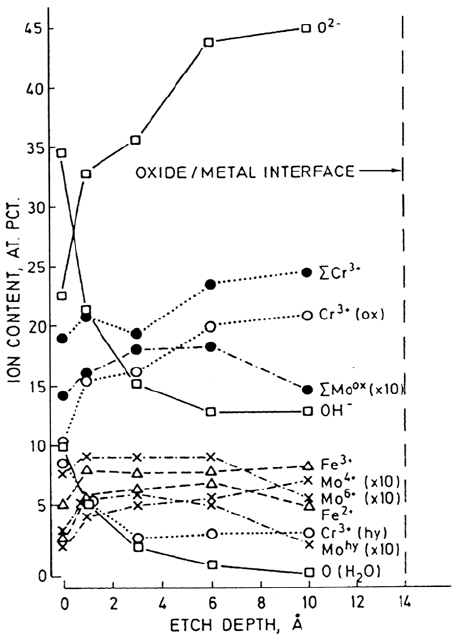
Figure 3 is a depth profile produced by argon ion sputter depth profiling of the
passive film formed at 500 mV (SCE) for the same alloy. From this figure it can be
seen that there exist two layers, an inner oxide-based layer (barrier layer) constituting
the majority of the film and an outer hydrated layer (deposit layer), which is also
richer in Mo
6+
than Mo
4+
. We shall discuss the role of Mo in the passivation process
later. A further striking observtion is that Cr
3+
is found in greater abundance in the
passive film in both the inner and outer layers than Fe, despite the fact that Fe is the
majority element in the alloy. Clearly, Cr is the main passivating species in stainless
steels.
BOUND WATER IN PASSIVE FILMS
A series of reported studies provide some insights into the nature and the role of bound
water in the passivity of austenitic stainless steels [4–6]. The studies primarily
Passivity of Austenitic Stainless Steels 221
Figure 3 Ion content vs. etch depth for the austenitic stainless steel Fe18Cr14.3Ni2.5Mo
polarized at 500 mV (SCE). (From Ref. 1.)
Copyright © 2002 Marcel Dekker, Inc.
focused on the passivity of type 304 stainless steel, passivated in deaerated 0.5 M
H
2
SO
4
. Radiotracer studies were conducted with tritiated water in order to determine
the quantity of water bound into the lattice following the formation of the passive
film. In addition, the rate of desorption of bound water from the film was determined
by dioxane solvent extraction. Dynamic rupture and self-repair of the passive film
were seen to be critically influenced by the nature of the bound water. Two classes of
bound water were determined from XPS, radiotracer, Coulombic titration, pitting
incubation, and noise analysis [4–6]. The two classes of bound water were of the
following types: (a) M-H
2
O and M-OH (aquo and olation groups) and (b) M-O or
M-OOH with oxo and olation bridges.
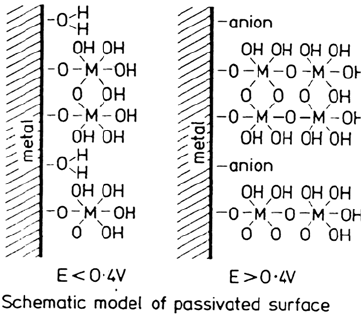
In Figure 4 the proposed structure of the passive films is presented
schematically [4]. The bound water was proposed to be associated largely with Cr
but with its nature governed by the applied potential, temperature, and time at
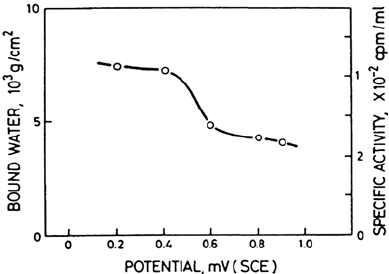
potential. It was established that the water content in the passive films formed on
304 stainless steel in deaerated 0.5 M H
2
SO
4
changed abruptly at the critical
potential of 400 mV (SCE) as shown in Figure 5 [5]. Later reflection high-energy
electron diffraction (RHEED) studies based on the same alloy composition and
test electrolyte [7] revealed that corresponding structural changes with potential
and time at potential were associated with chromium and iron products composing
the barrier and deposit layers, respectively. Passive films that were formed after 1
min were found to be amorphous. At longer times of passivation some crystallization
was revealed. The inner barrier film, which was found by XPS to consist mostly
of Cr
2
O
3
, was seen by RHEED to be amorphous at all potentials studied in the
passive range, except at the transpassive potential, where it crystallized. Low-
potential passive films (<400 mV) were seen to convert to the same structure as
higher potential passive films (>400 mV), given sufficient time for field-induced
deprotonation to take place. The chromium content of the deposit layer was
observed to be Cr(OH)
3
in the presence of acidic SO
2
4
/Cl and CrOOH in the presence
of 0.5 M H
2
SO
4
. Highly hydrated Fe compounds (including green rusts) were also
observed, which rapidly deprotonated with corresponding charge-balancing
oxidation of Fe
2+
at the critical potential of 400 mV (vs. SCE).
222 Clayton and Olefjord
Figure 4 The Okamoto model of the structure of the passive film. (From Ref. 4.)
Copyright © 2002 Marcel Dekker, Inc.
ROLES OF MOLYBDENUM IN THE PASSIVITY OF AUSTENITIC
STAINLESS STEEL
One of the most effective elements added to austenitic stainless steel, and for that
matter even ferritic stainless steel, in order to improve pitting resistance is Mo [8].
Molybdenum, however, is a highly versatile element, existing in the passive film in a
number of oxidation states. In the case of the hexavalent state it has been observed in
both the cationic and anionic states, namely as molybdenum trioxide and ferrous
molybdate. It has most commonly been reported to exist in the quadrivalent state as
molybdenum dioxide and oxyhydroxide.
The majority of the studies reported agree that Mo
4+
is incorporated into the
inner region of the passive film, whereas Mo
6+
is present in the outer layer. However,
the outer layer is variously defined as a salt layer or an extension of the barrier layer.
Numerous studies have attempted to elucidate the role of Mo in the passivity of
stainless steel. It has been proposed from XPS studies that Mo
6+
forms a solid
solution with CrOOH with the result that Mo is inhibited from dissolving trans-passively
[9]. Others have proposed that active sites are rapidly covered with molybdenum
oxyhydroxide or molybdate salts, thereby inhibiting localized corrosion [10]. Yet
another study proposed that molybdate is formed by oxidation of an Mo dissolution
product [11]. The oxyanion is then precipitated preferentially at active sites, where
repassivation follows. It has also been proposed that in an oxide lattice dominated by
three-valent species of Cr and Fe, ferrous ions will be accompained by point defects.
These defects are conjectured to be canceled by the presence of four- and six-valent
Mo species [1]. Hence, the more defect-free film will be less able to be penetrated by
aggressive anions. A theoretical study proposed a solute vacancy interaction model in
which Mo
6+
is assumed to interact electrostatically with oppositely charged cation
vacancies [12]. As a consequence, the cation vacancy flux is gradually reduced in the
passive film from the solution side to the metal-film interface, thus hindering vacancy
condensation at the metal-oxide interface, which the authors postulate acts as a
precursor for localized film breakdown [12].
The XPS spectra (Fig. 2) show that the passive film formed in Cl
–
-containing
solutions contains Cl
–
. It has been shown [13,14] that Cl
–
is present in both the
barrier layer and the hydroxide layer. It is believed that chloride ions substitute
Passivity of Austenitic Stainless Steels 223
Figure 5 Amount of bound water in the passive film vs. the potential. (From Ref. 5.)
Copyright © 2002 Marcel Dekker, Inc.
oxygen in the passive film. Thereby the stability of the film is reduced because the
number of cation-oxygen bonds decreases and dissolution of the oxide is
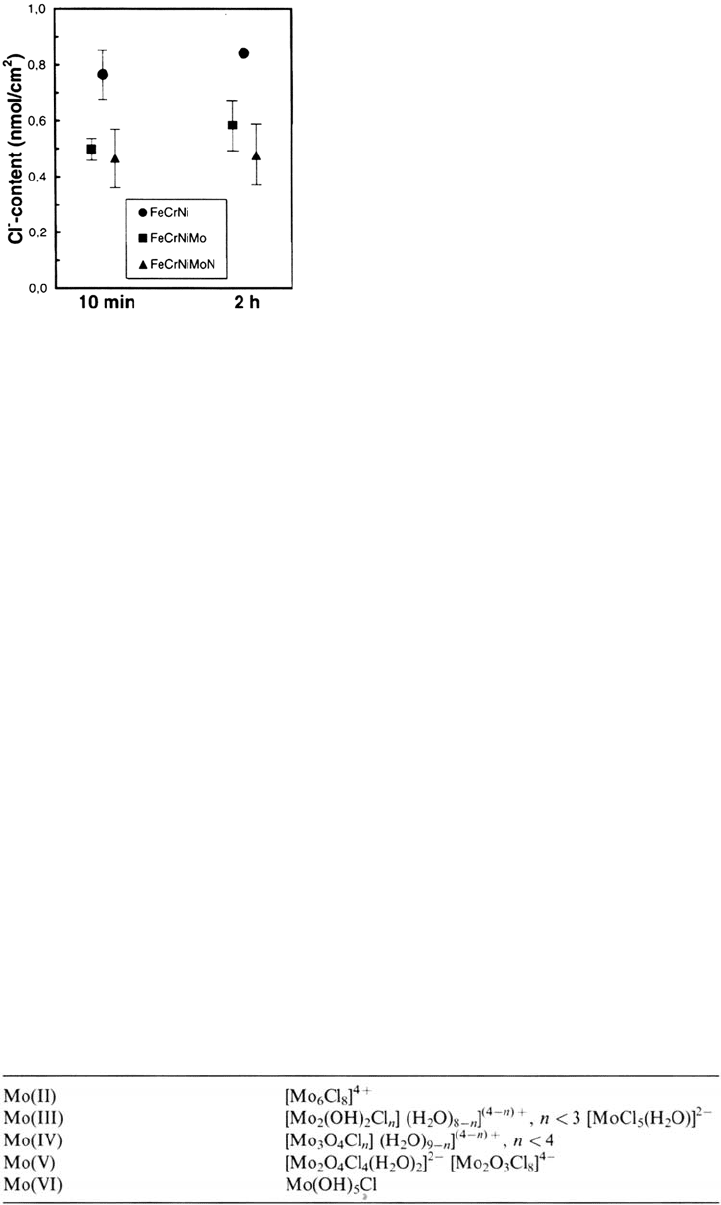
enhanced. In one study [14] high-alloyed steels, Fe20Cr20Ni, Fe20Cr20Ni6Mo,
and Fe20Cr20Ni6Mo0.2N were exposed to 0.1 M HCl + 0.4 M NaCl at –75 mV
(SCE) for 10 min and 2 h. It was found that the Cl
–
content of the Mo-free alloy
was considerably higher than the Cl
–
content of the Mo-containing alloy. Figure 6
[15] shows the experimental result. It was suggested [14,15] that the mechanism
for lowering the Cl
–
content in the film is due to the ability of Mo to form soluble
stable oxo-chloro complexes in diluted HCl solutions. Reference 16 gives details
about the Mo complexes. Table 2 summarizes some of the possible soluble stable
Mo-oxo-chloro complexes. Formation of these complexes, during the passivation
process, lowers the Cl
–
content in the passive film and thereby make the film more
resistant for pitting corrosion.
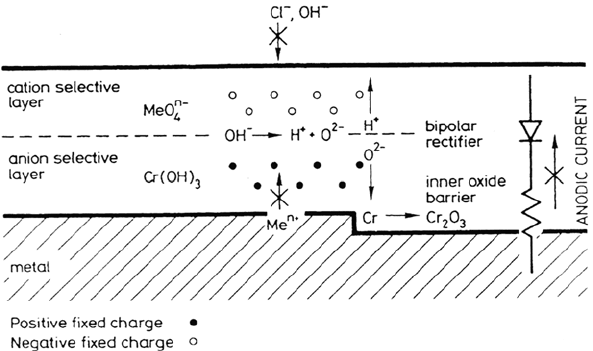
It has been suggested that molybdate ions may act as cation-selective species
in the deposit layer, thus producing a bipolar film with the anion- selective
component represented by the barrier layer, which in acidic media is predicted to
be anion selective [17]. The bipolar model of passivity is represented schematically
in Figure 7.
Evidence in support of several of these models has been reported. XPS studies
of the passive and transpassive films formed on Mo in deaerated 0.1 M HCl [3]
established that molybdate was absent from both surface films. In a later study the
same authors used a twin potentiostat arrangement, with a second working electrode
224 Clayton and Olefjord
Figure 6 Total amount of Cl
–
in the passive film formed at –75 mV (SCE) in 0.1 M HC1 +
0.4 M NaCl. (From Ref. 15.)
Table 2 Soluble Stable Mo-oxo-chloro Complexes in Aqueous HCl Solutions
Copyright © 2002 Marcel Dekker, Inc.
of either Fe, Cr, or Ni that was polarized near an Mo electrode at the same potential
(–180 mV vs. SCE) [18]. At this potential Mo and Cr are passive, while Ni and Fe
are active. In this work it was shown that for the Fe-Mo and Ni-Mo electrode
couples, iron or nickel molybdate was observed on the passive Mo surface. In the
case of the Cr-Mo couple, molybdate was observed only on the passive film of Cr.
This work was also able to show that transpassivity of Mo at 250 mV (SCE) was
suppressed in the presence of Fe, which formed a molybdate salt on the surface of
Mo. This indicated evidence of a possible mechanism by which Mo can remain
passive in stainless steels at higher potentials than the transpassive potential of Mo.
In addition, this work supported the idea that soluble molybdate anions can redeposit
at active sites.
In other work, a major prediction of the bipolar model of passivity [19] was
tested. This was that in the presence of a bipolar passive film with the cation-
selective layer (molybdate anions, for example) in the outer layer or deposit
film, there would be a tendency to increase O–H bond stretching and eventual
deprotonation due to the conjoint effect of the electric field associated with the
surface charge on the metal and the strong negative fixed charge on the oxyanion. As
a result of this work it was shown by XPS analysis that when a passive film formed
on an Fe-19Cr9Ni alloy was doped in solution with molybdate anions, there was
a significant decline in the hydroxyl concentration in the film in favor of oxide
anions. In addition, the concentration ratio of chromium oxide to hydroxide was
increased. This work has several ramifications. First, it provides some support for the
bipolar model of passivity, which would suggest that molybdate can rectify the
transport of ions through the passive film in favor of inhibiting egress of cations
other than protons as well as inhibiting the ingress of chloride ions that aid in the
dissolution of the film. Second, the promotion of deprotonation of the passive film
would favor oxidation of ferrous ions to ferric in order to establish charge neutrality.
This would also have the effect of reducing the defect concentration in the trivalent-
dominated oxide lattice in accordance with a previous suggestion [1].
Passivity of Austenitic Stainless Steels 225
Figure 7 Schematically illustration of the bipolar model of the passive film. (From Ref. 3.)
Copyright © 2002 Marcel Dekker, Inc.
So far, the role of molybdenum in the passive film has been discussed.
However, several workers have postulated that Mo may exclusively or additionally
control the kinetics of the active dissolution process. These models can be separated
into two kinds: (a) insoluble salt models and (b) surface alloy models. One of the
earliest reports to indicate the importance of insoluble salts in the repassivation
kinetics of ferrous alloys involved studies of Fe-Mo alloys [20]. It was shown that
when the alloy content exceeded 5% a protective chloride salt film stabilized by
ferrous molybdate precipitated from the test solution after the initial dissolution
of molybdenum. This work suggests that a similar mechanism may occur in
Mo-bearing austenitic stainless steels in chloride solutions. The stability of the
molybdenum-bearing passive film in chloride solutions has also been attributed to
the ability of Mo to form insoluble chloride complexes at the base of pits, thereby
arresting chloride ions in the pit solution, which in turn enables subsequent
repassivation to occur. This has been shown by Auger electron spectroscopy
(AES) of pit surfaces [21]. XPS analysis has revealed [15,22] the Mo(II) valent
compound β-MoCl
2
formed in the passive film on high-alloyed stainless steels
passivated in hydrochloric acid. β-MoCl
2
is the only one of the nonsoluble chloride
compounds that can easily be detected by XPS. Other insoluble high-Mo-valency
chloride compounds such as α-MoCl
3
, MoOCl, MoOCl
2
and MoO
2
Cl[16] are not
easily detected due to the overlap of their Mo signals with Mo-oxide peaks.
The activity of Cl ions in the pits of Mo-bearing stainless steels was also shown
by AES to be significantly reduced by precipitation of an insoluble chloride complex
containing Mo. This behavior is also reflected in the passivation of high-purity Moin
a deaerated solution of 4 M HCl [23]. It has been shown that the passive range of
potential for Mo in 4 M HCl is significantly greater than in 0.1 M HCl. XPS analysis
of the surface of Mo polarized in the passive range for deaerated 4 M HCl solution
provided evidence of a molybdenum oxyhydroxy chloride film. This product was not
observed in the exclusively oxide-based passive film formed on Mo in 0.1 M HCl
solution. The presence of such an insoluble film on Mo in 4 M HCl indicates that such
a film may be formed on the bottom of pits formed onMo-bearing stainless steels.
It has been shown that the low pH value of pitting solutions is caused by high
concentrations of metal chlorides [24]. Therefore the formation of insoluble chloride
complexes in pit bottoms suggests that lowering the concentrations of soluble metal
chlorides in the pitting solution would cause the pH to shift toward that of the bulk
solutions. The conjoint effects of raising pH and deposition of salt films provide
conditions more conducive to repassivation [25].
The effect of surface alloy modification by anodic dissolution on the
passivation of Mo-bearing austenitic stainless steels is discussed later in a separate
section.
ROLES OF NITROGEN IN THE PASSIVITY OF AUSTENITIC
STAINLESS STEEL
Nitrogen may be added to austenitic stainless steel (commonly 0.2 to 0.7 wt %) in
order to stabilize and strengthen the austenitic phase. However, in the presence of
Mo additions very significant improvements in general and localized corrosion have
226 Clayton and Olefjord
Copyright © 2002 Marcel Dekker, Inc.
been demonstrated by several workers [26–30]. This early work indicated a
strong synergistic effect of Mo and N in the corrosion resistance of austenitic
stainless steel.
More recently, it has been demonstrated [31] that N anodically segregates to the
oxide-metal interface during passivation. The dots with the error bars in Figure 8 [15]
show the measured intensity ratios, N/(O
2–
+ OH
–
), recorded by XPS as a function
of the take-off angle for an alloy (Fe20Cr20Ni6Mo0.2N) polarized in a 0.1 M HCl +
0.4 M NaCl solution at –75, 500 and 800 mV for 10 min. The distribution of N was
estimated by utilizing quantitative analysis described in Ref. 32. The thick solid lines
at the bottom of the three figures represent the expected intensity ratios one should
obtain if the nitrogen atoms (concentration 0.2 wt %) are uniformly distributed in
the phase below the passive film. However, the measured intensity ratios are
significantly higher, which implies that N is enriched at the surface. By assuming a
model shown in the upper right corner of the figure it was possible to find a
distribution of N that satisfies the measured data. The thin solid lines in Figure 8 are
theoretical intensity ratios calculated by assuming that N is enriched at the metal-oxide
interface and that the bulk concentration is 0.2 w % N. The theoretical ratios are
obtained for N coverage of 12, 17, and 20 at % at the interface.
It has been demonstrated [33] that a strong Mo-Ni-N interaction is likely to
occur. In a series of papers on experimental N-bearing alloys #30 and #30c, which
were compared with alloy AL6X, it was shown that nitrogen additions strongly
improved corrosion resistance [29,30] (Table 3). In tests conducted in several acidic
chloride solutions it was shown that nitrogen alloying was responsible for lowering
the critical current density and passive current density at room temperature and at
elevated temperature [29]. Alloy #30 was also tested at 80°C in a solution of 4% NaCl
+ 1% Fe
2
(SO
4
)
3
+ 0.01 M HCl in which it showed no evidence of pitting. This result
was all the more significant because it had previously been shown that for austenitic
alloys a linear relationship existed between the critical pitting temperature and the
compositional factor (wt % Cr + 2.4 wt % Mo) in the same solution [34]. The
inference therefore remains that the N-bearing alloy may protect against pitting via
an alternative overall mechanism.
In further studies of alloy #30 it was shown by Auger depth profiling [30] (see
Fig. 9) that nitrogen segregated to the alloy surface at the metal-oxide interfacedur-
ing passive dissolution at 500 mV (SCE) for 24 h in deaerated 0.5 M H
2
SO
4
. In later
studies it was shown that N additions strongly influence the alloy composition at the
metal-oxide interface [35]. Thus segregation of N was found to coincide with the
enrichment of Ni and Cr in 304(N) stainless steel and Ni, Cr, and Mo inMo-bearing
austenitic stainless steels such as 317LX(N), 904L(N), and AL6X(N) (Table 4).
It was determined from the N 1s photoelectron spectra that the form in which
nitrogen was segregated is a surface nitride [33]. XPS studies of surface nitrides
formed on Fe, Cr, Ni, Mo, and the stainless steels 304, 317LX, 904L, and AL6X
show that the nitride anodically formed on nitrogen-bearing austenitic stainless
steels was a mixed nitride. For this work and for the purpose of studying the
interaction of anodically segregated N with the individual alloying constituents, a
room temperature electrochemical nitriding process was developed [33]. The process
involved the cathodic reduction of nitrate ions. The outcome of the treatment was that
the same surface nitrides were formed on the stainless steels as formed by anodic
Passivity of Austenitic Stainless Steels 227
Copyright © 2002 Marcel Dekker, Inc.
