Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

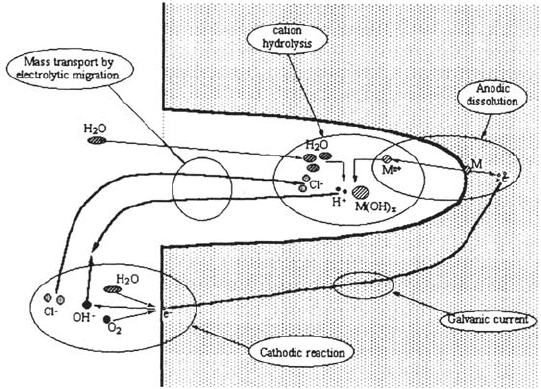
Indeed, numerous experimental studies have been performed to study the
evolution of the environment in a crevice. Most data were obtained in actively
corroding artificial crevices or pits, either by sampling the solution or by direct pH
and Cl
–
concentration measurements. Table 1 summarizes significant results that
confirm the foregoing trends. In crevice solutions, the drop of pH depends on the
hydrolysis constants of the metal cations. On stainless alloys, chromium and
molybdenum are considered to be the cause of the very low, sometimes negative, pH
observed. Iron, nickel, and aluminum exhibit much less acidic hydrolysis reactions
and the pH values in the crevices are higher: values of 3 to 5 are reported for iron,
values of 3 to 4 for the aluminum alloys.
It must be noted that the pH drop is due to the dissolution of the base material:
thus, the more resistant the material, the lower is the pH required for dissolution to
occur. Thus, the more acidic crevice pH values are observed on the more resistant
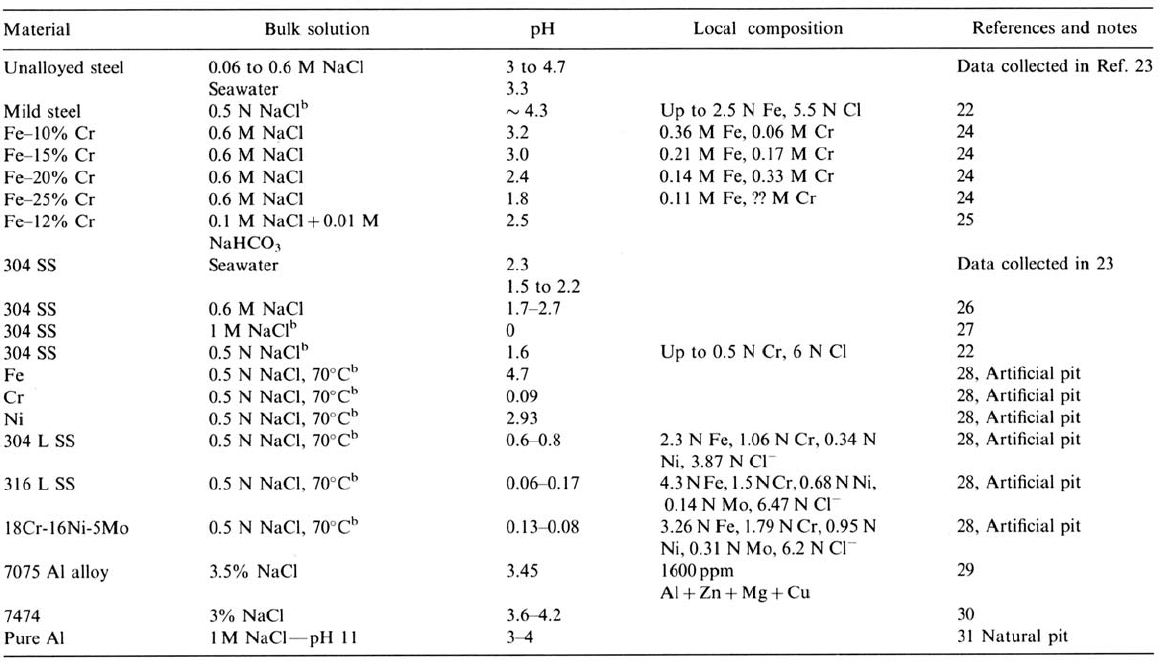
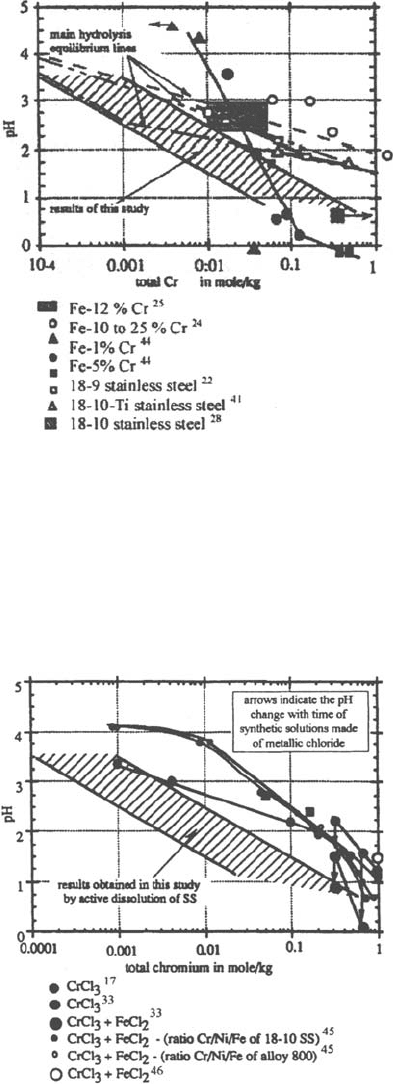
materials. This is shown, for example, in the studies of Bogar and Fujii (Fig. 14 [24])
on Fe-Cr ferritic stainless steels and Suzuki et al. [28] on 304L, 316L, and
18Cr-16Ni-5Mo austenitic stainless steels (Table 1).
The concentration of the ions in the crevices can reach very high values of the
order of several mol/L [28,33], and the solution may eventually become
saturated, which possibly limits the dissolution rate. In such concentrated solutions,
salt films may form on the dissolving metal surfaces: NiCl
2
has been identified in
nickel [34] and FeCl
2
in iron [35] and stainless steel [35] pits or crevices.
Several studies also outline the strong effect of the potential of the free surfaces
on the pH drop inside an actively corroding crevice: in particular, early work of
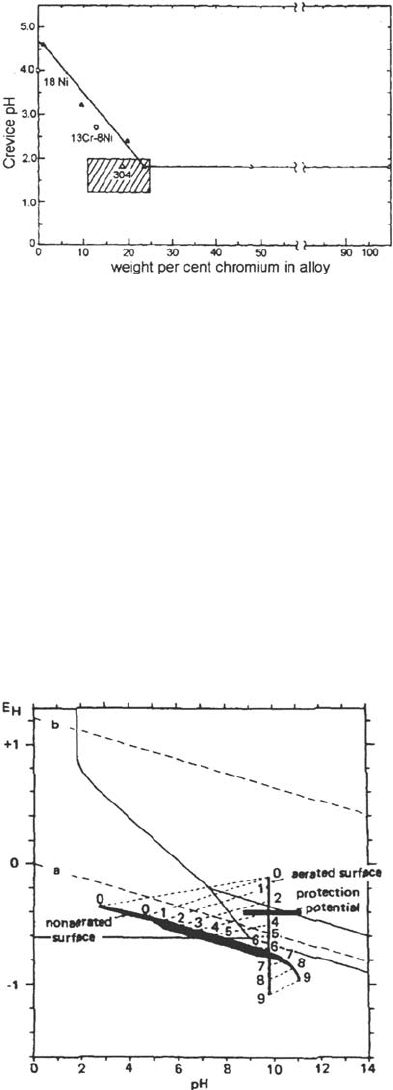
Pourbaix [36] on unalloyed steel showed (Fig. 15) that the higher the potential of the
external surfaces, the higher the potential and pH drops in the crevice. Similar results
were obtained by Zuo et al. [37] for stainless steel actively corroding in an artificial
crevice. Conversely, Pourbaix [36] and Turnbull and Gardner [38] on unalloyed
Crevice Corrosion of Metallic Materials 361
Figure 13 Sketch of the main processes leading to environment evolution in a crevice.
Copyright © 2002 Marcel Dekker, Inc.
362 Combrade
Table 1 Some Experimental Measurements of Local pH and Composition in Crevices
a
a
For more details see Turnbull [32].
b
Anodic polarization.
Copyright © 2002 Marcel Dekker, Inc.
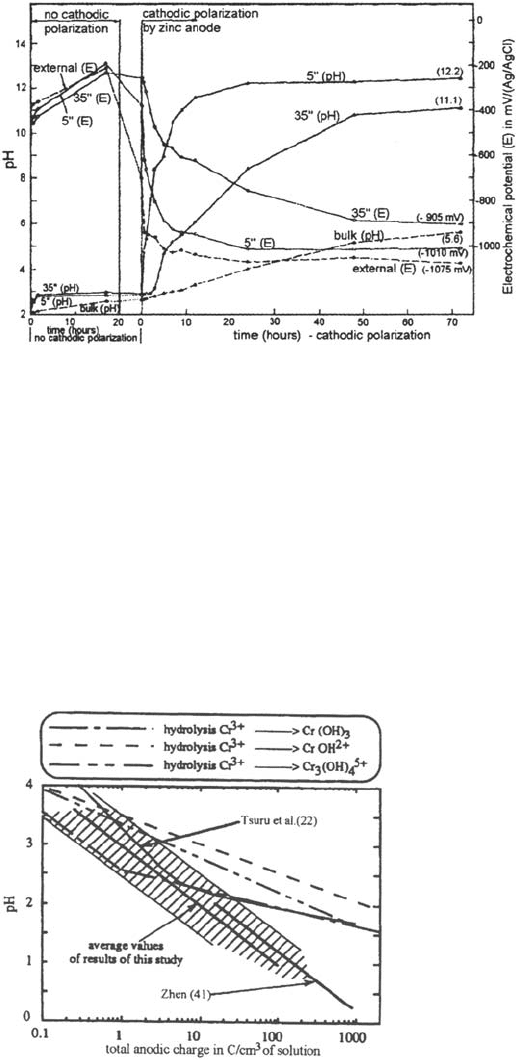
steels and Peterson and Lennox [39] on 304 stainless steel showed that cathodic
polarization of the free surfaces induced an increase of pH and a cathodic
polarization of the surfaces inside the crevice (Figs. 15 and 16).
In the artificial crevice experiments, several other features are interesting. As
an example, for the same 304 stainless steel:
The relationship between the total amount of anodic charge passed in an artificial
crevice and the pH is not unique (Fig. 17),
The relationship between the chromium content (and more generally the composition)
of the crevice solution and the pH is not unique and does not agree with any
simple hydrolysis reaction (Fig. 18),
Crevice Corrosion of Metallic Materials 363
Figure 14 Effect of chromium content of the alloy on the pH in the crevice. (From Ref. 24.)
Figure 15 Effect of the potential of the external surfaces on the pH and potential inside an
artificial C-steel crevice [36]. Same numbers refer to the corresponding conditions on the free
surface and inside the crevice.
Copyright © 2002 Marcel Dekker, Inc.
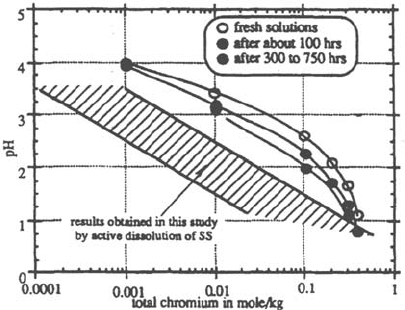
The pH of a synthetic solution made of reagent grade transition metals and sodium
chlorides does not have the same pH as the crevice solution of the same
composition (Fig. 19). In addition, this pH spontaneously decreases with time
[33,40] (Figs. 18 and 20) for periods of time of several weeks which should not
be neglected when modeling the crevice evolution. Indeed, Zhen [41] showed,
by ultraviolet (UV) spectroscopy, the presence of at least two different Cr
compounds in stainless steel crevice solutions and observed a spontaneous
evolution with time toward one of these species.
364 Combrade
Figure 16 Effect of cathodic polarization by zinc anode on the pH and potential inside
a 304 SS crevice [37].
Figure 17 Experimental relationships obtained by different authors between pH and
dissolution current 304 SS artificial crevice and comparison with simple hydrolysis
equilibria [40].
Copyright © 2002 Marcel Dekker, Inc.
Crevice Corrosion of Metallic Materials 365
Figure 18 Experimental relationship obtained by different authors between pH and Cr
content of artificial crevice solutions [40].
Figure 19 Comparison of artificial crevice solution with synthetic solution of the same
composition [40].
Copyright © 2002 Marcel Dekker, Inc.
Thermodynamics of the Crevice Environments for Fe-Ni-Cr Alloys
Thus it appears that the detailed chemistry of the crevice environments is not very
well known for the following reasons:
The ions and solid species formed may be more complex than the simple metal
hydroxides assumed in most models. For example, Tsuru et al. [22] and
Ogawa [42] have shown, in the case of stainless steel, the formation of
chloro-hydroxy complexes involving up to 20 chloride ions, although the
coordination number of chromium ions should not exceed 6. Tsuru et al.
[22] also showed that in iron and stainless steel crevices, iron is likely to
be complexed to chloride as [FeCl(H
2
O)
5
]
+
(i.e., FeCl
+
), but Brossia et al.
[35] found that CrCl
2+
is the most likely metallic chloride in stainless
steel crevices.
In concentrated solutions, the presence of large amounts of chloride has been
shown to decrease the pH [22,33,42] by increasing the activity coefficient of
the hydrogen ions.
Some reactions of hydrolysis and complexation are not as fast as usually assumed
(see earlier).
This may suggest that the nature of the cationic species produced by the
anodic dissolution may depend on the surface conditions of the alloy and on the local
environment. In particular, the analyses of passive films on stainless steels showed
[43] that the external layers are very hydrated and contain chloride. On the other
hand, the number of hydroxyl groups and chloride ions adsorbed on active surfaces
is likely to be dependent on the local pH, chloride content, and surface potential.
Thus, dissolution from a passive surface may produce metallic hydroxy-chlorides
different from those resulting from active dissolution, and active dissolution may
produce cations depending on the local conditions.
366 Combrade
Figure 20 Evolution with time of the pH of synthetic solution made of metallic chloride
salts [40].
Copyright © 2002 Marcel Dekker, Inc.
Other Environmental Evolutions in the Crevice Gap
In the case of stainless steels, Lott and Alkire [47] consider that the dissolution of
sulfide inclusions (MnS) inside a crevice is the main environment change before
passivity breakdown. They showed by direct analysis of the local environment in an
artificial crevice that manganese sulfides were oxidized to thiosulfates, which are
known to promote pitting in aerated chloride environments [48–50] and, more
generally, to inhibit film repair on stainless alloys. More recent work of Brossia and
Kelly [51,52] showed that the dissolution of manganese sulfides actually produced
sulfide rather than thiosulfate ions. Whatever the nature of the reactive sulfur species
produced by the degradation of MnS inclusions, they can be deleterious for the
passive layer, particularly because they may form on the bare metal surfaces an
adsorbed layer of sulfur that enhances the anodic dissolution and inhibits (or retards)
the formation of the passive film [53]. Indeed Crolet et al. [54] showed that in acidic
environments representing crevice solutions an increase in the sulfur content of the
stainless steels favored the onset of active dissolution.
In the case of dissimilar crevices where one of the crevice walls is made of
a nonmetallic material, such as a gasket, the possibility of dissolution of foreign
elements must also be considered.
Breakdown of Passivity inside the Crevice
The detailed mechanism of passivity breakdown inside a crevice is not clearly
established and different possibilities are still discussed. Indeed, it is quite possible
that different mechanisms may be involved depending on the material, bulk
environment, crevice geometry, and, possibly, surface condition of the metal.
“Classical” Mechanism”: General Breakdown of Passivity in Low
pH, High Chloride Solutions
The more “classical” mechanism involves general film breakdown in a critical
environment characterized by a low pH and a high chloride content. Low pH and high
chloride concentrations are known to be deleterious for the passivity of most alloys.
Thus, the progressive evolution of the crevice environment causes a degradation of
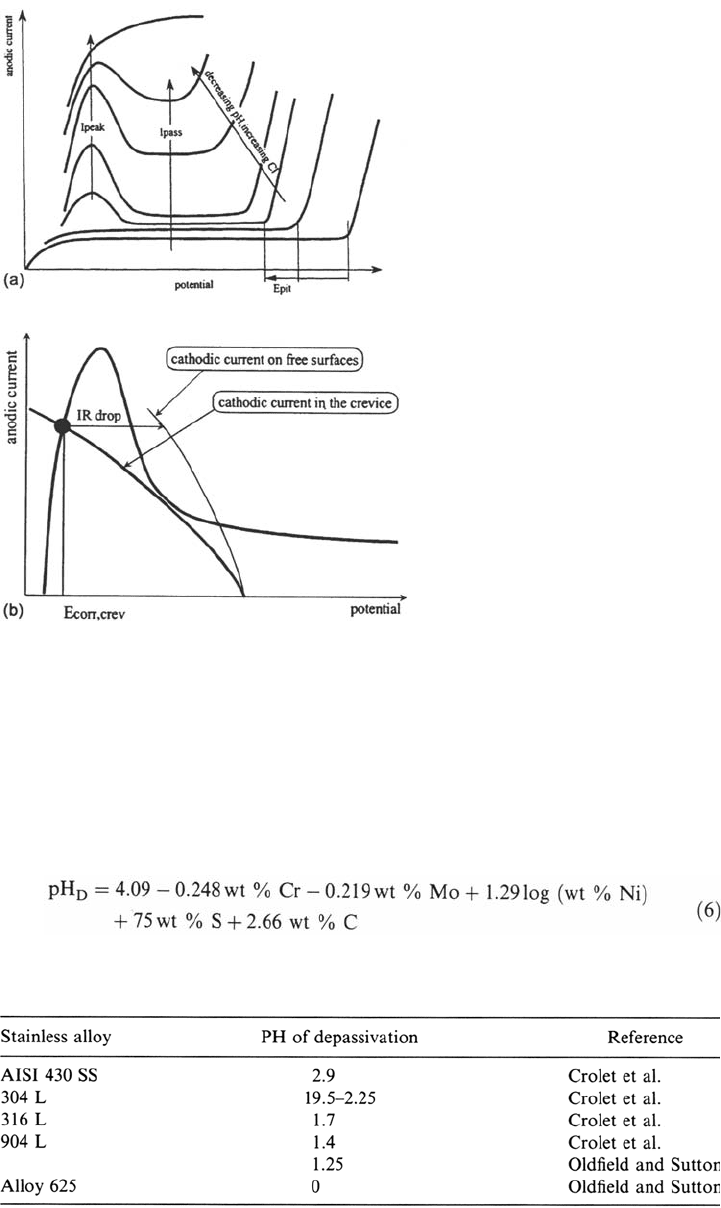
the passive film that may result in the following successive situations (Fig. 21a):
1. An increase of the passive current
2. The onset of an active dissolution domain with a peak current that increases with
decreasing pH and increasing chloride content (and, thus, increasing with time)
3. A complete inhibition of passivity
Passivity breakdown is thought to occur either when the corrosion potential of the
crevice surfaces is located in the active peak due to ohmic drop (Fig. 21b) or when
there is no more active-passive transition on the anodic curve.
This mechanism of initiation gave rise to the notion of critical pH for film
breakdow [55], which was extensively used as a criterion to rank the resistance of
stainless alloys to crevice corrosion. For Crolet et al. [55] the critical pH is the pH
corresponding to the onset of an active peak in the polarization curves, while Ogawa
[42] considered the pH of spontaneous film breakdown under free corrosion
conditions. These differences changed somewhat the critical pH values but usually not
Crevice Corrosion of Metallic Materials 367
Copyright © 2002 Marcel Dekker, Inc.
the alloy ranking. Table 2 gives some results of critical pH evaluated on stainless
alloys. Okayama et al. [56] performed measurements of the depassivation pH of over
50 stainless steels and nickel-base alloys and found that the effect of alloying
elements of austenitic materials was given by
368 Combrade
Figure 21 (a) Evolution of the anodic characteristic of passivated alloy when decreasing
the pH and increasing the chloride content. (b) Activation inside a crevice when the
corrosion potential is located in the activity peak due to ohmic drop in the crevice.
Table 2 Examples of Depassivation pH Measured according to Crolet et al. [47] and
Oldfield and Sutton [54] Criteria
Copyright © 2002 Marcel Dekker, Inc.

This relation showed the well-known beneficial effect of Cr and Mo and confirmed
the very deleterious effect of sulfur (see previous section).
On very resistant stainless alloys, the criterion of depassivation pH tends to
be replaced by a criterion of critical crevice solution (CCS) [19,57] and Gartland
assumes that crevice corrosion occurs when the solution is so aggressive that
passivity is no longer possible over the whole potential range.
However, this classical mechanism appears not to be consistent with different
observations, at least on less resistant materials:
Several experiments [58–60] with in situ local measurement of pH and/or chloride
concentration with microelectrodes showed that the large changes in crevice
pH and chloride content mentioned in the former paragraph occurred not
before but after passivity breakdown. In these experiments [58,61], the
potential difference between crevice and free surfaces (i.e., the ohmic drop) is
often very low before initiation (few mV) and becomes larger only after
crevice initiation.
This mechanism does not explain clearly why the initiation time is so dependent on
the potential of the external surfaces [60,62], particularly if the passive current
is not significantly dependent on the potential as is assumed in most crevice
models. This strong dependence on the applied potential would support the
idea that the pH drop rate in the crevice is controlled by the migration process
and/or by the available cathodic current. This is hardly understandable at
the very low current densities involved during the initiation stage, when
considering that dissolution rates several order of magnitude higher can be
sustained during the propagation stage.
The following points have been raised to account for these observations:
The corrosion initiates by local passivity breakdown in the crevice as a result of (a)
microcontacts due to wall asperity or (b) pitting inside the crevice.
The critical environment involves the buildup of deleterious species such as (c)
sulfur or (d) chloride species rather than low pH.
“Microcrevices” inside the “Macrocrevice” Gap
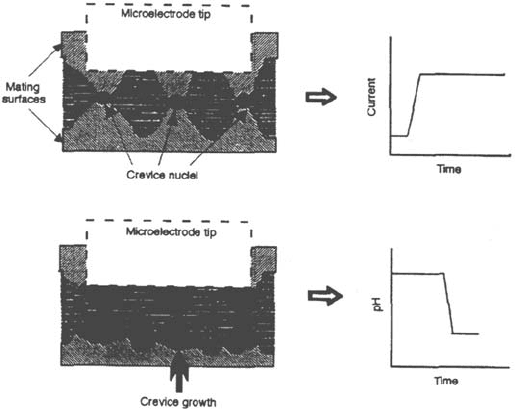
Several authors [1,14,60] raised the point that at the scale of the crevice gap, the
surface roughness may play a major role in the environment evolution. Local contact
between the opposite surfaces may constitute very tight local “microcrevices”
inside the “macrocrevice” (Fig. 22).
Due to their very narrow gap, these microcrevices could be very efficient in
promoting a very local pH change (see Modeling Crevice Corrosion later) and a local
Crevice Corrosion of Metallic Materials 369
Figure 22 Crevice formed by asperity contact [14].
Copyright © 2002 Marcel Dekker, Inc.
passivity breakdown by the mechanism presented in the former paragraph. As
soon as active dissolution occurs somewhere inside the macrocrevice, the breakdown
of passivity spreads out progressively to the whole crevice provided the potential
inside the crevice is high enough.
Thus, according to this view, the classical mechanism could operate at the scale
of the microcrevices because of asperity contacts rather than at the scale of the whole
crevice. Most of the “apparent” incubation period would be a period of very local
corrosion extending progressively into the whole crevice zone, and thus, it would be
sensitive to the potential because of the locally active dissolution.
This interpretation is also consistent with the observation that pH and chloride
microelectrodes detect only a significant environment evolution after a time tag
following the current increase because the size of the sensitive area of these electrode is
much larger than the size of the crevice nuclei inside the crevice. Thus, these microelec-
trodes detect only the spreading of the corrosion through the whole crevice (Fig. 23).
Pitting in the Crevice Gap
The initiation of crevice corrosion by pitting inside the crevice gap is mainly
invoked for stainless steels. Indeed, several investigators [23, 46, 63, 64] have
observed pitting and further coalescence of the pits inside stainless steel
crevices. Eklund [64] showed that pitting on MnS inclusions near the mouth of
the crevice may be the initial stage of crevice corrosion of stainless steels.
Oldfield and Sutton [46] have given a detailed description of the sequence of
micropitting, pit coalescence, and crystallographic etching that results in a
rapid dissolution stage in a stainless steel crevice in 1 M NaCl solution (Fig. 24).
In this case, early corrosion damages progressively spread through the crevice,
suggesting an effect of the environment evolution in the crevice. By using
370 Combrade
Figure 23 Development of localized corrosion inside a crevice gap formed by asperities
and corresponding response of current [60].
Copyright © 2002 Marcel Dekker, Inc.
