Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

remove it from the surface by dissolution of the cluster formed. More recently, the
molybdenum-induced removal of adsorbed sulfur has been observed in measurements
performed on single-crystal alloys with chemical compositions close to the
compositions of type 304 and 316 stainless steels, i.e., without and with molybdenum,
respectively [7]. After preadsorbing sulfur on the surfaces of the two alloys, the sulfur
coverage was measured as a function of time of exposure of the alloys to 0.05 M
H
2
SO
4
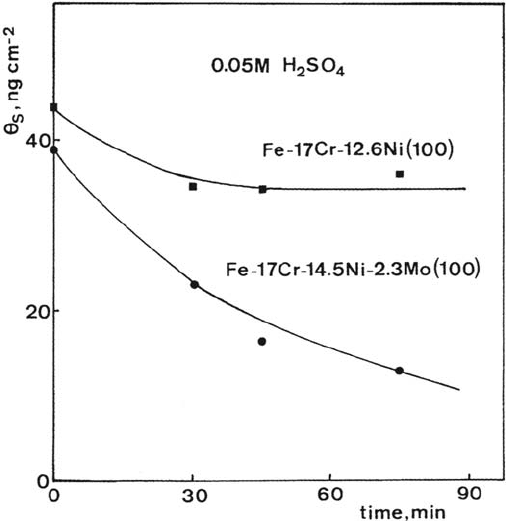
at the corrosion potential. The results are shown in Figure 11. The monolayer
of sulfur adsorbed on the 304-type stainless steel is stable in 0.05 M H
2
SO
4
at the
corrosion potential, whereas, owing to the presence of molybdenum, the desorption
of sulfur is observed on the 316-type stainless steel. The model used above gives a
fit of the experimental data for k ~ 1, instead of k = 2 for the Ni-Mo alloys. This
suggests that chromium lowers the number of Mo atoms required to promote the
dissolution of sulfur.
The Role of Chromium
The role played by alloyed Cr in the presence of sulfur has been investigated in detail
on Ni-based alloys with various concentrations of Cr in the range 8 to 34% (a range
of concentration within which alloys 600 and 690 are situated) [5,6,18]. The combined
electrochemical, radiochemical (
35
S), and spectroscopic (XPS) measurements
300 Marcus
Figure 11 Mo-induced removal of sulfur adsorbed on a single-crystal alloy of composition
close to 316 stainless steel. The measurements on an alloy of composition close to 304
stainless steel are shown for comparison. The coverage of sulfur was measured with
radioactive sulfur (
35
S).
Copyright © 2002 Marcel Dekker, Inc.
performed with sulfur preadsorbed on the surface and with bulk sulfur (introduced
in the alloy in controlled amounts by bulk diffusion of S at high temperature)
revealed that Cr allows the surface to be passivated under conditions in which the
passivation would be precluded by sulfur if there were no Cr. The surface analysis by
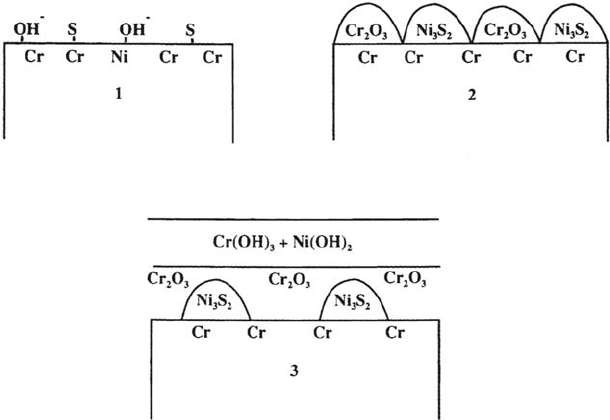
XPS [18] revealed the coexistence of nickel sulfide and chromium oxide on the
surface. The mechanism of this counteracting effect of Cr is totally different from the
mechanism of the Mo effect discussed earlier. With Cr the desorption of sulfur is not
the key factor. What Cr does is to react selectively with oxygen (i.e., with OH or
H
2
O) to give Cr oxide, whereas Ni reacts with sulfur. This leads to the coalescence of
S in small nickel sulfide islands that can be covered by the lateral growth of the
chromium oxide islands. In other words, the antagonistic effects of Cr and S are
based on the fact that, on the considered alloys, Cr acts as an oxide former whereas
Ni acts as a sulfide former. This competitive mechanism is represented schematically
in Figure 12. In agreement with this mechanism, it has been observed that H
2
S,
which accelerates the anodic dissolution of chromium, has no influence on passivated
chromium [37].
IMPLICATIONS IN AREAS OF PRACTICAL IMPORTANCE
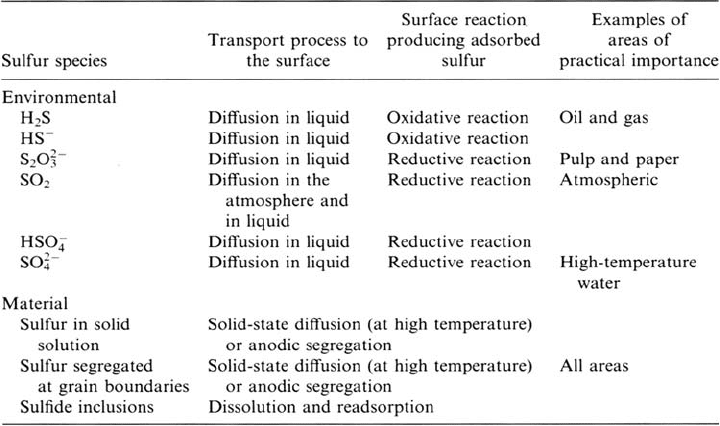
There is much evidence for the damaging effect of sulfur species in a wide range of
corrosion-related service failures. The relation between the sulfur-induced corrosion
mechanisms presented in this chapter and the implications in areas of practical
importance can be rationalized on the basis of (a) the source of sulfur, (b) the
transport process to the metal surface, and (c) the conditions of the reduction (or
Sulfur-Assisted Corrosion Mechanisms 301
Figure 12 The mechanism of the antagonistic effects of chromium and sulfur on the
corrosion resistance of Ni-Cr-Fe alloys.
Copyright © 2002 Marcel Dekker, Inc.
oxidation) of the sulfur species into the harmful chemical state of sulfur, i.e., the
adsorbed (chemisorbed) sulfur (or the sulfide if the concentration of the sulfur
species is high). Table 2 summarizes some of these considerations and gives a few
examples of practical areas in which detrimental effects of sulfur have been identified,
e.g., oil and gas, pulp and paper industries, power plants (high-temperature water
reactors), and atmospheric corrosion. Further details can be found in the other
chapters of this book.
Two categories of sulfur species may be considered according to the origin of
the species: (a) sulfur species (molecules or ions) in the environment, e.g., SO
2
(gaseous), H
2
S (gaseous or aqueous), HS
–
, HS
2
O
3
–
, S
2
O
2
3
–
,S
4
O
2
6
–
, HSO
4
–
, SO
2
4
–
, and
(b) sulfur in the material: sulfur in solid solution, sulfur segregated at the surface
or in the grain boundaries, and sulfur in sulfide inclusions. After considering the
source of sulfur, the process by which the sulfur species arrive at the surface (transport
process) has to be identified. For environmental sulfur species it is generally
diffusion in the liquid (e.g., aqueous solution). For sulfur present in the bulk metal
or alloy, it may be surface segregation by solid-state diffusion at high temperature
or anodic segregation (a process that has been defined in a preceding section). Higher
anodic dissolution rates of grain boundaries, further accelerated by sulfur (according
to the mechanism of sulfur-enhanced dissolution described earlier), result in rapid
surface enrichment of sulfur at grain boundaries exposed to the electrolyte. In the case
of sulfide inclusions, e.g., MnS, although their detrimental effect on corrosion of
steels and stainless steels has been extensively studied [30– 33,38–41], the exact
mechanism is not fully understood. The important case of sulfide inclusions in
stainless steels is discussed in detail in Chapter 10. On the basis of the
302 Marcus
Table 2 Sulfur Species that may be Present in the Environment or the Material, with the
Process of Transport to the Surface, Nature of the Surface Reaction Producing Adsorbed
Sulfur, and Examples of Areas of Practical Importance
Copyright © 2002 Marcel Dekker, Inc.
data reviewed here, other authors have invoked the role of adsorbed sulfur to explain
the detrimental effects of sulfide inclusions in stainless steels [38–40]. It must be
pointed out that early studies of the effects of H
2
S addition on corrosion of stainless
steels [41] have revealed the equivalence of the effects of sulfur added in the form of
H
2
S or present in sulfide inclusions. The effects of S
2
O
2
3
–
observed in the pulp and
paper industry [23] and of sulfur species in the steam generators of pressurized water
reactors [43] have also been interpreted on the basis of the data presented in this
chapter. Attempts to investigate the transport of sulfur from the inclusions to the
surrounding surface led to the conclusion that sulfur is cathodically deposited after
dissolution of the sulfide, producing rings of sulfur around the sulfide inclusion [38].
In a study of the mechanisms of pitting corrosion of stainless steels using submicron
resolution scanning electrochemical and photoelectrochemical microscopy [42] it
was concluded that the electrodissolution of certain MnS inclusions in stainless steel
is chloride catalyzed. The enchancement of the dissolution of sulfide inclusions by
Cl
–
had also been suggested in an earlier work [40].
After the process by which sulfur is transported to the surface, the nature of the
surface reaction producing adsorbed sulfur must be considered. Sulfur may be
adsorbed by electro-oxidation of sulfides [H
2
S (aqueous) or HS
–
] or by electro-
eduction of sulfates (HSO
4
–
or SO
2
4
–
) or thiosulfates (HS
2
O
3
–
, S
2
O
2
3
–
).
The oxidative or reductive reactions and the conditions of potential and pH in
which they may take place are detailed in the following. For SO
2
, the surface reactions
are presented in the chapter on atmospheric corrosion (chap. 15).
Once sulfur is present on the surface in the active chemical state (i.e., adsorbed),
it has the same effects (which have already been described) irrespective of its origin.
The stage at which the surface reactions become localized is not exactly known.
It is probably strongly related to the stage at which surface defects are crucial, which
is the case for the surface reactions that produce adsorbed sulfur.
THERMODYNAMICS OF SULFUR ADSORPTION ON METAL
SURFACES IN WATER
The principle of potential-pH (Pourbaix) diagrams has been extended to the case of
bidimensional layers of elements adsorbed on metal surfaces [44,45]. It allows us to
predict the conditions of stability of adsorbed sulfur on metals immersed in water
containing dissolved sulfur species such as S, H
2
S, HS
–
, HS
2
O
3
–
, S
2
O
2
3
–
, HSO
4
–
, and
SO
4
2–
. The calculated E-pH diagrams show that the stability domain of adsorbed sulfur
extends beyond the usually predicted range of stability of metal sulfides, and thus
adsorbed sulfur layers can exist under conditions in which no bulk sulfide is stable.
Potential-pH diagrams have been calculated for sulfur adsorbed on surfaces of iron,
nickel, and chromium, in water containing sulfides or sulfates [44–46], and in water
containing sulfides and thiosulfates [47]. They are now also available for copper [48].
Principle of E-pH Diagrams for Adsorbed Species
An element A (which may be O, S, N, or H) is adsorbed on a metal M in the form
of a monoatomic layer, with a valence state equal to zero [denoted A
ads
(M)]. The
Sulfur-Assisted Corrosion Mechanisms 303
Copyright © 2002 Marcel Dekker, Inc.

adsorption of A from a species dissolved in aqueous solution may be an electro-
oxidation or an electro-eduction reaction, depending on the valence state of A in
the dissolved species.
In contrast to adsorption in ultrahigh vacuum (UHV) and in gas phase,
described in Chapter 2, the adsorption of an atom or a molecule on a metal surface in
water involves replacement of adsorbed water molecules [H
2
O
ads
(M)] and competition
with the adsorption of oxygen [O
ads
(M)] or hydroxyl [OH
ads
(M)] resulting from the
dissociation of H
2
O molecules on the metal surface.
In a Langmuir model for adsorption, it is assumed that the two-dimensional
phase is an ideal solution, where water, oxygen or hydroxyl, and sulfur adsorb
competitively on the same surface sites and there are no interactions between
adsorbed species.
Under these conditions the chemical potential of each element A in the phase
adsorbed on a metal M can be expressed as follows:
304 Marcus
where θ
A
is the relative coverage of the surface of M by adsorbed A (0 ≤ θ
A
≤ 1;
θ
A
= 1 for the complete monolayer of A); μ
°
Aads(M)
is the standard chemical potential
of A, corresponding to saturation of the surface by A. The chemical potential of
adsorbed water is also given by:
with
As an example of the method of calculation of the E-pH relations, let us consider the
adsorption on a metal of oxygen from water:
H
2
O
ads
(M) = O
ads
(M) + 2H
+
+ 2e
–
The equilibrium potential of this half-reaction is obtained by applying the Nernst
law with the chemical potentials of adsorbed oxygen and water expressed as in
Eqs. (1) and (2):
with the standard potential E° given on the standard hydrogen scale by
with μº
H+
+ μº
e–
=
1
–
2
μº
H
2
(g)
.
Electrochemical experiments and surface analyses show that the adsorbed
oxygen species in solution on most transition metals at 25°C are likely to be
hydroxyls. Thermodynamic data obtained from electrochemical experiments are
presently available only for OH
ads
on copper [49]. Therefore O
ads
is considered here
on Fe, Ni, and Cr and OH
ads
on Cu. The standard Gibbs energies of formation
(chemical potentials) for sulfur and oxygen adsorbed on metal surfaces can be
calculated [44–46] from literature thermodynamic data for reversible chemisorption
at the metal-gas interface (see Chap. 2).
Copyright © 2002 Marcel Dekker, Inc.
E-pH Relations for the Equilibria between Dissolved and
Adsorbed Species
In water containing thiosulfates, the sulfur species to be considered are the
thermodynamically stable sulfide species H
2
S
(aq)
and HS
–
, and the metastable
thiosulfate species HS
2
O
3
–
and S
2
O
2
3
–
. The E-pH relations associated with the various
equilibria between water, the dissolved sulfur species and adsorbed sulfur, and
oxygen or hydroxyl adsorbed on Fe, Ni, Cr, and Cu at 25°C are as follows:
Sulfur-Assisted Corrosion Mechanisms 305
Copyright © 2002 Marcel Dekker, Inc.
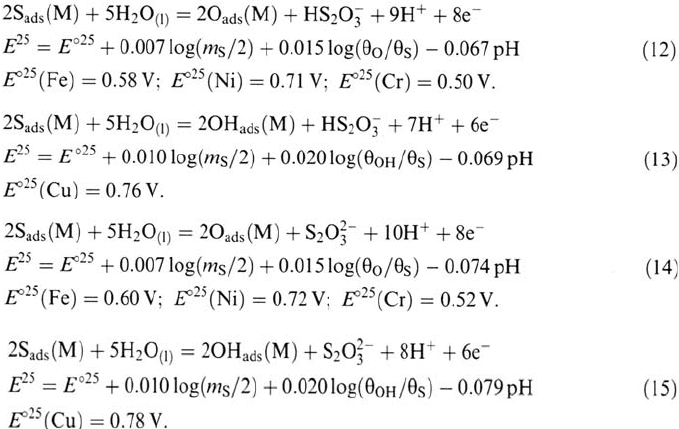
Potential-pH Diagrams
The preceding equations have been used to construct the potential-pH diagrams for
sulfur and oxygen (hydroxyl) adsorbed in water containing sulfides (H
2
S or H) or
thiosulfates (HS
2
O
3
–
or S
2
O
2
3
–
) on Fe, Ni, Cr, and Cu [44–48]. The diagrams are
shown in Figures 13 to 16 at 25°C for different sulfur coverages (θ
s
= 0.01; 0.5; 0.99)
and a molality of dissolved sulfur m
s
= 10
–4
mol kg
–1
. The diagrams are superimposed
on the S-M-H
2
O diagrams (M = Fe, Ni, Cr, Cu), calculated for a molality of dissolved
metal m
M
=10
–6
mol kg
–1
.
These diagrams allow us to predict the E-pH conditions in which sulfur is
adsorbed on a surface of Fe, Ni, Cr, or Cu in water by oxidation of sulfides or
reduction of thiosulfates. In aqueous solution, when the potential is increased in
the anodic direction, the adsorbed water molecules are replaced by sulfur atoms
adsorbed from H
2
S
(aq)
and HS
–
. At higher potentials, sulfur is oxidized in HS
2
O
3
–
or
S
2
O
2
3
–
and replaced by adsorbed oxygen or hydroxyl. The replacement reaction is
completed within a very narrow range of potential (~ 0.06 for O
ads
and 0.08 V for
OH
ads
). The domains of stability of the adsorbed S monolayer (for m
s
=10
–4
mol kg
–1
and m
M
=10
–6
mol kg
–1
) overlap the domains of stability of the metals (Fe, Ni, Cr,
Cu), the dissolved cations (Fe
2+
, Ni
2+
, Cr
2+
, Cr
3+
, Cu
+
, and Cu
2+
), and the oxides or
hydroxides (Fe
3
O
4
, Fe
2
O
3
, Ni(OH)
2
, NiO, Cr
2
O
3
, Cu
2
O, CuO). The stability
domains of S
ads
are significantly wider than the stability domains of the bulk metal
sulfides, and thus S
ads
is stable in E-pH regions where there is no stable metal sulfide,
which reflects the excess of stability of the chemisorbed state with respect to the
corresponding 3D compound. On this basis, detrimental effects of sulfur on the corrosion
resistance of metals are predicted even under potential and pH conditions where the
metal sulfides are not thermodynamically stable. The comparison of the behaviors of
the different metals in the presence of dissolved sulfur species shows that the
306 Marcus
Copyright © 2002 Marcel Dekker, Inc.
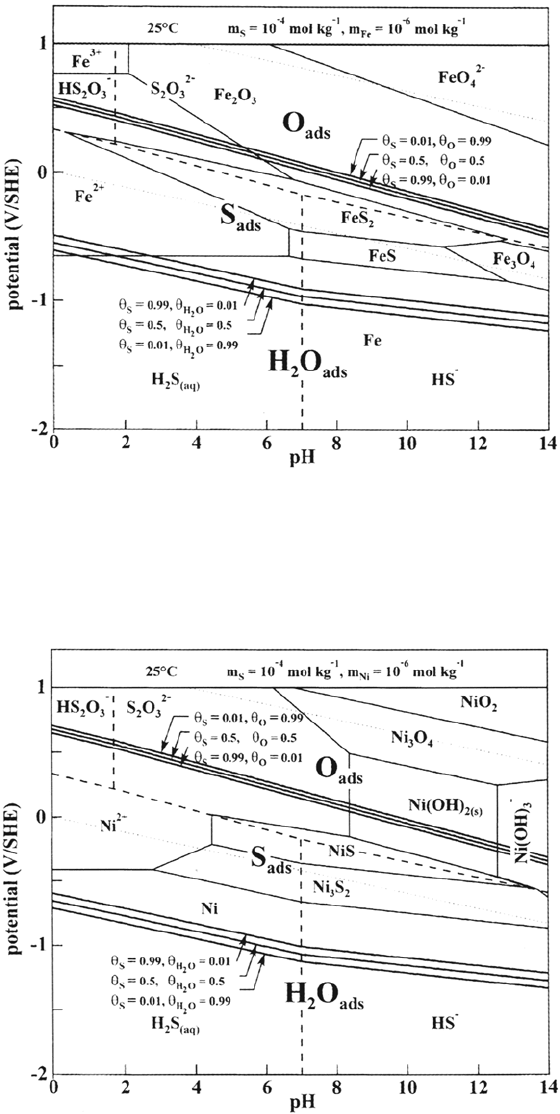
Sulfur-Assisted Corrosion Mechanisms 307
Figure 13 Potential-pH diagram for sulfur adsorbed on Fe (25°C, m
s
= 10
–4
mol kg
–1
).
Figure 14 Potential-pH diagram for sulfur adsorbed on Ni (25°C, m
s
= 10
–4
mol kg
–1
).
Copyright © 2002 Marcel Dekker, Inc.
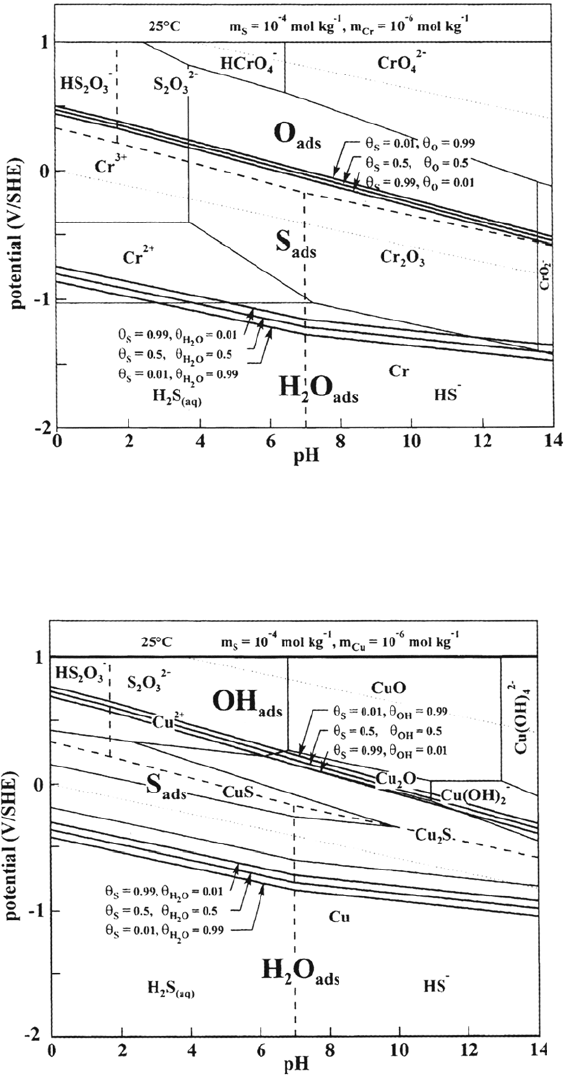
308 Marcus
Figure 15 Potential-pH diagram for sulfur adsorbed on Cr (25°C, m
s
= 10
–4
mol kg
–1
).
Figure 16 Potential-pH diagram for sulfur adsorbed on Cu (25°C, m
s
= 10
–4
mol kg
–1
).
Copyright © 2002 Marcel Dekker, Inc.
extent of the overlap of S
ads
with the stable metals decreases in the sequence Ni,
Fe, Cr, so the effect of thiosulfates on corrosion of these metals is expected to
decrease in the same order Ni > Fe > Cr. Prediction of S adsorption in the active
domains (i.e., during anodic dissolution) is also important because this is the
condition in which S-enhanced dissolution is experimentally observed. Another
effect of thiosulfates expected from the stability of S
ads
in the passive domains
is the blocking or retarding of passivation (or repassivation) of stainless steels.
Such diagrams are useful to assess the risk of corrosion of metals and alloys
induced by adsorbed sulfur produced by electro-oxidation of sulfides or electro-
eduction of thiosulfates.
CONCLUSION
The fundamental aspects of sulfur-induced corrosion have been reviewed. The
mechanisms have been derived from data obtained on chemically and structurally
well-defined surfaces using electrochemical and surface analysis techniques
(
35
S radiotracer and surface spectroscopies).
The data obtained show the direct link that exists between atomic-scale surface
reactions of sulfur and macroscopic manifestations (enhanced dissolution, blocking
or retarding of passivation, and passivity breakdown). The data provide the
fundamental basis required to rationalize the detrimental effects of sulfur species
encountered in a large number of service conditions.
REFERENCES
1. P. Marcus, N. Barbouth, and J. Oudar, C.R. Acad. Sci. Paris 280:1183 (1975).
2. J.Oudar and P. Marcus, Appl. Surf. Sci. 3:48 (1979).
3. P. Marcus and J. Oudar, Fundamental Aspects of Corrosion Protection by Surface
Modification (E. McCafferty C.R. Clayton, and J. Oudar, eds.), The Electrochemical
Society, Pennington, NJ, 1984, p. 173.
4. P.Marcus, A. Teissier, and J. Oudar, Corros. Sci. 24:259 (1984).
5. P. Combrade, M. Foucault, D. Vançon, J. M. Grimal, and A. Gelpi, Proceedings of the
4th International Symposium on Environmental Degradation of Materials in Nuclear
Power Systems-Water Reactors (D. Cubicciotti, ed.), NACE, Houston, 1990, p. 5.
6. D. Costa and P. Marcus, Proceedings of the European Symposium on Modifications of
Passive Films (P. Marcus, B. Baroux, and M. Keddam, eds.), The Institute of
Materials (EFC 12), 1994, p. 17.
7. A. Elbiache and P. Marcus, Corros. Sci. 33:261 (1992).
8. P. Marcus, Advances in Localized Corrosion (H. S. Isaacs, U. Bertocci, J. Kruger, and
S. Smialowska, eds.), NACE, Houston, 1990, p. 289.
9. S. Ando, T. Suzuki, and K. Itaya, J. Electroanal. Chem. 412:139 (1996).
10. G. Seshadri, H.-C. Xu, and J.A. Kelber, J. Electrochem. Soc. 146:1762 (1999).
11. H. Ma, X. Cheng, G. Li, S. Chen, Z. Quan, S. Zhao, and L. Niu, Corros. Sci. 42:1669 (2000).
12. H.C. Xu, G. Seshadri, and J.A. Kelber, J. Electrochem. Soc. 147:558 (2000).
13. V. Maurice, H. Talah, and P. Marcus, Surf. Sci. 284:L431 (1993).
14. V. Maurice, H. Talah, and P. Marcus, Surf Sci. 304:98 (1994).
15. D. Zuili, V. Maurice, and P. Marcus, J. Electrochem. Soc. 147:1393 (2000).
16. P. Marcus, I. Olefjord, and J. Oudar, Corros. Sci. 24:269 (1984).
Sulfur-Assisted Corrosion Mechanisms 309
Copyright © 2002 Marcel Dekker, Inc.
