Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

228 Clayton and Olefjord
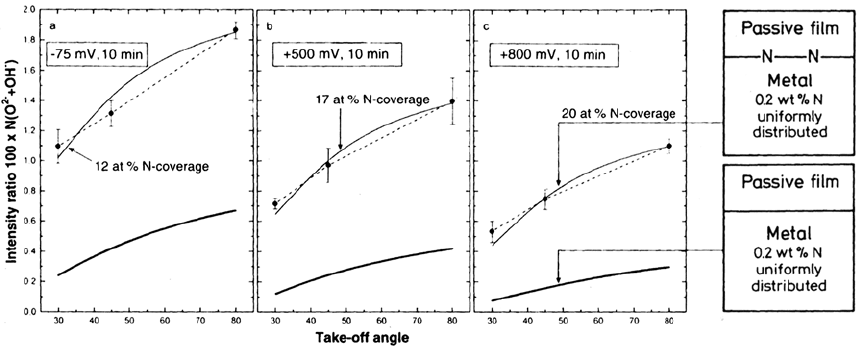
Figure 8 Measured intensity ratios, 100 × N/(O
2–
+ OH
–
), at the take-off angles 30°, 45° and 80° after
passivation of the Fe20Cr20Ni6Mo0.2N alloy at –75, 500 and 800 mV (SCE). The lower thick line and the
upper thin line represent the estimated intensity ratios from the N-distributions shown in the models to the
right of the figure. (From Ref. 15.)
Copyright © 2002 Marcel Dekker, Inc.
segregation. The benefit of the technique was that, unlike the case of ion implantation
and plasma or thermal nitriding, a room temperature surface phase could be
formed without expected surface damage or sensitization. The advantage of studying
surface nitrides over the nitrogen-bearing alloys was reported to be that, whereas
anodic segregation is a continuously regenerating process, surface alloying provides
a finite quantity of nitrogen that can be monitored by surface analysis after anodic
dissolution under active and passive conditions.
As seen in Figure 10, the potentiodynamic behavior in deaerated 0.1 M HCl of
austenitic stainless steels is beneficially modified by the surface nitriding treatment, in
agreement with previous studies of the same steels alloyed with nitrogen [33]. Each of
the steels showed that the surface nitride stifles active dissolution and that this effect
Passivity of Austenitic Stainless Steels 229
Table 3 Chemical Composition of Main Elements of Steels (wt%)
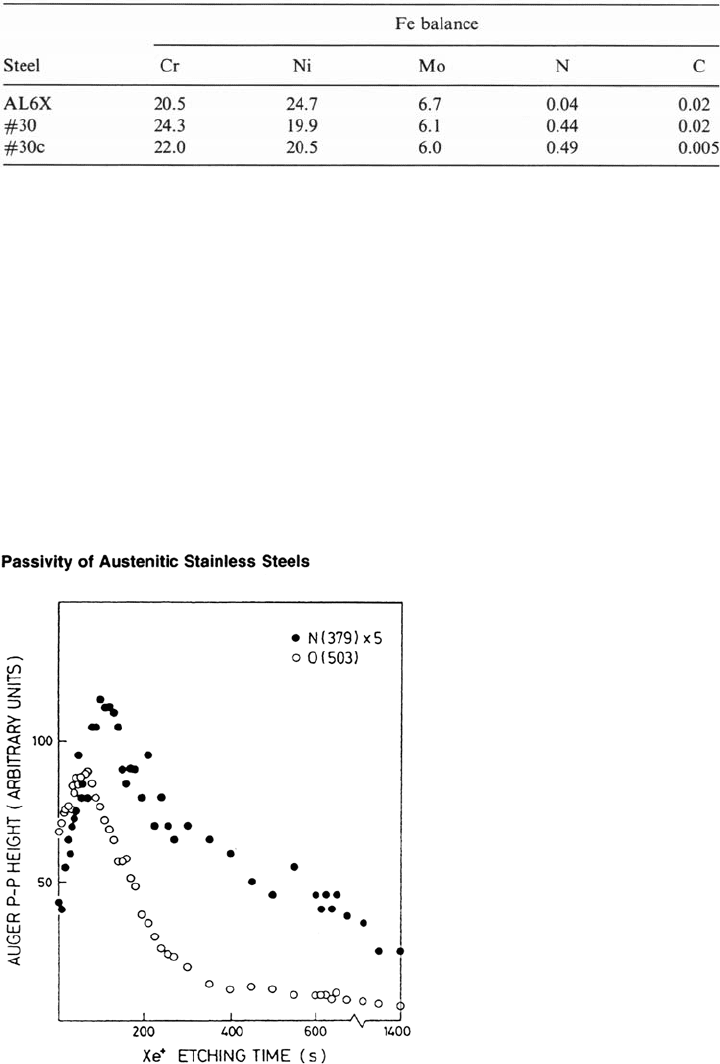
Figure 9 Auger depth profile for alloy 30 after passivation for 24 h in 0.5 M HCl + 2 M
NaCl. Approximate etching rate was 0.01 nm/s. (From Ref. 30.)
Copyright © 2002 Marcel Dekker, Inc.
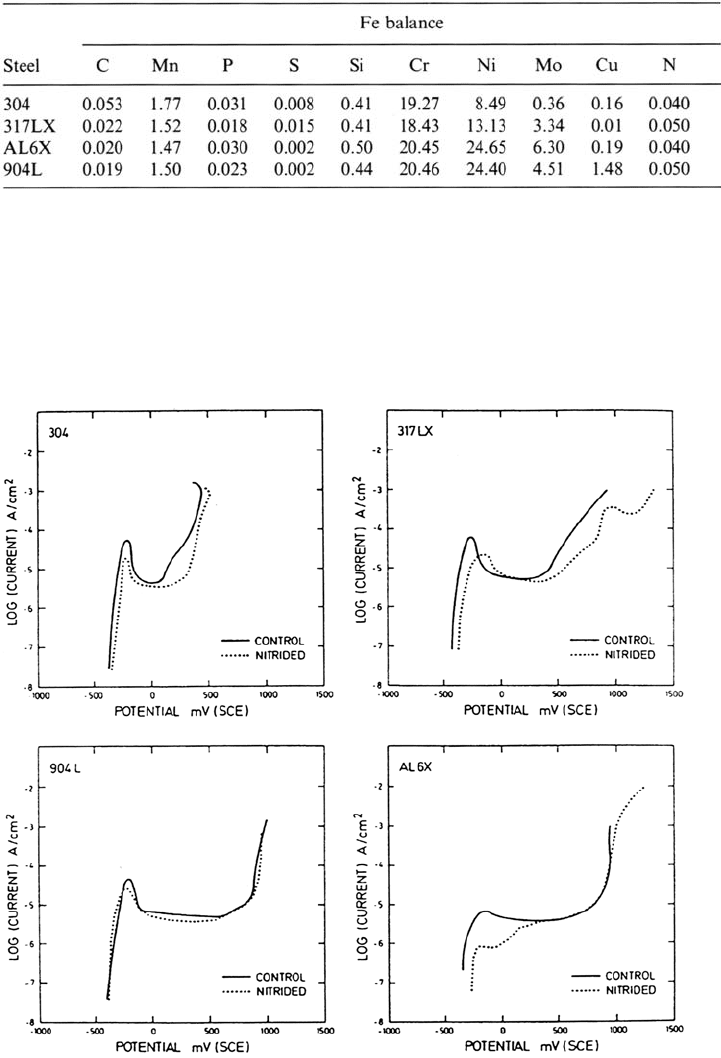
is more pronounced as the addition of Mo is increased. In the lower alloyed steels,
304 and 317LX, the greatest influence of nitrogen was reported to be in raising the
pitting potential. As shown by Figure 10, the pitting resistance of surface-nitrided
317LX steel is markedly better than that of type 304 due to the higher Mo content. It
230 Clayton and Olefjord
Table 4 Chemical Composition of Steels (wt%)
Figure 10 Polarization curves for surface-nitrided and untreated austenitic stainless
steels in deaerated 0.1 M HCl where the specimens were permitted to float to the open-circuit
potential before polarization. Sweep rate: 1 mV/s. (From Ref. 33.)
Copyright © 2002 Marcel Dekker, Inc.
is also apparent that the greatest anodic inhibition was achieved by the AL6X alloy,
which has the highest Mo content of the alloys studied and in particular is very
similar in composition to 904L except for an additional 1.8 wt % Mo. These
observations imply that N and Mo support the same mechanism of corrosion
inhibition.
The authors also noted that following potentiodynamic polarization from the
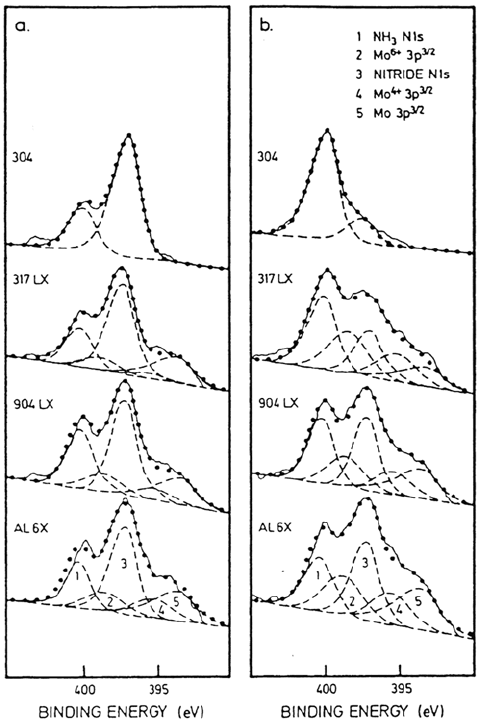
corrosion potential to 0 mV at a scan rate of 1 mV/s, XPS analysis was still able to
detect significant quantities of surface nitride. This is illustrated in Figure 11. The
most active of the alloys studied, type 304, was determined to have dissolved
approximately 20 monolayers. This suggested that the nitride may form a kinetic
barrier that is protected by the oxide passive film from rapid protonation to
ammonia and ammonium in the active range of potential. In the same study the
nitride phase formed on Ni had little effect on anodic behavior in 0.1 M HCl,
Passivity of Austenitic Stainless Steels 231
Figure 11 N Is spectra for austenitic stainless steels: (a) As nitrided; (b) follow polarization
from open circuit to 0 mV. Sweep rate: 1 mV/s. (From Ref. 33.)
Copyright © 2002 Marcel Dekker, Inc.
whereas Fe became anodically activated by nitriding. Chromium exhibited complete
removal of the active nose following nitriding. This was attributed to suppression of
Cr
2+
by direct chemical reaction of surface N and Cr forming a Cr
3+
state as CrN, a
precursor of Cr
2
O
3
, which was identified by XPS. Molybdenum, however, showed
some ennoblement of the corrosion potential and transpassive potential. This effect
on Mo was later found to be even more pronounced following thermal nitriding [36].
It has been reported that nitrogen alloying and surface nitriding result in a
higher metal oxyanion content in the outer layers of the passive films of stainless
steels formed in acidic solutions. By inspection of the E-pH diagrams of Mo and W,
for instance, it is seen that MoO
2–
4
and WO
2–
4
are both more likely to form in the
middle to high pH range [37]. The presence of the metal oxyanions in the outer region
of passive films formed on stainless steels indicates that the products are developed
during the active stage of repassivation, precipitating as salt films. The well-known
pitting inhibition derived from metal oxyanions such as molybdate suggests that the
presence of such anions in the outer layers of the passive films would tend to reduce
the probability of pit initiation.
ALLOY SURFACE LAYERS
A stainless steel is normally used at potentials corresponding to the passive range.
In this condition only a fraction of the oxidized atoms remain in the passive film;
most are dissolved into the solution. The oxidation rate of the metal corresponds
to the passive current density. As established earlier, the alloying elements Cr and
Mo are enriched in the passive film. The other two main alloying elements, Fe and
Ni, could in principle be found in the solution due to selective dissolution of these
elements. XPS spectra [1,38–40] recorded from stainless steels after polarization
to potentials in the active and passive regions show that the measured Fe content
in the metal phase is lower than the composition of the bulk alloy. Fe must therefore
be selectively dissolved and can thereby be found enriched in the solution. Ni and
Mo, on the other hand, are enriched in the metal phase underneath the oxide. It has
been proposed [1,38–40] that during active dissolution and passivation of stainless
steel a thin layer of an intermetallic compound is formed in the outermost layers
of the metal phase. Inhibition of the active dissolution of stainless steel by elemental
Mo has been considered [41]. It was proposed that Mo serves to decrease active
dissolution by binding to active surface sites such as kinks and thus increasing the
coordination of more active species. More recently, it has been shown [42] by cyclic
polarization and pit propagation rate tests on a series of Fe-Ni-Cr-Mo alloys having
Mo contents of 3, 6, and 9 wt % for three Fe/ Ni ratios that active dissolution was
governed by an Ni-Mo surface complex. These observations led us to the
conclusion that in describing the corrosion properties of stainless steel it is not enough
to describe the reactions leading to formation of the barrier layer; it is also necessary
to describe the reactions taking place in the outermost layers of the metallic phase.
The influence of the alloy composition on the polarization diagrams of
austenitic stainless steels polarized in 0.1 M HCl + 0.4 M NaCl is shown in Figure 12.
The curves represent the following steels: curve 1, 20Cr-18Ni-6.1Mo-0.2N (wt %);
curve 2, AISI 316, 18Cr-13Ni-2.7Mo; curve 3, AISI 304, 18Cr-9Ni. The polarization
curves show that the high-alloyed steel is passive within a broad potential range. The
232 Clayton and Olefjord
Copyright © 2002 Marcel Dekker, Inc.
other two alloys are sensitive to pitting corrosion in this solution. The critical current
density is about the same for the two Mo-containing alloys, and it is an order of
magnitude larger for the non-Mo-containing steel. The conclusion from the polar-
ization diagrams is that the composition of the metal influences markedly the passi-
vation and the pitting behavior of the steels. It will be shown in the following that syn-
ergistic effects exist between the alloying elements.
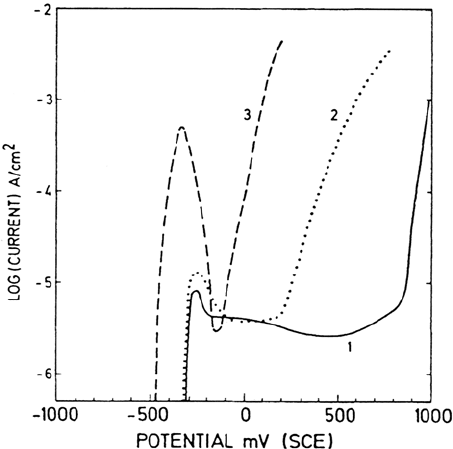
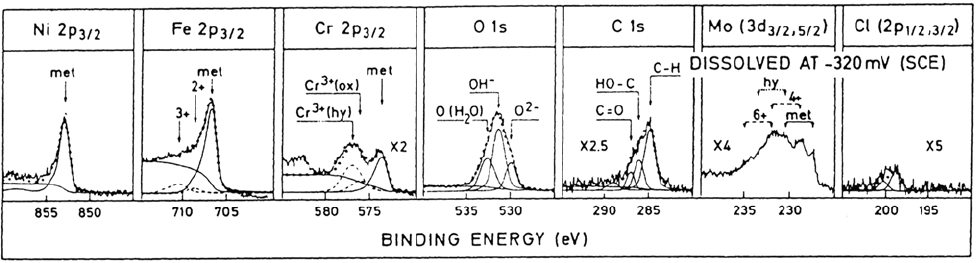
The spectra in Figure 13 were recorded from a high-alloyed austenitic
stainless steel (16.7Cr-15Ni-4.3Mo) after polarization in 0.1 M HCl + 0.4 M
NaCl at –320 mV (SCE). The potential represents the active dissolution potential
slightly above the corrosion potential. The result is from the same study as
Figure 2. It appears from the spectra of Figure 13 that the intensities of the
metallic states are much higher after polarization to the active potential
compared with the passive potentials of Figure 2. This is, of course, due to the
fact that the oxide film formed on the sample polarized at the active potential is
much thinner than on the passivated samples. One should in principle expect no
oxide or at most a very small amount of oxidized species on the alloy polarized
to the active region. However, during the rinsing and the transfer of the sample
from the electrochemical cell to the XPS analyzer, the surface is slightly oxidized.
The thickness of the oxide formed during handling of the sample polarized to
the active region is about 0.5 nm.
The composition of the metal phase can be estimated from the recorded
intensities. The dots in Figure 14a [1,40] show the apparent compositions of the metal
phases of the alloy after polarization to the active potential. The composition is
calculated from the measured peak intensities in Figure 13, taking into account the
Passivity of Austenitic Stainless Steels 233
Figure 12 Polarization diagrams obtained during exposure to 0.1 M HC1 + 0.4 M NaCl
of the following alloy steels: (1) Fe-200Cr-18Ni-6.1Mo-0.2N; (2) Fe-18Cr-13Ni-2.7Mo;
(3) Fe-18Cr-9Ni. (From Ref. 32.)
Copyright © 2002 Marcel Dekker, Inc.
234 Clayton and Olefjord
Figure 13 XPS spectra of an Fe-20Cr-18Ni-6.1Mo-0.2N (wt %) alloy recorded after polarization to the active potential
–320 mV(SCR). (From Ref. 1.)
Copyright © 2002 Marcel Dekker, Inc.
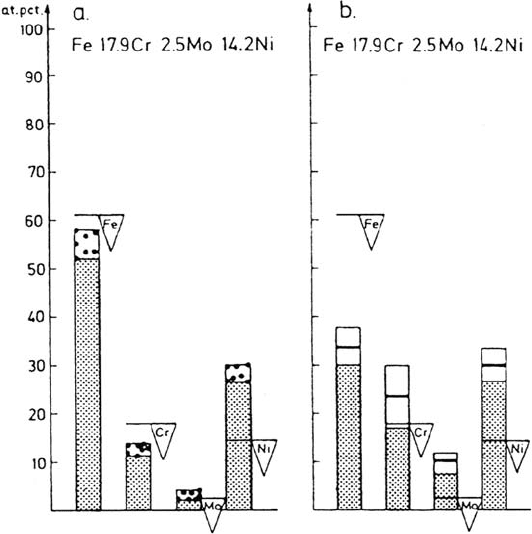
yields and the attenuation lengths of the photoelectrons. The horizontal lines in the up
and down triangles in Figure 14 show the composition of the alloy. It appears from
Figure 14a that Ni and Mo are enriched in the metal phase, while the elements Fe and
Cr are depleted. The depth to which the composition changes from the bulk
composition can be only a few atomic planes because of the very low diffusion rate
at room temperature. The depth of analysis, which is about three times the
attenuation length, is larger than the enriched zone. Hence, the bulk of the alloy
contributes to the recorded spectral intensity. Thus, the composition in Figure 14a is
an apparent composition. The actual surface composition can be found only if the
distribution of the elements in the surface region is known. Unfortunately, this is not
the case. It is therefore necessary to assume a realistic distribution function of the
elements in order to be able to calculate the surface composition of the metal phase.
The simplest distribution function describes enrichment of the elements Ni
and Mo in only one atomic plane. If this model is applied to the spectra shown in
Figure 13, it becomes apparent that it is not possible to obtain mass balance.
However, most of the oxide present on the surface during the analysis of the sample
polarized to the active potential is formed during transfer of the sample from the
cell. Therefore, to assess the composition of the surface during anodic dissolution,
the cations detected have to be converted to their metallic states and added to the
contribution from the metallic spectra. By assuming a model for the distribution of
the elements, the surface composition can be calculated. The details of the procedure
Passivity of Austenitic Stainless Steels 235
Figure 14 Composition of the metal phase during dissolution at an active potential.
(a) Apparent metal content; (b) estimated metal content in the outermost atomic layer [1,40].
Copyright © 2002 Marcel Dekker, Inc.

have been published [1,40]. Figure 14b shows the quantitative analysis of the metal
composition during anodic dissolution. The distribution function used was an error
function where the composition varies over three atomic planes. The composition
of the second plane is 50% of the difference between the bulk composition and the
composition of the surface. The values shown with the coarse solid line represent
the composition of the outermost atomic layer during anodic dissolution. As
already pointed out, it was assumed that most of the oxide observed on the surface
was due to the transfer of the sample. It appears from Figure 14 that Ni and Mo
are markedly enriched on the surface of the metal during anodic dissolution. Even
the Cr concentration in the outermost layer is significantly higher than the bulk
concentration. Because of the enrichment of Cr, it is easy to understand that the
oxide formed during handling of the sample was mainly Cr oxide. Furthermore,
Figure 14b shows that the Fe content in the outer surface layer is markedly lower
than the alloy composition.
It was shown before that the Ni content of the passive film is low. One can
therefore expect that the Ni content at the metal-oxide interface is still high after
passivation. Figure 15 [1] illustrates the measured apparent metal content of the
metal phase after polarization to the active potential, –320 mV (SCE), and the
passive potentials, –100 mV and 500 mV (SCE). It appears from the figure that Ni
largely remains in the alloy phase after passivation.
It has been pointed out that the passive currents for all three alloys whose
polarization diagrams were shown in Figure 12 are about the same. Even the passive
current for pure Cr (Fig. 1) is about the same as for the alloys. The noticeable
difference in electrochemical behavior between the alloys is in the passivation current
and the pitting potential. As shown earlier, the alloying elements are enriched on the
236 Clayton and Olefjord
Figure 15 Apparent metal content of the alloy vs. the potentials (SCE). (From Ref. 1.)
Copyright © 2002 Marcel Dekker, Inc.
surface in their metallic states during active dissolution. It has been suggested
[1,40] that an intermetallic surface phase is formed during dissolution.
The formation of an intermetallic surface phase can be understood from the
Engel-Brewer [43] valence bond theory of metallic bonding by considering the ground
state electronic configuration of the elements and the nature of the possible bonding
processes. The model predicts intermetallic bonding between “hyper” and “hypo”
d-electron transition metals, resulting in very strong bonding. Such bonding, in
principle, should result in low dissolution rates due to a higher activation energy for
anodic dissolution. Such systems are formed between transition metals from the left of
the periodic table having more vacant d electrons and those to the right having fewer
d-electron vacancies. The intermetallic bonding between elements suitablyseparated in
the transition row may result from penetration of an electron pair from the hyper
d electron into the d orbital of the hypo d-electron metal. XPS analysis [44] of thin
Ni-Mo intermetallic layers has confirmed the charge transfer. Compared with those of
pure metal lattices, the binding energies of Ni and Mo from the intermetallic layer are
shifted higher and lower, respectively. It has been pointed out that the strength of the
d-electron bonding increases from 3d to 4d, indicating that thestability of the inter-
metallic bond between Ni and Cr should be lower than that between Ni and Mo [33].
Therefore, it has been suggested that the overall latticeenergy increases during dissolu-
tion of Mo-alloyed austenitic stainless steel in the metal layers close to the passive film
due to formation of intermetallic bonds between Ni and Mo atoms [45]. Consequently,
the activation energy increases for anodicdissolution and the dissolution rate decreases.
According to this model, bonding between Mo and Fe is predicted to be weaker than
between Mo and Ni and Mo and Cr, and therefore Fe is selectively dissolved.
The Engel-Brewer model [43] of intermetallic bonding would explain how
Ni can lower the critical current density and elevate the pitting potential of
austenitic alloys, while playing no direct role in the construction of the passive
film. It is evident that Mo not only plays several direct roles in the formation and
stability of the passive film but also enhances Ni’s role by further enhancing the
anodic segregation of Ni. Therefore, it is proposed that through sluggish dissolution
kinetics alone, Ni, when bonded with Cr and more strongly with Mo, will lower
the rate of metal dissolution during the pitting process and thereby reduce the
maximum metal chloride concentration in the pit solution [45].
In the N-bearing stainless steels, N has a pronounced influence on the pitting
corrosion properties. It has already been pointed out [15,26,31] that the positive
effect of N is obtained for the Mo-alloyed stainless steels. The lower dissolution rate
of the Mo-containing alloys due to formation of intermetallic layer during active
dissolution provides a model for the synergistic effect between Mo and N. Figure 16
shows the proposed mechanism. Three alloys with and without Mo and N are
assumed to be exposed to an acid chloride–containing solution. Pits are initiated on
all three alloys. The pits formed on the Mo-containing alloys become smaller than
the pits on the Mo-free alloy because the Mo lowers the dissolution rate of the alloy
by formation of an intermetallic surface layer. If the acidity and/or the chloride
content of the solution is high enough, the pits formed on the Mo-free alloy will
become critical and grow. The dissolved ions are hydrated and the hydrolysis causes
increased acidity in the pits. The pH value in the small pits formed on the
Mo-containing alloys becomes low due to the outlet diffusion of H
+
ions. The
Passivity of Austenitic Stainless Steels 237
Copyright © 2002 Marcel Dekker, Inc.
