Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

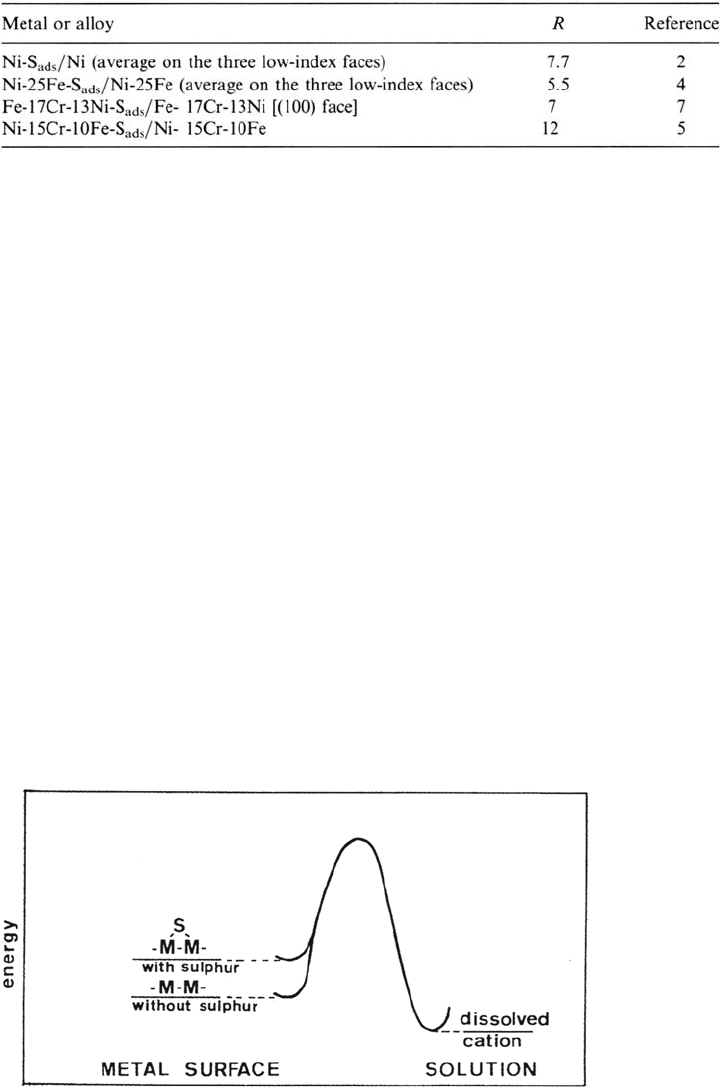
activation energy barrier for the passage of a metal atom from the surface to the
solution. This mechanism is shown schematically in Figure 3 [8]. The existence of a
dipole M
δ+
– S
δ–
associated with the presence of sulfur can also promote the
anodic dissolution.
The influence of sulfur can be strongly localized if the sulfur is not homogeneously
distributed over the surface but adsorbed in specific sites, such as surface defects,
where sulfur atoms are more tightly bonded.
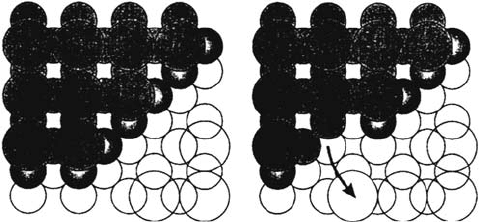
Direct observation of the dissolution of sulfur-modified Ni(100) [9] using in situ
scanning tunneling microscopy (STM) is in agreement with the previously shown
effects of adsorbed S on dissolution (including the enhanced dissolution and the
stability of the S layer). It also provides structural data at the atomic scale, indicating
that the dissolution of nickel atoms is taking place at step edges, with a displacement
of adsorbed S atoms from the edge of the upper terrace to the adjacent lower terrace,
as shown in Figure 4.
On Cu, it was also shown that the anodic dissolution, in a mildly alkaline
borate buffer solution, is catalyzed by a submonolayer of sulfur and that S remains
adsorbed on the copper surface [10].
The anodic dissolution of iron in acidic medium is also accelerated by H
2
S [11].
290 Marcus
Table 1 Ratio (R) of the Current Densities at the Maximum of the Active Peaks with and
without Adsorbed Sulfur
a
a
Coverages of the surfaces by preadsorbed sulfur are (in 10
– 9
g/cm
2
): 42 on Ni(100), 47 on Ni(111), 44
on Ni(110), 40 on Ni-25Fe (100), 42 on Ni-25Fe(111), 41 on Ni-25Fe (110), 40 on Fe-17Cr-13Ni(100),
and 40 on Ni-15Cr-10Fe.
Figure 3 Weakening of the metal-metal bond by adsorbed sulfur and acceleration of the
dissolution of the metal.
Copyright © 2002 Marcel Dekker, Inc.
The major conclusion of the results reviewed in this section is that a
monoatomic layer of sulfur can promote the dissolution of macroscopic amounts
of material. This gives evidence of a direct link between the atomic-level interactions
of adsorbed sulfur atoms with metal surfaces and their macroscopic manifestations in
corrosion.
Blocking or Retarding Effect of Adsorbed Sulfur on Passivation
Effect on the Growth of the Passive Film
Another important effect of adsorbed sulfur that was observed first on Ni [1–3]
and later on Ni-Fe alloys [4] is the poisoning of passivation. This effect takes place
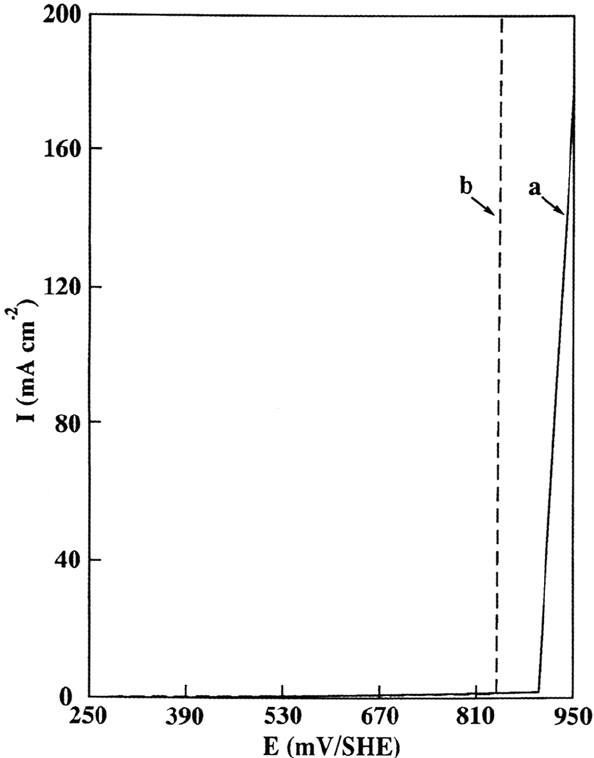
above a critical coverage of the surface by sulfur that was found to be 0.7–0.8
monolayer. This effect of sulfur is observed in the i-E diagram shown previously in
Figure 1, where the passivation potential of the surface covered by a complete
monolayer of sulfur (θ = 1) is shifted (by ~100mV) to a more anodic value with
respect to the sulfur-free surface. In using the
35
S radiotracer, it was demonstrated
that the desorption (or electro-oxidation) of ~20% of the full monolayer is
necessary to allow the passive film to be formed. The retarding effect of sulfur on
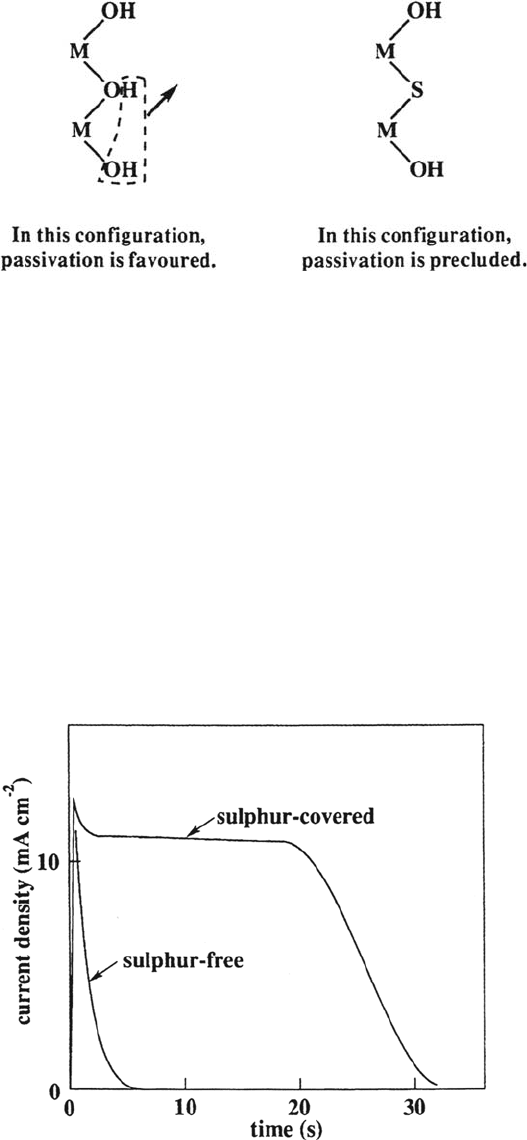
the growth of the passive film is caused by blocking the sites of adsorption of
hydroxyl ions, which are the precursors in the formation of the passive layer. X-ray
photoelectron spectroscopy (XPS) measurements have revealed that in the
presence of adsorbed sulfur the adsorption of OH groups is not totally inhibited,
but the OH groups on the surface are more diluted and thus the disproportionation
reaction of adjacent OH groups is prevented. A schematic representation of this
mechanism is given in Figure 5. In this way the oxide film, which would normally
passivate the surface, is not formed.
Effect on the Passivation Kinetics
Measurements of the passivation kinetics by means of potential steps applied to
surfaces without or with adsorbed sulfur have revealed that the time to complete
passivation increases when the surface is covered by adsorbed sulfur. This effect is
Sulfur-Assisted Corrosion Mechanisms 291
Figure 4 Model of the anodic dissolution process of sulfur-covered Ni(100) electrodes,
showing dissolution of two Ni atoms from the step edge and displacement of an S atom from
the upper terrace to a fourfold hollow site on the lower terrace (small circles denote Ni atoms,
large circles denote S atoms, the gray color indicates atoms of the upper terrace). (Adapted
from Ref. 9.)
Copyright © 2002 Marcel Dekker, Inc.
represented in the i-t diagram of Figure 6. A rapid decrease of the current
immediately after the potential step is observed for the clean surface, whereas for
the sulfur-covered surface the current density decreases slowly, indicative of a slow
process of lateral growth of the film on top of the remaining fraction of a monolayer
of adsorbed sulfur. This is consistent with the view that a complete monolayer of
sulfur inhibits the nucleation of the oxide, as observed on nickel, whereas a fraction
of a monolayer does not inhibit the nucleation but may diminish the density of
nucleation sites for the oxide and make the lateral growth of the film more difficult.
These two effects can slow down the passivation kinetics.
Such kinetic effects have been observed on sulfur-contaminated nickel [8], on
nickel alloy 600 [6], and on copper [12].
292 Marcus
Figure 5 The mechanisms of the blocking effect of adsorbed sulfur on passivation.
Figure 6 The effect of adsorbed sulfur on the passivation kinetics of nickel [Ni(111), 0.05 M
H
2
SO
4
, 540 mV/SHE].
Copyright © 2002 Marcel Dekker, Inc.
Effect on the Structure and Properties of the Passive Film
Below the critical sulfur coverage of 0.7–0.8 monolayer on Ni, the passive film grows
on top of the remaining adsorbed sulfur. However, the structure and the properties
of the film formed on the sulfur-contaminated surface are modified. A study [2] by
reflection high-energy electron diffraction (RHEED) of the structure of the passive
films formed on nickel single crystals [Ni(111)] has shown that, in the absence of
sulfur, a crystalline film epitaxial with the substrate is formed (a fact that has been
confirmed by both ex situ and in situ atomic resolution imaging of the film by
scanning tunneling microscopy [13–15]), whereas in the presence of sulfur the
epitaxial growth is disrupted and a more defective polycrystalline film is obtained.
Accordingly, the current density in the passive state is about four times larger for a
film formed with sulfur at the metal-oxide interface. XPS measurements of the
binding energy of sulfur (S 2p) provide evidence that it remains at the interface
because the binding energy remains close to that of sulfur bonded to the metallic
surface (~ 162 eV).
Anodic Segregation of Sulfur
The concept of anodic segregation was first proposed after the discovery that
sulfur present as an impurity in the bulk of Ni and of Ni-25Fe alloys is enriched on
the surface during anodic dissolution [2,4,16]. This phenomenon was also
observed on Fe-50Ni [17] and on alloy 600 [5,18]. The experiments were performed
on Ni and Ni-Fe alloys that had been doped with sulfur prior to the electrochemical
treatments. The introduction of radioactive S into the bulk was performed by
exposing the samples to an H
2
S-H
2
gas mixture (with
35
S-labeled H
2
S) at high
temperature (1000 to 1200°C) under thermodynamic conditions in which sulfur is
in solid solution in the metal or the alloy. Rapid quenching to room temperature
after the high-temperature treatment avoids sulfide precipitation. The i-E curves
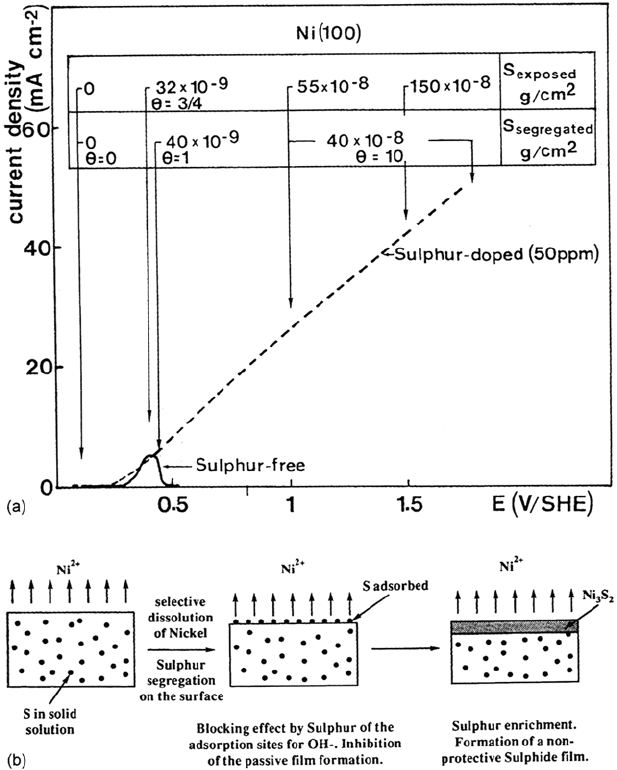
recorded on a sulfur-free (i.e., annealed in pure hydrogen) Ni electrode and on Ni
containing 50 ppm sulfur are shown in Figure 7a. The sulfur-free Ni sample
exhibits the normal active-passive transition observed in 0.05 M H
2
SO
4
. The
sample with bulk sulfur exhibits a completely different behavior: there is no active-
passive transition, and the current density increases with increasing potential in the
whole range of potentials corresponding to the passive state of sulfur-free Ni. The
results of the measurements of the sulfur concentration on the surface, using
radioactive sulfur, are shown in Figure 7a. They demonstrate that sulfur segregates
on the surface during dissolution of the electrode with bulk sulfur (the measured
values of the surface sulfur concentration are denoted S
segregated
in Fig. 7a). The
values denoted S
exposed
in Figure 7a represent the integrated amount of sulfur that
became available on the surface due to the dissolution of the metal matrix.
Comparison of the data for S
segregated
and S
exposed
revealed that all the sulfur present
in the bulk remains on the surface during dissolution, up to a surface concentration of
40 × 10
–9
g/cm
2
, which corresponds to a complete monolayer of sulfur on Ni(100).
Above this surface coverage, nickel sulfide precipitates and then a thin layer of nickel
sulfide is formed. The amount of sulfur in this layer is ~40 × 10
–8
g/cm
2
, which corresponds
to ~25 Å of Ni
3
S
2
. Sulfur in excess of this amount is dissolved. The formation, before
reaching the passivation potential, of a complete monolayer of sulfur and the subsequent
Sulfur-Assisted Corrosion Mechanisms 293
Copyright © 2002 Marcel Dekker, Inc.
growth of a sulfide layer preclude the formation of the passive oxide film. The
sulfide layer is not protective and thus high corrosion rates are obtained in the range
of potentials in which pure Ni is normally passivated. This phenomenon was called
anodic segregation [3,4]. The anodic segregation rate depends on the sulfur content
of the material and on the rate of anodic dissolution. Because of differences in
dissolution rates for single-crystal surfaces of different crystallographic orientation,
the sensitivity to sulfur increases in the order (111) ≤ (100) < (110), which is the order
of increasing dissolution rates observed for Ni in 0.05 M H
2
SO
4
[2]. The mechanism
of anodic segregation is represented in Figure 7b.
294 Marcus
Figure 7 (a) Surface enrichment of sulfur during anodic dissolution: i-E curves and radio-
tracer measurements of the sulfur concentration on the surface of Ni(100) containing 50 ppm
of sulfur (0.05 M H
2
SO
4
, 1 V h
–1
). (b) The mechanism of anodic segregation.
Copyright © 2002 Marcel Dekker, Inc.
Passivity Breakdown Caused by Interfacial Sulfur
After the formation of the passive film on a bare metal surface, slow dissolution of
the metal cations continues, involving dissolution at the passive film surface and
transport of ions through the oxide (see Chap. 6). The question then arises as to where
the bulk impurities, e.g., sulfur, go during this process. This question was addressed
in a detailed investigation of Ni and Ni-Fe alloys that were doped with radioactive
sulfur in order to trace the path taken by the sulfur atoms during the slow dissolution
in the passive state [19]. The radiochemical results gave direct evidence that sulfur
present in the bulk accumulates at the metal–passive film interface. The enrichment
rate was shown to be proportional to the sulfur content in the metal and to the
dissolution current density. Above a critical concentration of sulfur at the interface
(metal-oxide), breakdown of the passive film was observed. This critical concentration
of interfacial sulfur was measured with radioactive sulfur and found to be close to one
monolayer of sulfur (i.e. ~ 40 × 10
–9
g/cm
2
). The loss of adherence (or the decohesion)
at the interface for this critical coverage has been attributed to aweakening of the
bonding of the oxide to the substrate caused by sulfur. The nucleation of the sulfide
that is expected to take place when the sulfur concentration exceeds a
complete monolayer may be responsible for the observed local breakdown of the film
and pitting. The defects that are likely to exist at the interface could serve as specific
sites for the nucleation of the sulfide, once a quasi-complete monolayer of sulfur has
been accumulated at the interface. A schematic representation of the proposed
mechanism of the sulfur-induced breakdown of the passive film is shown in Figure 8.
It is to be noted that the three following factors are in favor of sulfur remaining
at the metal–passive film interface: (a) the sulfur-metal chemical bond is very strong
(see Chap. 2), (b) the solubility of S in nickel oxide (which constitutes the inner part
of the passive film on Ni and Ni-Fe alloys) is very low, and (c) the electric field across
the passive film, which assists the passage of cations from the metal–passive film
interface to the passive film–solution interface, should impede the transport of sulfur,
which would be negatively charged.
The Joint Action of Sulfur and Chloride Ions
The passivity breakdown and pitting caused by Cl
–
ions has been the subject of an
enormous amount of work. This area has been reviewed in Chapter 8.
Sulfur-Assisted Corrosion Mechanisms 295
Figure 8 Mechanism of the breakdown of the passive film induced by enrichment of sulfur
at the metal–passive film interface.
Copyright © 2002 Marcel Dekker, Inc.
In the preceding parts of this chapter we have seen that S alone can enhance the
dissolution, block or retard the growth of the passive film, and cause passivity
breakdown and pitting by enrichment at the metal-film interface. All these effects can
evidently also take place in the presence of Cl
–
. A major difference between the
mechanisms of action of Cl
–
and S is that S does not seem to interact directly with
the oxide surface as strongly as Cl
–
.
The effect of sulfur species and chloride ions has been studied in some detail in
the case of corrosion of stainless steels in solutions containing thiosulfate (S
2
O
2
3
–
)
and chloride (Cl
–
) ions [20–27].
It has been demonstrated [27] that S
2
O
2
3
–
is not reduced on the surface of the
passive film formed in neutral solution on an Fe-17Cr alloy, whereas the reduction to
adsorbed sulfur or sulfide does take place on the nonpassivated alloy surface. In this
study, the combined action of chloride and sulfur was also investigated. The
conclusion was that with 30 ppm thiosulfate added to the Cl
–
-containing solution
(0.02 M) at pH 7, the initial breakdown of the passive film is caused by Cl
–
and the
thiosulfates do not play a major role in this step, whereas the presence of thiosulfate
has a drastic effect on the subsequent step, the occurrence of stable pits. With Cl
–
, and
without S
2
O
2
3
–
, unstable pits (i.e., pits that are repassivated) are formed over a wide
range of potentials below the classical pitting potential corresponding to the formation
of stable pits, whereas with the thiosulfates, the reaction on the bare metal surface
produces reduced sulfur (adsorbed sulfur or sulfide), which precludes repassivation
and causes a marked increase of stable pits at low potential. This effect is shown in
Figure 9. The formation of adsorbed sulfur by reaction of S
2
O
2
3
–
with the bare
metal surface was demonstrated by XPS measurements of the binding energy of the
sulfur core level electrons (S2p), which was found to be 169eV for adsorbed thiosulfate
on the passive film surface and 162eV after reduction of the thiosulfate on the
Fe-17% Cr alloy surface. In previous works on the effects of addition of S
2
O
2
3
–
on
corrosion of stainless steels [21,25], such a mechanism had been hypothesized, but
direct experimental evidence was lacking. A synergistic effect of Cl
–
and S
2
O
2
3
–
on
pitting corrosion was also observed on 310 stainless steel [28]. Type 304L stainless
steel suffers stress corrosion cracking in chloride solutions with 10
–3
to 10
–1
M S
2
O
2
3
–
(at 353 K). It was shown that the cracks initiated at pits [29] and the authors
concluded that S
2
O
2
3
–
affects the initiation process of pitting and cracking.
To summarize, a likely mechanism for the combined action of chlorides and sulfur
is the local breakdown of the film by Cl
–
, which then permits all the effects of S shown
above on metal and alloy surfaces to take place. A major consequence is the stabilization
of otherwise unstable (or metastable) pits. This mechanism is consistent with the lower
pitting potential and/or shorter incubation time observed experimentally.
Sulfur species in aqueous solution may also cause directly the breakdown of the
film if the sulfide is thermodynamically more stable than the oxide, a case that would
require a high concentration of the sulfur species in solution to provide a reaction of
the type M
x
O
y
+ zH
2
S↔M
x
S
z
+ yH
2
O+2(z–y)H
+
+2(z–y)e
–
.
Sulfide inclusions have been known for many years to be detrimental to the corro-
sion resistance of steels and extensive studies have been performed on
stainless steels ([30–33] and references in Chapter 10). Preferential adsorption of Cl
–
on
sulfide inclusions has been suggested to be an important step in the corrosion mechanism.
The precise mechanism of the joint action of Cl
–
and the sulfide is not fully elucidated.
296 Marcus
Copyright © 2002 Marcel Dekker, Inc.
COUNTERACTING THE SULFUR EFFECTS WITH ALLOYED
ELEMENTS
The Role of Molybdenum
Numerous electrochemical and surface analytical studies have been performed to
understand the effect of alloyed molybdenum on the corrosion resistance of stainless
steels (see, e.g., the section on the role of Mo in Mo-containing austenitic stainless
steels in Chapter 7).
Sulfur-Assisted Corrosion Mechanisms 297
Figure 9 The joint action of S
2
O
2
3
–
and Cl
–
on passivity breakdown and localized corrosion
of an Fe-17Cr alloy. (a) 0.02 M Cl
–
; (b) 0.02 M Cl
–
+ 30 ppm S
2
O
2
3
–
. S
2
O
2
3
–
is reduced on the
alloy surface, as evidenced by the difference in the binding energy of S 2p for S
2
O
2
3
–
(169 eV)
and S
ads
(162 eV).
Copyright © 2002 Marcel Dekker, Inc.
In order to reach a better understanding of the role of molybdenum in the
presence of sulfur, studies were undertaken on simple systems, i.e., binary and ternary
single-crystal alloys on which the surface concentrations of sulfur and of Mo could be
precisely measured by radiochemical (
35
S) and spectroscopic (XPS) techniques. The
experiments performed on nickel-molybdenum alloys [Ni-2Mo and Ni-6Mo(at %)] pro-
vided the first direct evidence of a specific surface interaction between molybdenum and
adsorbed sulfur leading to the removal of sulfur from the surface and thereby the atten-
uation or the disappearance of the detrimental effects of adsorbed sulfur [34,35].
The most conclusive experiments were done in (a) preadsorbing, in a gas
mixture of H
2
S and H
2
, a known amount of sulfur on the well-defined surface of an
Ni-2 at % Mo alloy [the (100) crystallographic orientation of this fee alloy was used]
and (b) measuring the variation of the sulfur coverage during anodic dissolution in the
active state (at 320mV/SHE) in 0.05 M H
2
SO
4
. The first and essential observation was
that the sulfur coverage decreases, whereas it had been found to remainconstant on pure
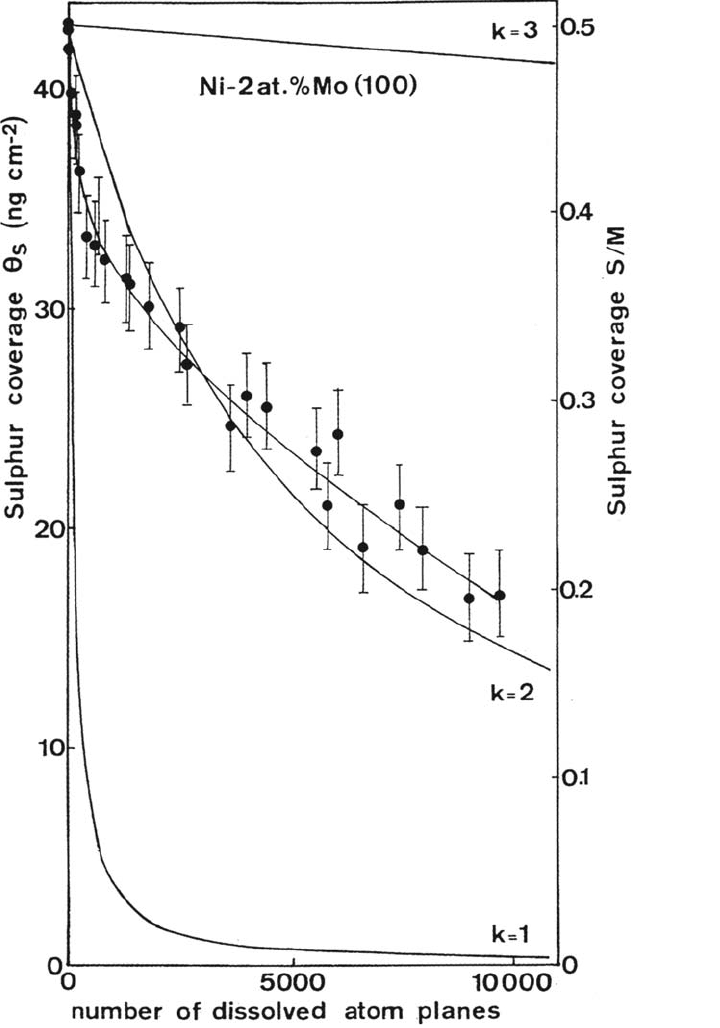
Ni (discussed earlier in this chapter). The precise measurement of the desorption kinet-
ics is shown in Figure 10 in terms of sulfur coverage (weight per cm
2
and number of
sulfur atoms per metal atom on the surface) versus the amount of dissolved material
(expressed in number of dissolved atom layers). The initial coverage corresponds to
saturation of the surface by adsorbed sulfur [42 ng/cm
2
, one S atom for two M atoms
on the surface of the (100) face]. Because sulfur was initially present only on the
surface, the results demonstrate that a surface reaction between Mo and sulfur is the
cause of the loss of the surface sulfur. This is a dynamic process in which the dissolu-
tion of the alloy brings Mo to the surface, which bonds to adsorbed sulfur and is then
dissolved with it. A theoretical model was proposed for the mechanism of the effect of
Mo. In this model, one S atom adsorbed on the surface reacts with k Mo atoms of the
surface plane and the cluster that is formed (in which O or OH may participate) is
dissolved. It was shown that the sulfur coverage on the surface after dissolution of
(n+ 1) atom planes of the alloy, denoted θ
s
(n + 1), has the following expression [34]:
θ
s
(n + l) = θ
s
(n) – [(θ
s
(n))
2
(C
s
Mo
)
k
]
where C
s
Mo
is the surface concentration of Mo, which was assumed to be identical to
the bulk content of Mo, and k is the number, defined above, of Mo atoms required
to form with sulfur a cluster that is dissolved. This equation was used to fit the
experimental variation of the sulfur coverage. The results for k = 1, 2, 3 are computed
in Figure 10. The curve with k = 2 provides a very good fit to the experimental curve.
Similar findings were obtained with Ni-6% Mo (100). These results strongly support
the proposed mechanism in which, on a (100) surface, two Mo atoms bond to and
remove one adsorbed S atom. It is to be noted that in this mechanism the passive
film is not directly involved, but of course the major consequence of the removal
of sulfur by Mo is that the passive film may be formed on an otherwise blocked
surface. The same model has been used successfully to interpret in a quantitative
manner the fact that on other Ni-Mo alloys the surface enrichment of sulfur by
anodic segregation of bulk sulfur was very limited [36] compared with what had been
reported previously for Ni [2]. On Ni-6%Mo (100) with 32 ppm sulfur in the bulk,
the coverage by sulfur measured after different times of anodic polarization in the
active region (in 0.05 M H
2
SO
4
) was always less than a complete monolayer. The
theoretical amount of sulfur, calculated using an equation similar to that given
298 Marcus
Copyright © 2002 Marcel Dekker, Inc.
above for the Mo-induced desorption of adsorbed S, but in which a term accounting
for the supply of sulfur by the dissolution of the alloy was added, gave a good fit to
the experimental values when the value of k was taken equal to 2. This confirmed
the validity of the mechanism in which two Mo atoms bond to one sulfur atom and
Sulfur-Assisted Corrosion Mechanisms 299
Figure 10 The loss of surface sulfur induced by molybdenum (experimental and
theoretical curves).
Copyright © 2002 Marcel Dekker, Inc.
