Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

of this dissolution process will finally result in breakdown of the passive layer and
exposure of bare metal surface to the electrolyte. For F
–
this occurs after intermediate
breakdown and repair events at the total surface. For the other halides, local
effects and the formation of pits are found corresponding to their less pronounced
complexing tendency. They will form complexes and cause their effect only at
special active sites of the oxide surface. It should also be mentioned that fluoride
causes a reduced local attack in more alkaline solutions such as phthalate buffer
pH 5 [42], which finally leads to the formation of corrosion pits and not a general
attack as observed in strongly acidic electrolytes, The thinning of the oxide layer
is also much less pronounced or hardly detected for pH>5. For a higher pH the
passive layer gets to or close to its dissolution equilibrium in the sense of the
thermodynamically deduced potential-pH diagrams. In this situation, the tendency
for the formation and transfer of soluble fluoride complexes will be reduced so
that again only local effects are observed.
In this regard, the special situation of Cr should be discussed. This metal does
not show pitting and the passive layer is not attacked in the presence of chloride. The
stability constants of CrX
2+
complexes are smaller than 1. Besides that, it is well
known that the exchange of Cl
–
ligands and H
2
O between the inner and the outer
sphere is an extremely slow process with a half-time of several hours [49]. This is a
consequence of the large ligand field stabilization of the Cr(III) complexes with an
octahedral coordination shell with three electrons in the lower t
2g
and none in the e
g
level. This situation causes “insolubility of CrCl
3
and Cr
2
O
3
in cold water” as
reported in literature [50]—however, it is rather the slow dissolution rate of the Cr
3+
salt or oxide than their low solubility. The exchange of a ligand of the inner with one
of the outer coordination shell requires a large activation energy, which makes the
Mechanisms of Pitting Corrosion 259
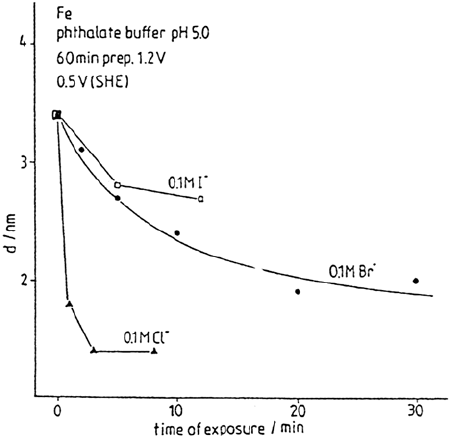
Figure 8 Decrease of oxide thickness with time of halide exposure at 0.5 V after 60 min
prepassivation at 1.20 V for different halides, deduced from XPS measurements. (From
Ref. 48.)
Copyright © 2002 Marcel Dekker, Inc.
dissolution process even via complex formation extremely slow. Therefore one
has to conclude that the presence of a CrCl
2+
complex at the surface will not
increase the dissolution rate because it will form and dissolve very slowly by
itself. In contrast to this situation, the exchange is rapid for Fe
3+
complexes. Thus,
a chemical change of Cr
3+
ions from a part of the oxide matrix to a CrCl
2+
complex
will not increase the dissolution rate. Besides these circumstances, the smaller stability
constants of the Cr
3+
complexes are also in favor of the stability of the passive
layer. In consequence, the tendency for Cr
3+
-halide complexes to form is negligibly
small, and once they have formed their dissolution rate is not increased relative to
Cr
3+
within an oxide matrix. Therefore the halides will not attack the passive layer
of chromium and pitting cannot occur, in agreement with the experimental findings.
For similar reasons, the dissolution rate of Cr(III) oxide is extremely slow in the
passive state. Additions of Cr therefore stabilize the passive behavior of Fe-Cr alloys
and stainless steel. Fe-Cr alloys are more resistant to pitting in chloride-containing
electrolytes with more positive pitting potentials compared with pure Fe. The Cr
concentration is increased within the passive layer relative to the composition of
the bulk metal. Thus Fe-Cr alloys are more protected against the attack of aggressive
anions and pitting by the beneficial effect of Cr.
Comparison of the Different Nucleation Mechanisms
The discussion of the different nucleation mechanisms on the basis of experimental
results for iron and nickel leads to the conclusion that the film breaking and
adsorption mechanisms are very effective. As usual in kinetics, the fastest reaction
path is dominating. This, however, depends on the experimental or environmental
conditions. For a stationary state of the passive layer the adsorption mechanism
seems to be most effective, as demonstrated for Fe in weakly acidic electrolytes. If a
nonstationary state is attained by a fast change of the potential, film breaking is most
probable. Of course, other nucleation mechanisms may contribute as well, such as
mechanical damage of the surface, dissolution of inclusions, and last but not least the
penetration mechanism. Penetration is believed to be the leading mechanism for
pitting of Ni in Cl
–
-containing electrolytes. A conclusive critical experiment to
determine whether penetration of Cl
–
is an initial step for breakdown of Ni passivity
is still missing. The role of inclusions is the subject of another chapter in this book
and will not be discussed in detail here. It was the aim of this chapter to discuss effects
related to pure or at least single-phase metals. In the technical world, however, these
other effects are very important. As chemistry plays a decisive role in the pitting
process, one should discuss the tendency of the different cations to form complexes
with halides. Thus, one has to include the properties of the aggressive anions and of
the specific metals under study as well. This idea is often neglected but seems to be
a key question for breakdown of passivity and the stable growth of corrosion pits, and
it will be discussed again in the next section.
TRANSITION FROM PIT NUCLEATION TO PIT GROWTH,
MICROSCOPIC OBSERVATIONS
In recent literature the transition of nucleation to a stable growth of corrosion pits
is examined with the scanning tunneling microscope (STM) [51,52]. These studies
260 Strehblow
Copyright © 2002 Marcel Dekker, Inc.
try to follow the development of corrosion pits from a diameter of some few
nanometers to several micrometers using besides the STM the scanning electron
microscope (SEM). The results thus give an extension from previous investigations
with the SEM and the light microscope [6] from the μm to the nm range. STM
studies may trace corrosion pits down to a size of some few nm. In principle, these
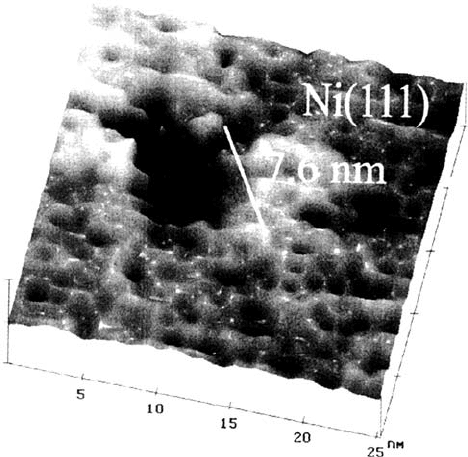
effects have been followed in two different ways. Maurice et al. [51] passivated
Ni(111) single crystals in ~ 0.1 M sulfate solution of pH 3 for 30 min at E = 0.85
and exposed them finally to this solution after the addition of 0.05 V NaCl at the
same potential (pitting potential 0.75 V). Numerous small pits (5–9 × 10
10
cm
–2
)
with a preferential round or some with a triangular shape aligned at steps of
terraces of the (111) plane could be found (Fig. 9). They have an average lateral
size of 20 nm and a depth of 2 nm, which is in the thickness range of the passive
layer of ~ 1 nm. A comparison of the total area of these pits (number and size)
leads to a charge of metal dissolution of ~ 0.7 mC cm
–2
, which is about one order
of magnitude less than the directly measured charge of 1.6 to 8 mC cm
–2
. This
result leads to the conclusion that increased metal dissolution in the passive state
occurs, which is expected according to the adsorption mechanism for pit nucleation.
These small pits are interepreted as pit nuclei that apparently did not grow further.
About 1% of these pit nuclei grow larger (70 nm) but with the same depth and a
triangular shape oriented still parallel to the step edges at the Ni surface.
Mechanisms of Pitting Corrosion 261
Figure 9 STM topographic image of a pit formed on Ni(111) passivated for 1800 s at
0.85 V (SHE) in 0.05 M Na
2
SO
4
, pH 3 and subjected to pitting in the same solution with
additions of 0.05 M NaCl for 4500 s at the same potential. (From Ref. 51.)
Copyright © 2002 Marcel Dekker, Inc.
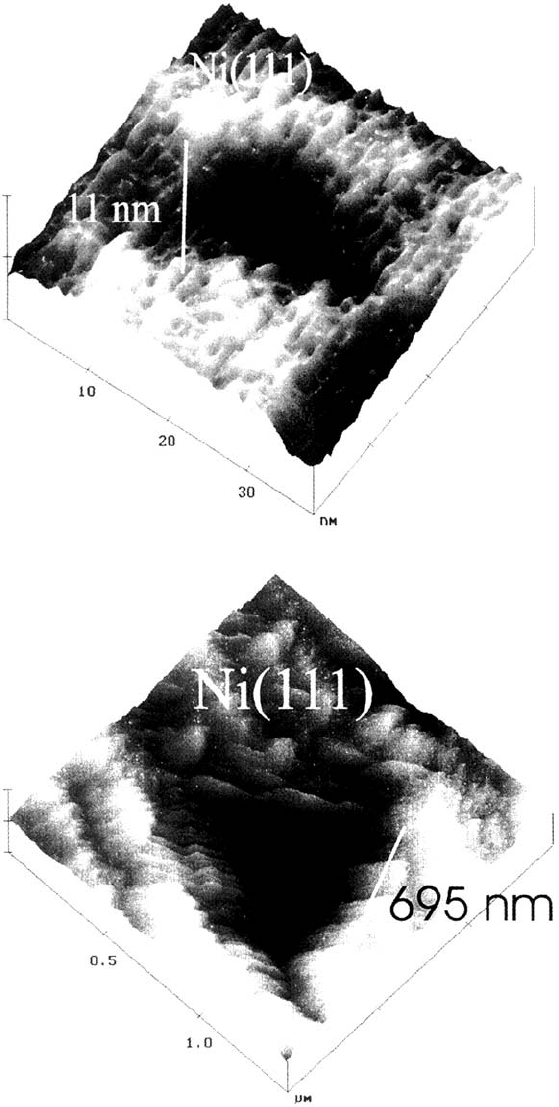
They presumably are repassivated pits. About 0.1% of the pits grow to a size of
some few 100 nm with an elongated irregular shape parallel to the steps. These
pits are seen as repassivated, i.e., metastable pits and grow when current transients
are observed in the potentiostatic current-time curves.
Another approach is the growth of corrosion pits at potenials several hundred
mV more positive than the pitting potential for short times in the ms range. Kunze
and Strehblow prepassivated Ni(111) surfaces by changing the potential from –0.1 V
to values below the pitting potential (E = 0.39 V) for a few seconds in a 0.2 M
NaCl solution of pH 5.6 to prepassivate the surface [52]. Then the electrode was
pulsed to potentials in the range 1.0 to 1.5 V for some few ms to grow pits and
finally stepped back to –0.1 V to stop pit growth. These pulse measurements
facilitated pit nucleation and thus caused a large density of very small corrosion
pits [30,53] that could be studied in situ. The sulfate-free NaCl solution enhanced
pit nucleation and hindered the early change of their shape from a polygon to a
hemisphere [6]. Figure 10 presents a sequence of typical corrosion pits in different
stages of development [52]. Small pits of less than 10 nm lateral size and 1.5 nm
depth have an irregular slightly elongated shape. They change to triangular pits
during further growth. This shape is easily explained by (111) crystal planes
forming the pit surface and their intersection with the (111) Ni surface. They are
the most densely packed crystal planes of face-centered cubic (fcc) Ni and thus
have the largest resistance to metal dissolution. As a consequence, these planes
remain during the dissolution of an actively corroding surface site and thus form
the inner pit surface. They still grow further as shown on the SEM micrograph of
262 Strehblow
Figure 10 Sequence of in situ STM topographic images of growing pits formed on
Ni(111) by potentiostatic pulses in 0.2 M NaCl. pH 5.6 during 50 ms at 1.1 V (SHE) after
3 s prepassivation at 0.39 V. (From Ref. 52.)
Copyright © 2002 Marcel Dekker, Inc.
Mechanisms of Pitting Corrosion 263
Copyright © 2002 Marcel Dekker, Inc.
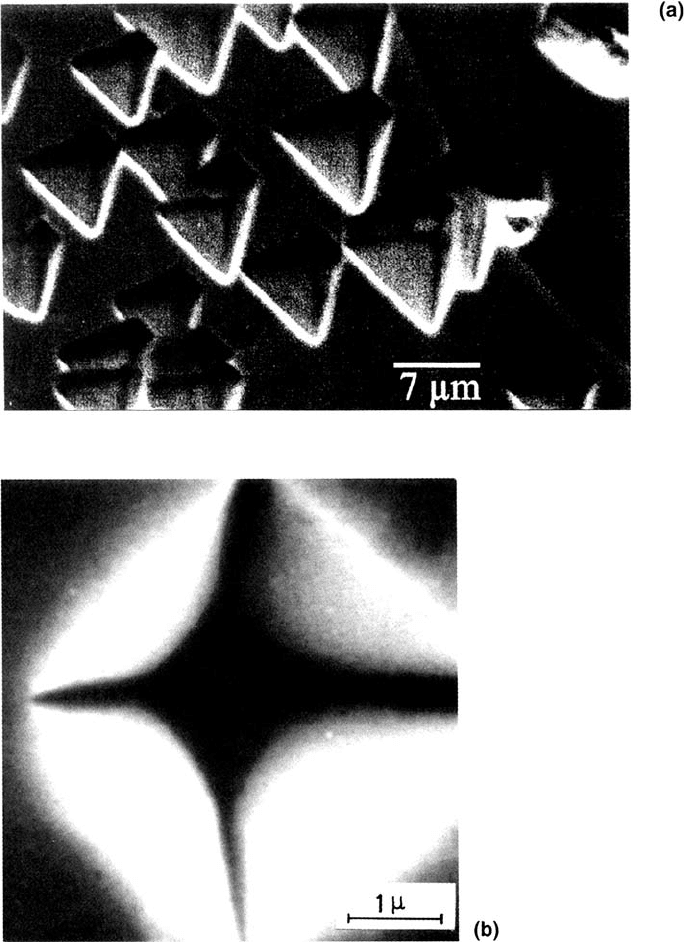
264 Strehblow
Figure 11 SEM images of pits formed on polycrystallinc Ni electrodes: (a) formed in
0.2 M NaCl, pH 5.6 for 5 ms at 1.5 V (SHE) after 10 s prepassivation at 0.5 V (from Ref.
52); (b) formed in 0.05 M phthalate buffer, pH 5.0 with addition of 0.1 M KCl for 200 ms
at 1.00 V (SHE) after preactivation at –0.5 V and 1 s prepassivation at 0.50 V (from
Ref. 58).
Copyright © 2002 Marcel Dekker, Inc.
Figure 11a for a similar experiment on polycrytalline Ni [52]. Depending on the
orientation of the crystallite surfaces, other shapes of the pit orifice also appear
such as squares (Fig. 11b) [58] or triangles with cut-off edges approaching a hexagon.
Figure 12 presents examples of the combination of (111) and (100) planes for
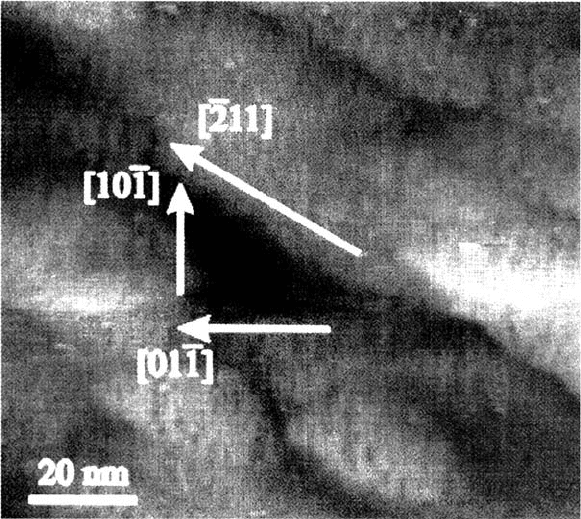
pits on crystallites of body-centered cubic (bcc) Fe grown with the potentiostatic
pulse technique described earlier [6]. These polygonal pits change their shape to
hemispheres when the increasing concentration of corrosion products leads to the
preciptation of a salt film. This situation causes electropolishing of the pit surface by
diffusion-limited metal dissolution within the pit. It starts at the pit bottom, where the
electrolyte is concentrated first, and finally includes the whole pit surface, changing the
polygonal form to a hemisphere [55]. These electropolished hemispheres keep their
form when crossing a grain boundary, whereas the polygons may change their shape
because of the different orientation of the adjacent crytallite (Fig. 13a and b) [6]. The
change of the pit surface from a polygon to a hemisphere will be discussed later in
detail together with the calculation of the electrolyte composition for later stages of
pit growth.
The change of the pit shape with its size and thus its age gives support to the
film breaking mechanism. A rapid change of the electrode potential causes small
cracks within the passive layer with a size similar to the thickness of the passive
layer of 1 to 2 nm. This leads to small elongated pits of some few nm, which then
change to polygonal forms during their growth to some 10 nm (Fig. 10a–c). If the
potential is high and thus the local current density at the pit surface is large
enough, the increasing concentration of corrosion products finally leads to a round
and electropolished hemisphere. Low potentials with smaller pit current densities
do not reach this stage at all or after a longer time so that large polygonal pits may
be observed for these conditions. In the vicinity of the pitting potential, pit growth
becomes instable. As a consequence, a metastable pit growth with a stop and go
sequence will lead to the observed irregular shape.
The presence of large amounts of nonaggressive anions in the bulk electrolyte
also causes their accumulation within the pits. This competition leads to smaller
halide concentrations with a less stable condition for pit growth. The smaller
dissolution current in the presence of these nonaggressive anions (see Fig. 15) further
supports unstable growth and possible repassivation. As a consequence,
discontinuous growth of pits with irregular shapes is observed. Figure 14 gives an
example of a pit grown for 10 s on Ni in 0.05 M phthalate buffer, pH 5 with
0.1 M KCl and 0.1 M K
2
SO
4
. For these conditions pits grow into the metal leaving
a metal cover that is perforated from their inner surface. Apparently, this irregular
shape compensates for the lower current density. The covered pit keeps the
accumulated halide inside and also causes a large ohmic drop that stabilizes its
growth under less favorable conditions. At the inner surface of this larger pit a
small triangular pit is observed, which supports the explanation of continuous
passivation and nucleation for irregular growth. The formation of small corrosion
tunnels with (100) surfaces during pitting of Al in the vicinity of its critical pitting
potential further supports the dicontinuous irregular growth at microscopic and
submicroscopic sites [54]. At more positive potentials, repassivation no longer
occurs and pits with a hemispherical and electropolished surface are obtained.
Mechanisms of Pitting Corrosion 265
Copyright © 2002 Marcel Dekker, Inc.
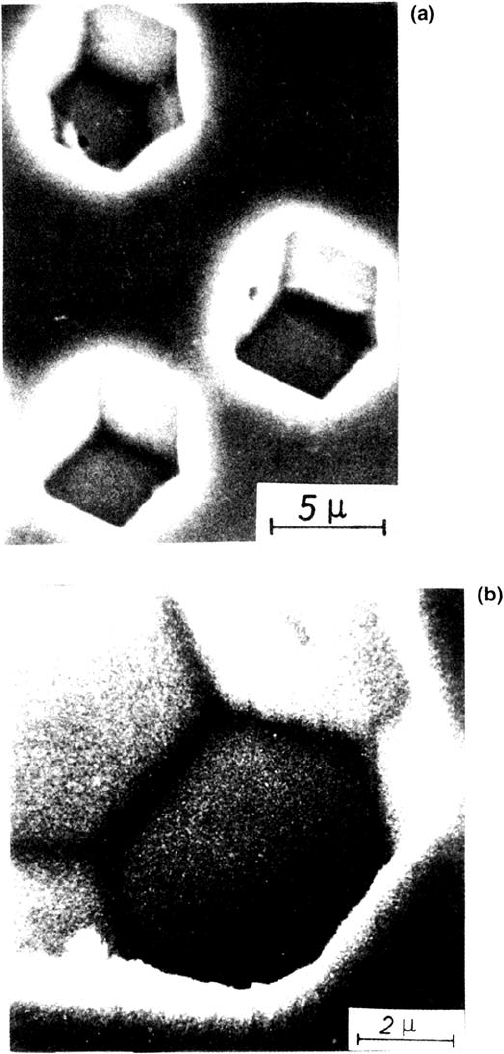
266 Strehblow
Figure 12 SEM images of pits on polycrystalline Fe formed in phthalate buffer, pH 5.0
with addition of 0.01 M KCl for 3 s at 1.10 V on crystallites with (111) orientation. (a)
(110) planes at pit surface; (b) combination of (110) and (100) planes at pit surface. (From
Ref. 6.)
Copyright © 2002 Marcel Dekker, Inc.
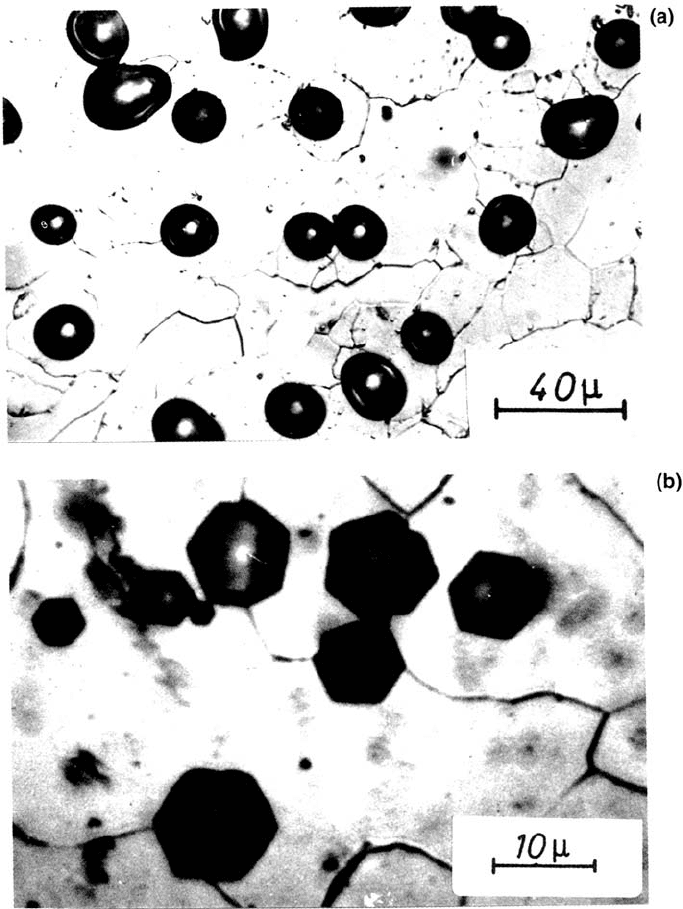
PIT GROWTH
As visualized in the previous section, different stages of pit growth have to be
distinguished, which are closely related to the question of the stability of localized
corrosion. A pit will run through these different stages, each of which has its own
Mechanisms of Pitting Corrosion 267
Figure 13 Microscopic images of pits on polycrystalline Fe formed in phthalate buffer
pH 5.0. (a) With 0.01 M KCl and 0.1 M K
2
SO
4
for 30s at 1.10 V (SHE). (b) with 0.01 M
KCl only, for 3 s at 1.10 V. (From Ref. 6.)
Copyright © 2002 Marcel Dekker, Inc.
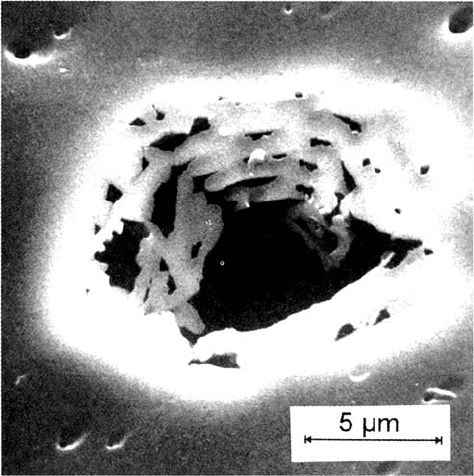
characteristic conditions of stability. Once a pit has nucleated, its local current
density refers to the applied electrode potential. For very positive potentials well
within the passive range, extremely high dissolution current densities of several
tens of A/cm
2
up to more than 100 A/cm
2
have been deduced from the growth of
the corrosion pits using Faraday’s law and taking into account their size and form
(Fig. 15) [53,55]. Equivalent current densities are measured directly on small wire
electrodes of <1 mm diameter embedded in resin when the passivation of the
whole electrode surface is prevented in solutions of high chloride content (>1 M)
starting with a preactivated oxide-free surface [53,56]. Extrapolating the dissolution
kinetics of free metal corrosion to potentials in the passive range of the polarization
curve leads to extremely high local current densities of 10
3
to 10
6
A/cm
2
[57].
These large current densities would cause precipitation of a salt film within 10
–4
to 10
–8
s. Current densities of more than 10
3
A/cm
2
are possible in principle but
only up to 120 A/cm
2
has been observed for a free corroding metal surface [53,55].
The local current density increases with the chloride concentration, whereas
the critical pitting potential decreases with increasing chloride concentration. If,
however, i
c,p
is presented relative to E
p
, the data fit a single curve. Apparently an
increasing chloride concentration shifts the whole i
c,p
/(E – E
P
) curve in the negative
direction [58, p. 55]. The addition of nonaggressive anions such as sulfate or nitrate
shifts the exponential current increase to positive potentials with a large potential-
independent plateau at low potentials [53,58, p. 57]. These plateau values are in the
range of a few A/cm
2
, i.e., 5 A/cm
2
for Ni in 0.1 M KCl + 0.1 M K
2
S0
4
and 2 A/cm
2
268 Strehblow
Figure 14 Microscopic image of a metal-covered pit with irregular shape on
polycrystalline Ni formed in 0.05 M phthalate buffer, pH 5.0 with 0.1 M K
2
SO
4
and 0.1 M
KCl for 10 s at 0.90 V (SHE), Small triangular pit at inner pit surface. (From Ref. 58.)
Copyright © 2002 Marcel Dekker, Inc.
