Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.


of a galvanic cell between crevice and external surfaces with two consequences: (a)
a difference in the corrosion potential inside and outside the crevice due to the
solution resistivity
*
and (b) an additional source of environment modification caused
by the electrolytic migration between the crevice and the bulk solution. This second
step is self-accelerated if the local environment becomes more severe and tends to
increase the galvanic current and the electrolytic migration.
Crevice Corrosion Due to IR Drop
In some instances, the increase of the corrosion rate in the crevice may be due
only to the IR drop. The more classical case where the IR drop is responsible for
crevice corrosion is the corrosion by differential aeration of unalloyed steels in
near-neutral water.
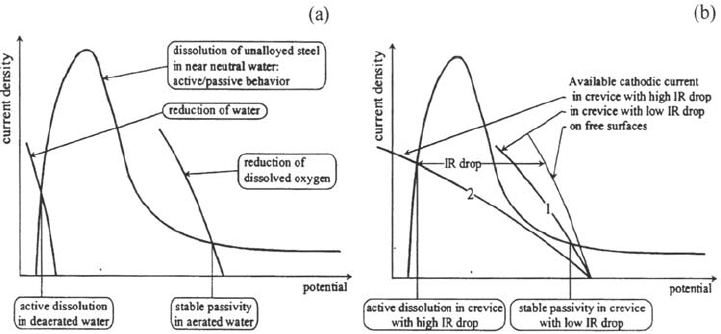
Unalloyed steels exhibit an active-passive transition in near-neutral water
(Fig. 3). In aerated environments, the availability of a cathodic current from oxygen
reduction allows steel passivity to be stable, whereas active dissolution prevails in
deaerated conditions (Fig. 3a). Inside a crevice, the local change of environment is due
to the rapid consumption of the dissolved oxygen. However, a cathodic current from
oxygen reduction may be still provided by the external surfaces. If the IR drop is low
enough, the surface may remain passive inside the crevice (curve 1 in Fig. 3b). If the IR
drop is too large (more efficient crevice effect), however, the corrosion potential inside
the crevice is too low for the passivity to be stable (curve 2 in Fig. 3b) and the surfaces
inside the crevice gap become active. In this case, the corrosion rate in the crevice is
usually much higher than the corrosion rate of the free surfaces in a deaerated environ-
ment because of the supply of cathodic current by the external surfaces.
Crevice Corrosion Due to Local Changes of Anodic Behavior
of the Material
In most cases, crevice corrosion also requires a local change of the environment that
results in a change of the material anodic behavior. For example, crevice corrosion of
stainless steels in aerated chloride environments requires a local depletion of the oxi-
dizing species, but it is triggered by a second step of environment modification that
causes a pH drop and an increase of chloride concentration in the crevice (see later).
Local pH drop is not the single cause of crevice corrosion. For example, local
depletion of passivating species may be the origin of crevice corrosion as is the case
for steels in cooling water with anodic inhibitors such as nitrite or chromate ions.
†
Initiation and Propagation
Because of the general mechanisms described previously, crevice corrosion always
includes two steps: (a) an initiation or incubation period required for the local
environment to undergo a critical change during which no significant dissolution
damage occurs in the crevice and (b) a propagation period during which the corrosion
Crevice Corrosion of Metallic Materials 351
* This potential difference is usually referred to as IR drop.
†
Note that cathodic inhibitors do not cause such severe crevice corrosion because their local
depletion does not significantly increase the cathodic current available in a deaerated crevice.
Copyright © 2002 Marcel Dekker, Inc.
damages occur. Depending on the cause of the localized corrosion, the initiation time
may or may not be a significant part of the lifetime of a structure suffering crevice
corrosion.
Parametric Effects
Crevice Geometry
The severity of the crevice regarding the initiation stage strongly depends on its
geometry, i.e., the crevice gap (h) and the crevice depth (L).
The crevice gap h (Fig. 2) controls the volume/surface ratio of the crevice: the
smaller this ratio, the faster the environment changes due to surface reactions.
However, the crevice gap must be wide enough for the environment to enter but it
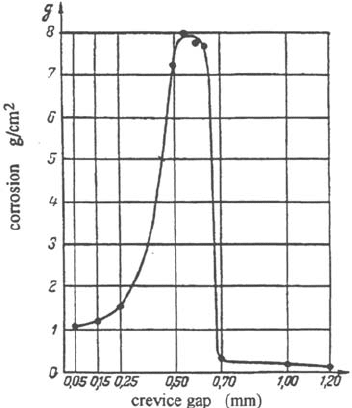
becomes inefficient if it is too wide. Figure 4 [1] shows the extent of crevice
corrosion of unalloyed steel in nitric acid as a function of the gap. For stainless steels
in chloride environments, short incubation times are obtained for crevice gaps of the
order of a micrometer or less [2,3] and the incubation time increases rapidly with the
crevice width. As a consequence, surface roughness is of major importance, and, for
example, crevice corrosion can be avoided by polishing the surfaces in contact with
seals to a smooth finish.
The crevice depth L (Fig. 2) controls the transport processes: the deeper the
crevice, the slower the transport kinetics between the crevice tip and the bulk
environment. A minimum crevice depth is often required for corrosion to occur [2].
Bernhardsson et al. [4] introduced the parameter L
2
/h as a geometric criterion
of crevice severity. A similar r
0
2
/h ratio is included in one of the nondimensional
parameters introduced by Alkire et al. [5].
External Surfaces
The free surfaces outside the crevice gap constitute the cathode, which provides
cathodic current to balance the anodic dissolution in the crevice gap. Thus, the larger
352 Combrade
Figure 3 Crevice corrosion of unalloyed steel in near-neutral water. (a) Behavior of
“uncreviced” steel surface in aerated and deaertated water. (b) Behavior in presence of
crevice—role of IR drop.
Copyright © 2002 Marcel Dekker, Inc.
the free surfaces and the higher the bulk solution conductivity, the higher the
available cathodic current and the higher the corrosion potential in the crevice. Except
in the cases where the IR drop is the driving factor for crevice corrosion (see earlier),
this usually shortens the incubation period and increases the corrosion rate in the
propagation stage.
IR drop
The IR drop depends on both the crevice geometry and the solution conductivity.
Most of the IR drop usually occurs inside the crevice gap, but the IR drop in the bulk
solution may be of major importance in dilute solutions and limit the effective area
of the cathode.
It is worth noticing that the IR drop and the diffusion fluxes between the crevice
and the bulk solution are controlled by the same restricted transport path. Thus, the
more limited the diffusion transport, the larger the IR drop.
The IR drop is responsible not only for the buildup of a potential difference
between the crevice and the free surfaces but also for potential gradients inside the
crevice. If large enough, the IR drop could make possible water reduction near the
crevice tip and this may create a more complex situation, the crevice tip becoming an
additional source of cathodic current and reversing the environmental evolution [6].
PHENOMENOLOGY OF CREVICE CORROSION OF PASSIVE ALLOYS
IN AERATED CHLORIDE ENVIRONMENTS
The first point to emphasize is that crevice corrosion of passive alloys does not occur
in environments deprived of oxidizing species other than water. Generally, it does not
occur in deaerated solutions. On the contrary, oxidizing agents such as many biocides
(hypochlorite, chlorine, chlorine dioxide, etc.) may cause crevice corrosion.
Crevice Corrosion of Metallic Materials 353
Figure 4 Effect of crevice gap on the amount of corrosion of unalloyed steel in nitric acid [1].
Copyright © 2002 Marcel Dekker, Inc.
As the other crevice corrosion phenomena, the development of crevice
corrosion on passive alloys in aerated (and more generally in oxidizing) chloride
environments involves two steps:
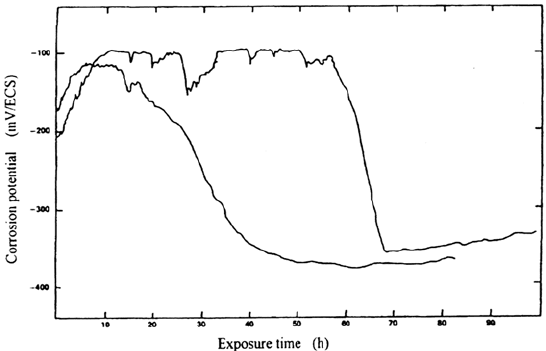
First, there is an incubation period, which may be very long for the more
resistant materials. During this period, short corrosion potential transients can
possibly be observed (Fig. 5), but, when examining the surface inside the crevice,
no significant traces of corrosion are observed.
Then, initiation of rapid corrosion occurs inside the crevice and the
propagation period begins during which the corrosion proceeds quite rapidly. The
formation of deposits outside the crevice allows visual detection of the corrosion.
Initiation of corrosion in the crevice is also marked by a significant drop in the
corrosion potential of the material [3] (Fig. 5). Thus, monitoring of the corrosion
potential can be an efficient way to detect crevice corrosion (as well as other types of
localized corrosion) in its early stages or even during the incubation period.
Figure 6 shows an example of severe crevice corrosion of stainless steel
bolts in seawater.
Effect of Potential: Critical Potentials for Initiation and
Repassivation
For a given bulk environment, crevice initiation appears to be dependent on the
corrosion potential of the free surfaces: initiation occurs when this potential exceeds
a critical value E
crev
. The propagation may occur at potentials significantly lower than
the potential that caused the initiation to occur, and indeed, the potential of a freely
corroding material is generally lower during the propagation compared with the
incubation period (Fig. 5). However, propagation stops below a critical potential for
the crevice repassivation E
rp
.
354 Combrade
Figure 5 Potential drop due to initiation and propagation of crevice corrosion (crevice
area ratio 1:1) in two different tests on a 316 stainless steel in 1 M NaCl, pH 6 solution at
room temperature. (From Ref. 3.)
Copyright © 2002 Marcel Dekker, Inc.

As a consequence, the polarization curves drawn on a specimen equipped with
a crevice former device exhibit (Fig. 7) features very similar to those typical of
pitting corrosion. During the upward potential scan, crevice corrosion initiates quite
abruptly at a critical potential E′
crev
that is generally lower than the pitting potential.
Then, during a subsequent backward potential scan, corrosion stops at a critical
potential E′
rp
significantly lower than E′
crev
.
However, both crevice and repassivation potentials are dependent on the
experimental conditions and particularly on the scan rate when determined by using
potentiokinetic techniques. Nevertheless, long-term experiments tend to show that
there is a minimum value of E
crev
above which the incubation time decreases as the
Crevice Corrosion of Metallic Materials 355
Figure 6 Severe crevice corrosion of stainless steel bolts in seawater (after A. Désestret).
Figure 7 Polarization curves of alloy 825 in chloride solution: comparison of curves for
pitting and crevice corrosion. (From Ref. 8.)
Copyright © 2002 Marcel Dekker, Inc.

potential increases (Fig. 8 [8] and 9 [7]). Thus, adding an oxidizing agent (for
example, biocides) in a chloride environment strongly enhances the crevice initiation
(as well as the pitting probability).
Conversely, there is some agreement that a critical value E
pr
(usually referred to
as protection potential) exists that is the maximum possible value of E
rp
below which
no crevice corrosion may propagate significantly. This is consistent with the fact
that crevice corrosion of passive alloys in chloride environments does not occur in
deaerated environments.
Figure 8 suggests that long-term crevice and pitting potentials tend to the same
value [8], but this still needs confirmation. In particular, it remains to be established
whether this common value is intrinsic to the alloy-environment system or whether it
depends on the crevice geometry.
356 Combrade
Figure 8 Effect of time on the crevice and repassivation potentials of alloy 825 in chloride
solutions. (From Ref. 8.)
Figure 9 Effect of potential on the initiation time of crevice corrosion on 316 stainless
steel (SS) in 3% NaCl solution. (From Ref. 7.)
Copyright © 2002 Marcel Dekker, Inc.

Effect of the Environment
For a given crevice geometry, the critical potentials for crevice initiation and
repassivation decrease with increasing chloride content (Fig. 10) and increasing
temperature (Fig. 11) of the bulk solution. This means that the susceptibility to
crevice corrosion of passivated alloys increases with the chloride content and the
temperature. For example, titanium alloys become sensitive to crevice corrosion only
in hot concentrated chloride solutions around 100/150°C [9,10]. Propagation rates
also increase with temperature.
The presence of sulfate ions or buffering species may retard crevice initiation,
at least in dilute chloride solutions [13].
Crevice Corrosion of Metallic Materials 357
Figure 10 Effect of chloride concentration on the crevice and repassivation potential of
alloy 825 at 95°C [11].
Figure 11 Effect of the temperature on the critical potentials for crevice and pitting
corrosion on 304 SS in 0.5 M NaCl solution [12].
Copyright © 2002 Marcel Dekker, Inc.

Geometric Factors
The incubation time is strongly dependent on the crevice geometry (see also
Parametric Effects earlier). The initiation time decreases with decreasing crevice gap.
By changing the crevice gap from about 0.2 to 5 μm, Oldfield [3] raised the initiation
time from 6.5 to 350 h on AISI 430 stainless steel.
The crevice gaps that exhibit the greater efficiency in crevice initiation are of
the order of tenth of micrometers to a few micrometers. At this scale, surface
roughness is of major importance.
The area of the free surface surrounding the crevice zone is also of importance: a
large free surface area causes shorter crevice initiation time, faster propagation rate,
and smaller potential drop at the crevice initiation.
Alloy Composition
Chromium, molybdenum, and nitrogen are the most efficient alloying elements for
increasing the resistance to crevice (and to pitting) corrosion initiation of stainless
alloys (Fe-Ni-Cr-Mo-N- … alloys). A pitting index has been derived that characterizes
the resistance of stainless alloys to these forms of corrosion [14] (Fig. 12 [15]):
PREN = (wt %Cr) + 3.3(wt %Mo) + 16 to 30(wt %N)
As an example, PREN values of about 40 to 45 are requested for a stainless alloy to
resist crevice corrosion in natural seawater. This leads to the use of sophisticated
alloys containing usually about 23 to 25% Cr, up to 7% Mo, up to 0.45% N, and very
often Cu and W additions.
The harmful effect of sulfur [16] as an alloy impurity has been mainly attributed
to the presence of reactive sulfide inclusions (usually MnS), which constitute
very efficient sites for initiation of localized corrosion (see later). Low sulfur or Ti
alloying* improves the alloy resistance to crevice corrosion [16].
358 Combrade
Figure 12 Effect of composition on the resistance to crevice and pitting corrosion of
stainless alloys in chloride environments—the PREN index. (From Ref. 15.)
* The beneficial effect of Ti alloying is probably due to the formation of sulfide compounds
more stable than mangnese sulfide.
Copyright © 2002 Marcel Dekker, Inc.
The propagation rate also depends on the composition of the alloy, but Oldfield
[3] observed that the ranking of alloys may be different because the effects of
elements are different for initiation and propagation. For example, he claimed that Cr,
which is very efficient in retarding the initiation, favors fast propagation rates Mo
definitely has a positive effect on both stages [17,18], but Ni seems to have a positive
effect only on the propagation rate [16,18].
Propagation of Crevice Corrosion
Crevice corrosion occurs at very high rates, which make this form of corrosion very
difficult to manage as the lifetime of a corroding structure may be determined by the
initiation period.
Laboratory studies showed that for most alloys, the corrosion rate is higher
near the mouth of the crevice [19,20]. However, alloys such as ferritic stainless
steels may corrode faster near the dead end of the crevice [20].
An interesting observation on long-term crevice tests of Fe-Ni-Cr-Mo-N alloys
[21] is that well-developed crevice corrosion may stop spontaneously even though
the environmental conditions do not change. If confirmed, such behavior could be of
major importance because it would mean that crevice corrosion may not necessarily
cause the failure of structures, particularly in applications involving thick materials
such as the containers for long-term storage and disposal of radioactive wastes.
PROCESSES INVOLVED IN CREVICE CORROSION OF PASSIVE
ALLOYS IN AERATED CHLORIDE ENVIRONMNENTS
The generally accepted scenario is that the environment in the crevice suffers a
progressive evolution that leads to the breakdown of passivity and to a propagation
stage during which the metal inside the crevice undergoes active dissolution. The
following paragraphs briefly describe the current understanding of the different
processes, i.e., environment evolution, passivity breakdown, and active dissolution in
the crevice gap.
Environment Evolution in the Crevice Gap
The following mechanism is generally accepted to describe the evolution of the
crevice environment on passivated alloys exposed to aerated chloride environments.
Step 1: Deaeration of the Crevice Environment
On the free surfaces, the cathodic reaction is the reduction of oxygen. However, the
environment in the crevices becomes deaerated after periods of time that can be very
short, at least compared with the lifetime of an industrial apparatus. As an example,
a passive current of 10 nA/cm
2
will cause the deaeration of a crevice with a
surface/volume ratio of 10
3
cm
–1
(i.e., a crevice gap of 20 μm between two metal
surfaces) in about 3 h.
The lack of oxygen causes the inhibition of the cathodic reaction inside the
crevice. Thus, the local anodic reactions must be balanced by cathodic reactions
occurring on the surfaces exposed to the bulk solution. This builds up a galvanic cell
Crevice Corrosion of Metallic Materials 359
Copyright © 2002 Marcel Dekker, Inc.
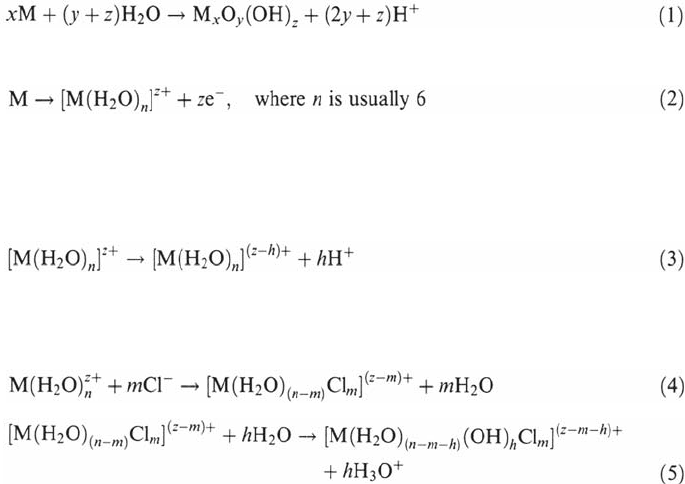
between the inside and the outside of the crevice. As a consequence, the IR drop
between the crevice and the bulk is responsible for the buildup of a corrosion
potential difference between the inside and outside surfaces, the former becoming
more anodic. However, the contrary to what happens on unalloyed steels (see
earlier), the stainless alloys exposed to neutral chloride solutions are passive in both
aerated and deaerated solutions and the local oxygen consumption has no significant
effect of the dissolution kinetics. Thus, this step does not induce any corrosion
damage but it is essential to trigger the following process.
Step 2: pH Evolution and Concentration of Dissolved Species
The anodic dissolution of the metal inside the crevice produces metallic cations
(and/or oxides that contribute to thickening the passive layer, at least before initiation
of the propagation stage).
Production of solid oxides or oxyhydroxides
360 Combrade
Production of solvated metallic cations
These metallic cations cannot concentrate indefinitely and they undergo
hydrolysis.
The earlier theories assumed the formation of simple hydroxides:
It is now recognized that metallic cations may also form metallic chlorides and/or
complex oxy-hydroxy-chlorides according to reactions such as
The formation of metallic polycations should also be considered [4].
These anodic processes result in a local excess of cations inside the crevice.
Because there is no cathodic reaction inside the crevice, the restoration of electrical
neutrality requires the onset of a galvanic current in the base metal and a migration
flux in the liquid. At least in the early stages of crevice evolution, most of the ions
conveyed by this migration are chloride ions that are transported from the bulk
solution into the crevice [22].
All these processes are summarized in Figure 13, where hydrolysis reaction (3)
is assumed. As a consequence, the crevice environment becomes more and more
acidic and more and more concentrated in metallic ions (including metal chloride
complexes) and free chloride ions. We will see later that, in some instances, this
pH evolution, combined with an IR drop, can make possible the reduction of water in
the crevice.
Copyright © 2002 Marcel Dekker, Inc.
