Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

The first idea to be proposed to account for the effect of soluble sulfides on
pitting initiation was that MnS dissolution provokes locally the formation of a virgin
metal surface. This microarea is exposed to an acidified and sulfur species–enriched
environment produced by the sulfide dissolution. When the solution near the
microarea has reached a certain composition, the contacting metal can no longer
repassivate and the metal starts to dissolve [6]. Following other workers [8], complete
MnS dissolution is not needed, as the pits preferentially initiate at the metallic matrix-
MnS interface. This model keeps open the questions of (a) the MnS dissolution
mechanisms, (b) the nature and the effect of the dissolved sulfur species which form
during this dissolution and (c) the role played by the passive film, either close to or
possibly on the inclusion. From this last viewpoint, the surface condition of the steel
is of major importance. The effect of MnS is probably not the same on freshly
mechanically polished surfaces or after various surface treatments, including
pickling, bright annealing, or electrochemical treatments.
There is some experimental evidence that Cl
–
ions cluster either on the
nonmetallic inclusions [10b] or at their boundaries with the metallic matrix [12b].
When the MnS is directly exposed to the electrolyte, Cl
–
can preferentially adsorb on
the inclusion due to its higher electronic conductivity, giving stronger electrostatic
image forces than on the surrounding oxide film [8]. In our opinion, Cl
–
adsorption
is followed by the potential-assisted formation of the adsorbed complex MnCl
+
,
which then dissolves in the aqueous solution according to (MnCl)
+
ads
+ Cl
–
→ Mn
2+
+ 2Cl
–
. Because their is some evidence that pitting initiation on MnS-containing
steels is strongly dependent on the solution pH (see below), it is suggested that Cl
–
and OH
–
adsorption compete on Mn sulfide surfaces, resulting in pH-dependent
dissolution kinetics.
The MnS dissolution provokes the formation of an Mn
2+
- and Cl
–
- enriched
local electrolyte [10c] (the Cl
–
ions being attracted by the local excess in positive
changes in the solution). As long as the concentration remains below a critical
level, the pit walls can repassivate, but beyond this critical level an MnCl
2
salt layer
is formed which may prevent the repassivation. The question of what happens at
the metal–salt layer interface has been discussed [16a] and it was suggested that an
FeCr oxychloride could form, whose properties, and also possible remnance once
the salt layer has dissolved, could play a determining role in repassivation mecha-
nisms. Following another idea, MnS would be covered with a defective passive
film [lc] on which the chloride ions first adsorb and then penetrate, leading to the
formation of a nonprotective salt chloride layer. The hydrolysis of this salt layer
would then increase the local acidity, resulting in the dissolution of the defective
passive film. Whatever the proposed mechanisms, chloride adsorption seems to be
the first stage of the sulfide dissolution.
Several models have been proposed for the dissolution of sulfur from MnS
inclusions. The first idea [6,8] is that, in acidified media, MnS dissolves according to
MnS + 2H
+
→ H
2
S + Mn
2+
or [17]
MnS + 2H
+
→ S + Mn
2+
+ H
2
These reactions can be split into the anodic reaction [6,8,10b]
320 Baroux
Copyright © 2002 Marcel Dekker, Inc.
MnS → S + Mn
2+
+ 2e
–
,
whose potential is V(volts) = –0.12 + .0.03 log [Mn
2+
], and the cathodic ones,
H
+
+ e
–
↔ 1/2H
2
or [6] 2H
+
+ 2e
–
+ S ↔ H
2
S
with V = 0.14 – 0.06pH – 0.03 log [H
2
S], and possibly [5c]
H
2
S ↔ H
+
+ HS
–
, then H
+
+ 2e
–
+ S ↔ HS
–
Direct action of water to form sulfates, sulfites or thiosulfates should also be
considered [6,8,10b,15b,17].
MnS + 4H
2
O ↔ Mn
2+
+ SO
2–
4
+ 8H
+
+ 8E
–
with V = 0.23 – 0.06pH – .0074 log[Mn
2+
][SO
2–
4
],
MnS + 3H
2
O ↔ Mn
2+
+ HSO
–
3
+ 5H
+
+ 6e
–
then
HSO
–
3
+ 5H
+
+ 4e
–
→ S + 3H
2
O,
2MnS + 3H
2
O ↔ 2Mn
2+
+ S
2
O
2–
3
+ 6H
+
+ 8e
–
S
2
O
2–
3
+ H
2
O → S
ads
+ SO
2–
4
+ 2H
+
+ 2e
–
All these reactions are potential and/or pH dependent, so they can occur or not
according to the actual conditions in, or close to, the pit embryo. This makes rather
complex the interpretation of the pitting potential pH dependence when sulfides act
as pitting sites (see below). Furthermore, they do not explicitly take into account the
effect of chloride ions for the dissolution of Mn
2+
; they should then be slightly
modified for this purpose, introducing the solution chloride content as the third
critical parameter for the MnS dissolution kinetics.
It should be noted that photoelectrochemical investigations [15b] suggest
that the last two reactions, involving the formation and then dismutation of S
2
O3
2–
3
and resulting in the formation of adsorbed sulfur, are operating in some cases.
Photoelectrochemical microscopy shows the deposition around the inclusions
of a ring of material deduced to be sulfur, which is consistent with the results of
several other studies [6,10b]. A detailed analysis of the fundamental aspects of the
sulfur-assisted corrosion mechanisms is presented in Chapter 9.
AN EXAMPLE OF THE EFFECT OF NONMETALLIC INCLUSIONS
Studied Steels and Their Nonmetallic Inclusions
In the following, the steels under consideration are mainly some annealed AISI
430-type FeCr ferritic alloys containing also either Nb (steels A and A') or Ti
(steels B and B') additions. Also, some solution-treated 304 and 321 AlSI-type
steels were considered (C and D), in order to separate the matrix and inclusion
effects. These two steels are austenitic and Ni bearing but the latter contains Ti and
is MnS free, whereas the former contains MnS inclusions.
Pitting Corrosion of Stainless Steels 321
Copyright © 2002 Marcel Dekker, Inc.
Except for steel A, some Al additions are used as deoxidizing agents during the
melting process, which leads to Al ~ 0.030% and induces the presence of some Al
2
O
3
inclusions. Note the low sulfur content, which is easily attained with modern
steelmaking techniques, and the presence of some stabilizing elements (Ti or Nb),
which trap carbon and avoid the formation of chromium carbides. For steels A and
A', sulfur is trapped under the form of manganese sulfide. The difference between
steels B and B' is their Cr content; both contain Ti, which also traps nitrogen in the
form of titanium nitrides and sulfur in the form of Ti sulfides; this trapping occurs
during the steelmaking process before the steel solidification. Concerning steel A',
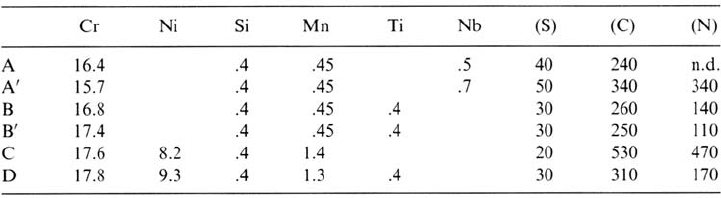
some Mn sulfides are found around aluminum oxides (Fig. 5). Because these oxides
have a poorer ductility than the metallic matrix, the cold-rolling process provokes
some microdecohesions around the inclusion, where some manganese sulfides are
often located, leading to the possible formation of noxious microcrevices. This
phenomenon is not observed on Al-free steels, where oxides are mainly malleable
silicates. However, the art of the steelmaker consists in avoiding the formation of Cr
oxides, which could produce a similar but worse effect than Al oxides. In every case,
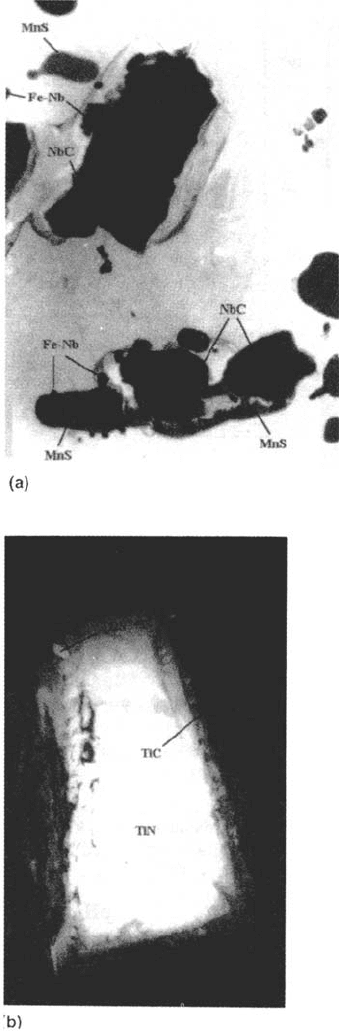
some Mn sulfides are also found closely stuck to Nb carbonitrides (Fig. 6a). Because
these carbonitrides precipitate at ~1200°C, this shows that (for the sulfur content
considered), the high-temperature sulfur solubility is sufficient for MnS precipitation
to occur in the solid steel (lower than 1200°C) while for higher sulfur contents MnS
is generally considered to precipitate at the end of the steel solidification. Note that
very few isolated MnS precipitates are found, probably because Nb(C,N) act
as precipitation sites. This situation contrasts with the behavior of steel C (304),
for which (a) no carbides may nucleate the MnS precipitate and (b) sulfides can
precipitate in austenite at the delta→gamma transformation temperature.
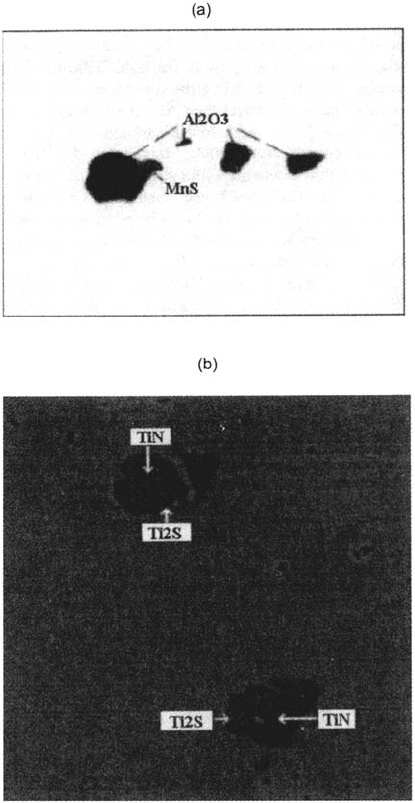
Figures 5b and 6b show the location of Ti sulfides on steel B, i.e., around the
titanium nitrides, embedded in a Ti carbide belt. Detailed examination suggests that
al high temperature a homogeneous Ti carbosulfidc belt precipitates around the Ti
nitrides (which formed in the liquid steel). Then, lowering the temperature, titanium
sulfides and carbides separate at some points as shown in Figure 5b, producing the Ti
carbide TiC and a Ti sulfide, identified in some cases (using electron diffraction) as
the hexagonal phase Ti
2
S. As another result, note that Al oxides have been identified
in the core of the Ti nitrides (which is not known in the figures), playing the role of
nuclei for TiN precipitation.
322 Baroux
Table 1 Steel Composition in wt % or (ppm)
Copyright © 2002 Marcel Dekker, Inc.
pH Effects and Pitting Sites
The pitting potentials were measured for all these steels using the potentiokinetic
method in NaCl aqueous media whose pH was varied from 3 to 6.6. The results for
steels A' and B in NaCl (0.02 M) are shown in Figure 7a. No pH dependence is
observed for steel B. In contrast, for steel A', a sharp pitting potential decrease is
evident when the pH is lowered below a critical value pH
c
ranging between 4.5 and 5.
Since the main difference between the two steels is the presence or absence of MnS,
and Ti sulfides are known to have better stability in aqueous electrolytes than MnS,
Pitting Corrosion of Stainless Steels 323
Figure 5 Nonmetallic inclusions (before pitting). Scanning electron microscopy, ×3000.
(a) Steel A': MnS nucleated on an aluminum oxide. (b) Steel B: Ti nitride surrounded by
sulfur compounds.
Copyright © 2002 Marcel Dekker, Inc.
324 Baroux
Figure 6 Nonmetallic inclusions (before pitting). STEM (×18,000) on thin foils. (a)
Steel A': MnS nucleated on an Nb carbonitride. Intermetallic (Fe, Nb) phases are also
observed, (b) Steel B: Ti nitride surrounded by a Ti carbide, in which some Ti sulfides
are embedded.
Copyright © 2002 Marcel Dekker, Inc.
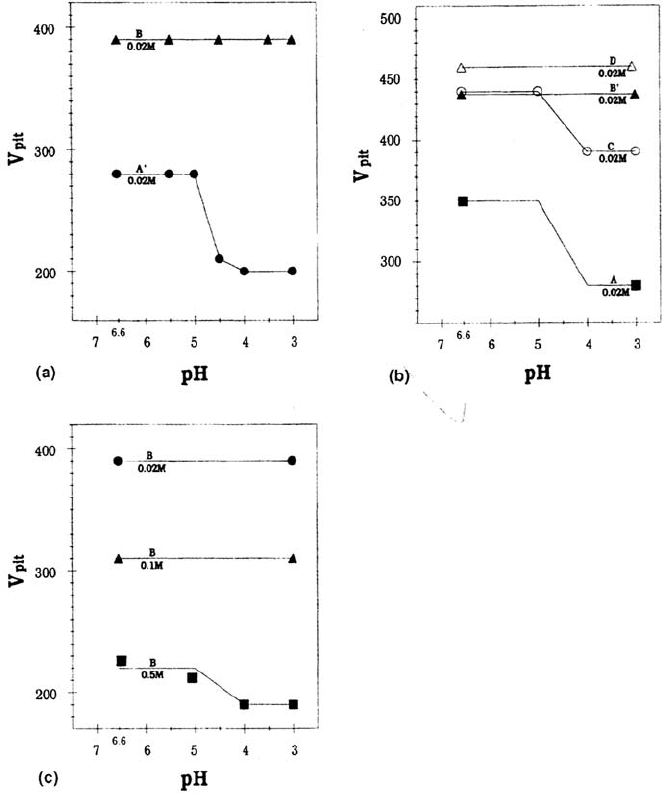
one can assume that this decrease is due to the pH-assisted MnS dissolution. Note
that the same phenomenon is observed (Fig. 7b) when comparing the two steels C
and D, which are respectively MnS containing the MnS free, showing that the
observed effect is related to the nature of sulfides present in the steel and not to other
metallurgical factors. Figure 7b also shows the results obtained for steels, A and B',
which confirm the above findings. The slight difference between steels B and B' may
be attributed to the difference in Cr concentration. The strong difference between
Pitting Corrosion of Stainless Steels 325
Figure 7 Effect of the solution pH on the pitting potentials. (a) Steels A' and B in NaCl
(0.02 M). (b) Steels A, B' (FeCr steels) and C, D (FeCrNi steels) in NaCl (0.02 M). (c)
Effect of the solution chloride content dependence for a Ti-containing steel. For low
chloride contents (0.02 and 0.1M), there are no pH effects. For 0.5 M, lowering the pH
decreases the pitting resistance.
Copyright © 2002 Marcel Dekker, Inc.
steels A and A' may be due to the difference in Cr concentration and sulfur contents
but also to the Al oxides present in steel A' and around which MnS are found. The
effect of the solution chloride content was also investigated between 0.02 and 0.5 M.
Figure 7c shows that for high enough chloride concentrations, a pitting potential pH
dependence is found even for Ti-containing steels, suggesting that Ti sulfides are not
so stable in such electrolytes. The discontinuity of the pitting potential versus pH
variations should then be related to the dissolution of sulfur species, which occurs
easily for MnS-containing steels (whatever the steel matrix composition) but only for
high enough chloride contents for Ti-bearing steels. Note that the critical pH which
is found (4.5<pH<5) is the same for Ti-free and Ti-bearing steels and is close to the
one deduced from the potential-pH equilibrium diagrams in some chloride-
containing aqueous solutions [6,8]. This could support the idea that the critical pH
can be deduced from the sulfur species-pH equilibria, regardless of the nature of
the dissolved cation (manganese or titanium), once the conditions are reached for
the sulfide to dissolve.
Figure 8 shows the typically observed pit initiation sites. For steel A', whatever
the pH or the chloride content, pits initiate either around Al oxides (Fig. 8a), where
some MnS is located, or on MnS inclusions (Fig. 8b) around Nb(C,N) or (seldom)
isolated. This confirms the preceding hypothesis. For steel B in NaCl (0.5 M)
solution, pitting generally occurs at the TiN boundary (Fig. 8c), where Ti sulfides are
present. This shows clearly that for such chloride concentrations, Ti sulfides act as
pitting sites and becomes unstable when the potential increases. For lower chloride
concentration (0.2 M NaCl), the situation is not so clear and pits could initiate
directly on the metallic matrix, with no direct relation to nonmetallic inclusions, or in
some cases on titanium nitrides. In the two situations, however, Ti sulfides do not
seem to act as pitting sites, which is consistent with the absence of any pitting
potential pH dependence.
A practical consequence can be drawn from these results: Ti-stabilized steels
exhibit better pitting resistance than Ti-free ones, provided that the corrosive medium
is not too severe, i.e., the chloride content does not exceed a critical value, depending
on the solution pH, which could cause the Ti sulfides destabilization. In the case of
crevice corrosion, the situation is more complex. Inside a crevice, the pH decreases
and the chloride concentration increases with time. It is an accepted idea that
corrosion occurs when the pH becomes lower than a critical value, which is referred
to as the depassivation pH. However, at least for the less resistant steels, pitting
corrosion may occur in the crevice before this general depassivation. From these
results, it is concluded that MnS-containing steels are much more sensitive to this
pitting-induced crevice corrosion than Ti-bearing ones. For very severe crevices,
however, the chloride content can drastically increase with time Ti-stabilized steels
are no longer different from Ti-free ones.
Inhibitive Effect of Sulfate Ions
Sulfate ions are known for inhibiting the pitting corrosion in chloride-containing
media. Figure 9 presents the elementary pitting probability–potential variations
obtained for steels A and B in 0.1 M NaCl. Three points are noticeable: (a) SO
2
4
–
additions decrease the pitting probability (for a given potential) and increase the
conventional pitting potential (for a given chloride concentration). This effect is
326 Baroux
Copyright © 2002 Marcel Dekker, Inc.
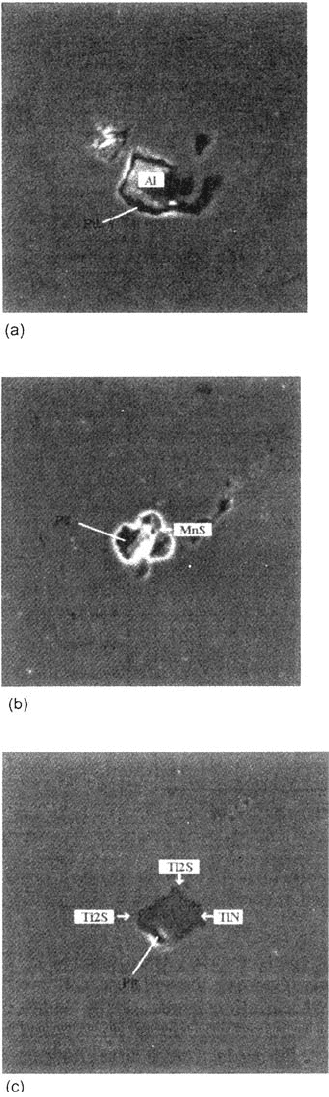
Figure 8 Scanning electron microscopy (×3000) on pitted samples. (a) Steel A': pitting
at the boundary of an alumina inclusion. (b) Steel A': pitting on a manganese sulfide. (c)
Steel B: pitting at the boundary of a Ti nitride.
Copyright © 2002 Marcel Dekker, Inc.
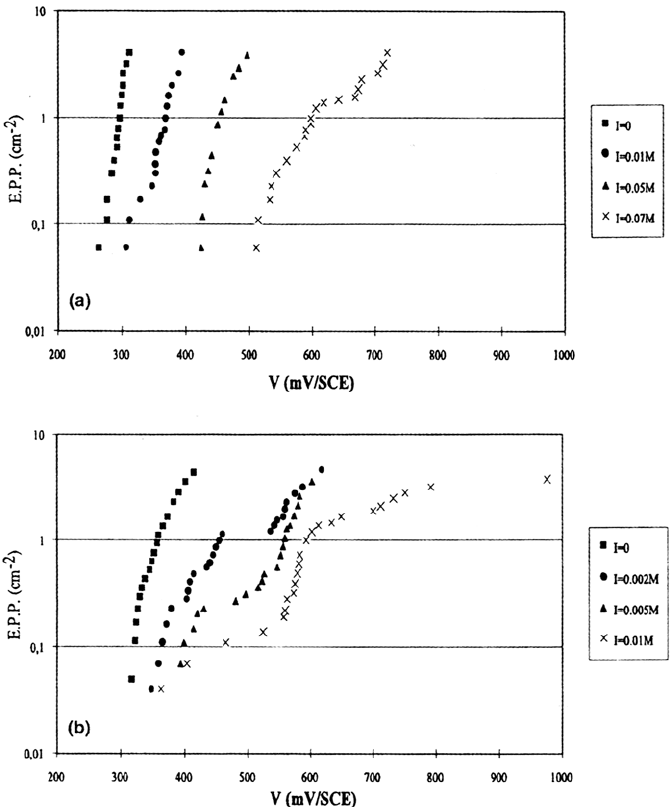
more marked for steel A than for steel B, but complementary tests in 0.5 M NaCl
show that the difference between the two steels regarding the sulfate addition
efficiency becomes smaller when the chloride concentration increases. (b) Sufficient
sulfate additions result in a deviation from the standard exponential ω(V) law. The
sulfate amount necessary to produce a noticeable deviation is much larger for steel A
than for steel B. (c) For high enough sulfate amounts, steel B exhibits a bimodal
behavior: exponential ω(V) laws are found both for high and for low electrode
potentials. Between these two domains a transition behavior is noted. Further work is
needed to interpret these results, but the difference between the MnS-containing and
MnS-free steels is now well established.
328 Baroux
Figure 9 Effect of sulfate ions on the pitting probability in 0.1 M NaCl (pH 6.6). I is the
sodium sulfate molarity and V the electrode potential. (a) Steel A; (b) steel B.
Copyright © 2002 Marcel Dekker, Inc.
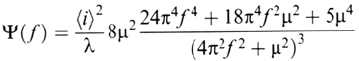
Prepitting Events
Recording the anodic current i(t) during a polarization below the conventional pitting
potential (Fig. 10a) provides two types of information. First, the average anodic
current decreases with time, probably corresponding to the onset of improved
passivity. Second, some oscillations of this anodic current are observed, which,
through the same SEM observations as above, were shown to correspond to some
initiated and then repassivated pits. The problem which arises for analyzing these
oscillations is to separate them from the average anodic current decay (the “anodic
current baseline”), which probably corresponds to some passive film modifications.
The baseline is determined by associating to each point [t, i(t)] the point [t, i
'(t) = min
{i(t
'), t' = t– τ/2 to t + τ/2}] where τ is an arbitrary time constant. The frequency 1/τ
should be smaller than the characteristic frequencies of the prepitting noise. Figure 10b
shows the typical reduced signal obtained for steel A' using this method. For MnS-
containing steels (A or A'), the prepitting events are numerous and produce what one
may call a prepitting noise. In contrast, for MnS-free steels the anodic events are
much less numerous and well seperated, as shown in Figure 10c. It is therefore
logical to relate the so-called prepitting noise to the dissolution of manganese
sulfides. For MnS-containing steels, the current time signature of the individual
prepitting events is found to be a current increase varying approximately as t
2
,
followed by a sharp current decrease (Fig. 11a). The peak amplitude at 200mV/SCE
is of the order of 0.1 to 1 μA. For the MnS-free steels, the form of the i(t) transient is
quite different (type II; see Fig. 11b) and the peak intensity is smaller (10 to 100nA
at 200mV/SCE). In neutral NaCl (0.02M) solution, the transient is characterized by
a sharp increase followed by a slow current decay, as expected for local passive film
breakdown followed by passive film healing.
A simple way to analyze the reduced signal corresponding to the prepitting
noise (Fig. 10b) is to build up a “distribution function” N(I) which counts, for a given
time period T(~ 8 min), the number of events for which the anodic intensity i is
larger then I. This function N decreases with I (Fig. 12a). One sees that the prepitting
noise increases with the electrode potential and the solution chloride content.
Moreover, the solution pH acts in the same way as for the pitting potentials, since a
discontinuity of N between pH 4 and 5 is once more evidenced (Fig. 12b). Last, the
prepitting noise decreases with the polarization time (Fig. 12c).
Another way is to calculate the power spectral density (PSD) associated with
the anodic current fluctuations. Let us consider a Poissonian series of birth and
death events (birth frequency λ, death frequency μ), with a parabolic growth law
between birth and death. This situation can be modeled as follows:
i(t)=∑
n
i
n
(t) with i
n
(t)=
α
–
2
(t – t
n
)
2
H(t – t
n
)[1 – H(t – t
n
– θ
n
)]
where t
n
and Θ
n
are, respectively, the induction time and the lifetime of the event
number n, H is the step function [H(x) = 0 when x < 0 and 1 when x > 0], and α is a con-
stant. The average current 〈i〉 and the PSD Ψ(f ) are found [4a] to be 〈i〉 = αλ/μ
3
and
Pitting Corrosion of Stainless Steels 329
Copyright © 2002 Marcel Dekker, Inc.
