Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

a moiré fringe technique to observe directly the metal dissolution in a crevice,
Shinohara et al. [65] confirmed the localized character of the early dissolution events
and their lateral extension and coalescence.
It is worth noting that the crevice may favor pit initiation in two ways: (a) the
pitting potential of a stainless alloys decreases with increasing chloride content
(Fig. 21a); (b) the restricted transport conditions may contribute to stabilize pit
embryos at potentials lower than on the free surfaces [66]. This last point is supported
by the experimental and modeling work of Laycock et al. [62]. Indeed, the deleterious
effect of anodic polarization on the initiation time is obviously consistent with a
pitting process.
Role of Sulfide Inclusions on Stainless Steel
Besides their possible role in pit initiation (see earlier), the dissolution of the MnS
inclusions can generate thiosulfates [47, 67] or more likely sulfide [51, 52] ions,
which are known to favor passivity breakdown. Indeed, Crolet et al. [54] observed
higher depassivation pH on stainless steels with high sulfur content. According to
Brossia and Kelly, the initiation involves a critical [Cl
–
]/[HS
–
] ratio. This effect of
MnS is unlikely in modern stainless alloys with very low sulfur contents.
Passivity Breakdown Due to High (Metal) Chloride Concentration
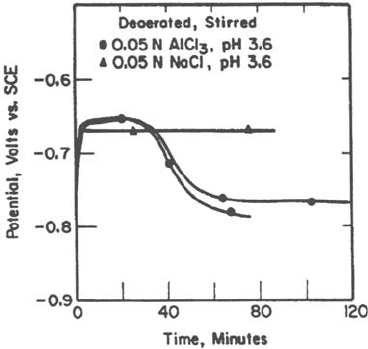
On pure aluminum, Hebert and Alkire [68] proposed that passivity breakdown
occurred when a critical concentration of aluminum chloride is attained in the
crevice environment. Indeed, they showed convincingly (Fig. 25) that the presence of
aluminum cations in the solution caused passivity breakdown for a pH and chloride
concentration at which solutions containing only sodium cations did not.
Based on an experimental work on alloy 7475 crevices and propagating cracks,
Holroyd et al. [30] agree with this assumption. The critical aluminum concentration
required decreases as chloride content increases [68] but low pH and high chloride
content are not a prerequisite for passivity breakdown.
The possibility of the existence of a critical concentration of chloride rather than
a critical pH has been proposed for stainless steels, for example, by Zakipour and
Leygraf [69] in relation to a critical composition of the passive film (chromium
content of about 48%).
Crevice Corrosion of Metallic Materials 371
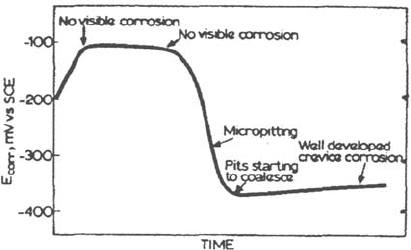
Figure 24 Crevice initiation by micropitting observed by Oldfield and Sutton [46] on
316 SS in 1 M NaCl, pH 6 solution.
Copyright © 2002 Marcel Dekker, Inc.
On stainless steels, several authors [70–73] showed that a minimum
concentration of metallic chloride is required for stable propagation to occur. As
mentioned previously, the external layer of the passive films on stainless steels
contains hydroxyl groups and chloride. Thus, it may be possible that the presence in
the solution of metal complexes modifies the external layers of the passive films and
contributes to lowering their stability.
The Role of IR Drop in the Initiation of the Crevice Corrosion
The role of IR drop in crevice initiation is not clear. Different authors [58,60,61]
observed crevice initiation on stainless steels at a very low IR drop level. It is clear
that initiation processes can be separated into two classes: (a) those that operate at
relatively high potentials (pitting in the crevice gap), which cannot be enhanced by
large ohmic drops, and (b) those that occur at low potential (general passivity
breakdown), which are favored by large IR drops. However, on stainless steels and
nickel-base alloys, there is at present no direct evidence to support the last type of
process, mainly because high free surface potential always enhances crevice
initiation of passive alloys.
On titanium alloys, however, the metal still has an active-passive transition in
the crevice environment and the surface potential must be low enough for the active
corrosion to occur. Thus, IR drop is required to stabilize the active dissolution [9,74],
particularly when large cathodic currents are available.
Propagation of Crevice Corrosion
Propagation of crevice corrosion occurs by active dissolution in an occluded cell
and it is generally considered analogous to the propagation stage of pitting corrosion.
372 Combrade
Figure 25 Effect of dissolved Al on the activation of pure Al in pH 3.6 solution [68].
Test under galvanostatic conditions.
Copyright © 2002 Marcel Dekker, Inc.
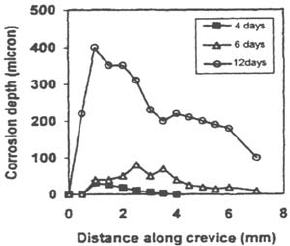
At least in a first stage, corrosion may not affect the whole crevice gap: it has
been shown that on austenitic stainless steels, dissolution is mainly located near the
mouth of the crevice [3,19] (Fig. 26), whereas on ferritic stainless steels the
maximum of dissolution rate is located deeper in the crevice [3].
As a consequence of rapid corrosion inside the crevice, the rate of evolution of
the environment toward more acidic and more concentrated solutions increases, and
indeed several experiments confirmed this point. However, the dissolution rate and
the environment evolution may be limited by the following factors:
Immediately after passivity breakdown, the dramatic increase of the dissolution
current has two consequences that contribute to limit the dissolution rate by
decreasing the corrosion potential of the active surfaces in the crevice:
1. The cathodic reaction kinetics increase on the free surfaces to balance the
increased anodic dissolution and this induces a drop of their corrosion
potential. Potential drops of several hundreds of mV can be observed on the
free surfaces due to the initiation of crevice corrosion (see Fig. 5) Large
cathodic areas, high solution conductivity, and cathodic reaction depolarizers
(such as carbon deposits) can increase significantly the amount of available
cathodic current and thus the crevice propagation rate.
2. The migration current from the crevice gap increases dramatically and this also
increases the ohmic drop: a role of the ohmic drop in the control of the
propagation rate is consistent with a maximum of corrosion rate located near
the crevice mouth as observed by several author [3,19]. One must notice that
ohmic drop is not limited to the solution inside the crevice. Significant ohmic
drops may also occur in the bulk solution near the crevice mouth, particularly
in dilute environments.
Later, several other events may also occur to limit the environment evolution
and the dissolution rate:
The solution inside the crevice reaches saturation and a salt film is formed on the
active surface. The corrosion rate is then limited by mass transport trough the
salt layer. Recent results [34,35,73,76–78] confirm this possibility, which was
not as clearly established as it is for pit propagation.
Crevice Corrosion of Metallic Materials 373
Figure 26 Preferential propagation near the crevice mouth [75].
Copyright © 2002 Marcel Dekker, Inc.
The pH and the potential in the crevice may become low enough for local water
reduction to be possible. This stifles further pH decrease as the local cathodic
reaction is able to balance the production of cations by anodic dissolution.
Balance between production and outward transport of cations and dilution due to
geometric changes of the crevice [79] are also invoked as possible limiting
processes. However, on the simple basis of the difference of density between
the dissolved metal and the saturated crevice solution, Hakkarainen [80]
pointed out that at least 97% of the dissolved cations must be evacuated out
of the active crevice. This weakens the preceding point. But, on the contrary,
the possible limitation by the diffusion of water through a saturated salt layer
has not been considered.
Thus, even though the corrosion occurs by active dissolution, its rate is not
controlled by an activation process and, generally, it remains limited to current
densities of the order of tens of mA/cm
2
in the saturated solutions, which prevail
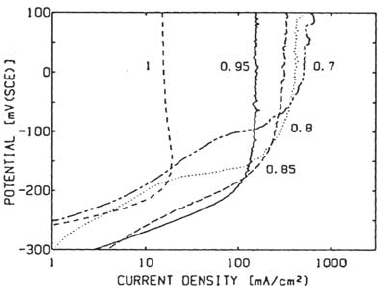
on well-developed crevices [19,33,57,70,71]. Figure 27 shows how the dissolution
rate decreases when saturation of the solution is approached.
When discussing the propagation of crevice corrosion, it is worth noting that the
analogy with the propagation of pits may have limitations. Most of the experimental
work and many simplified calculations refer to a geometry of the corroding crevice
with an aspect ratio L/h close to 1. This may have consequences for conclusions
regarding the distribution of the dissolution current inside the crevice gap and the
rate-controlling processes because an almost uniform corrosion potential on the
corroding surface and an almost uniform transport path for the diffusion and
migration processes can be assumed in pits. In very deep crevices, the potential and
the transport path vary along the crevice profile; thus, the evolution of the local
environment may vary. Consequently, the saturation and the formation of salt films
leading to a dissolution process controlled by diffusion may not occur simultaneously
on the whole crevice surfaces. In addition, the increasing IR drop near the tip of the
crevice gap may allow the reduction of water to occur, changing the local pH
evolution before any saturation has been reached.
374 Combrade
Figure 27 Anodic dissolution of 304 SS in simulated crevice solutions [71]. The numbers
indicate the degree of saturation of the solution.
Copyright © 2002 Marcel Dekker, Inc.
Crevice Repassivation
Spontaneous Crevice Arrest
Observations (see Propagation of Crevice Corrosion earlier) suggest that, under
constant bulk environment conditions, crevice corrosion may spontaneously cease.
This has been taken into account in a model developed by Gartland [19]. Gartland
attributed this behavior to an environment dilution caused by the geometric change
of the crevice, but we have seen that this argument is not always likely. It also seems
appropriate to invoke salt precipitation and/or local dryout due to the slow diffusion
of water through a salt film of increasing thickness. This suggests that mass transport
could not be efficient enough to eliminate such a large amount of corrosion products
and this could lead to the precipitation of solid products that would fill the crevice
gap. Another cause of self-arrest, or at least of kinetic limitation, could be lack of
sufficient water in the crevice gap. Further studies are required to establish the
conditions and the mechanisms involved in this spontaneous arrest, in order to take
advantage of it in practical situations.
Repassivation Potential
The propagation of crevice corrosion can also be arrested by decreasing the potential
of the outside surfaces below a critical value (see earlier). The existence of a
“repassivation” or “protection” potential was recognized very early, in particular by
Pourbaix et al. [81] for pitting corrosion. From a practical point of view, the existence
of a protection potential below which no crevice corrosion is possible is of major
importance because it guarantees the immunity of passivated alloys in near-neutral
chloride solutions in the absence of oxidizing species and because it makes possible
the cathodic protection of structures.
However, the significance and the intrinsic nature of this potential are still under
discussion. There are at least two possible causes for active corrosion inside crevices
to stop below a critical potential:
First, the critical potential may be a “deactivation potential”, i.e., a potential that
corresponds to a cancellation of the overpotential required for dissolution in
the crevice. According to Starr et al. [82], if active corrosion stops by
deactivation with decreasing potential, a further increase of the corrosion
potential would cause an immediate reactivation of the crevice. Indeed, Starr
et al. [82] and Dunn and Sridhar [83] observed such a reactivation on
low-grade stainless steels in acidic environments.
Second, the critical potential may be a repassivation potential. It has been shown
in artificial active crevices that lowering the potential of the free surfaces
causes the local environment to become less aggressive (see, for example,
Pourbaix [36]). Thus, at some point, the environment becomes not aggressive
enough for active dissolution to be sustained and the metal surface in
the crevice becomes passive. In this case, a subsequent increase of the
corrosion potential does not produce immediate reactivation inside the
crevice. Starr et al. [82] observed this situation on 12% Cr stainless
steels in near-neutral environments; Dunn and Sridhar [83] observed the
same behavior on alloy 825. However, the repassivation may be
attributed to different environment changes: an increase of local pH in
the crevice [82,84], a destabilization of the salt film that controls the
Crevice Corrosion of Metallic Materials 375
Copyright © 2002 Marcel Dekker, Inc.
dissolution kinetic in the crevice [77,85,86], or a decrease of the chloride
concentration below a critical value [34,35,71–73,77,78,83]. Recent experi-
mental observations [34,35,78] show that corrosion may be stable even
after dissolution of the salt film.
In fact, studies of Cragnolino et al. [8,11] suggest that, depending on the
conditions of the experimental determination, the “arrest” potential measured by
backscan polarization may be close to either potential: using a relatively high scan
rate, which does not allow time for the local environment to be significantly
modified, the measured arrest potentials are close to the deactivation potentials and
they are significantly lower than those measured under a low potential scan rate. The
latter are more likely to be due to a modification of the local environment toward less
aggressive conditions. We have already seen that the maximum repassivation
potential seems to be almost identical to the minimum initiation potential (Fig. 8).
This observation has been reported for a relatively resistant stainless alloy (alloy 825)
and it would support the idea of Gartland [19,57] (see earlier) that there is a critical
environment for passivity breakdown and that the local environment required for
repassivation is not very different. This could be an argument against the mechanism
of initiation by pitting in the crevice and in favor of a mechanism involving a critical
environment.
A pending question is still to know whether this repassivation potential has
a unique value or depends on corrosion damages and crevice geometry. The following
arguments are in favor of such a unique value:
1. For a given bulk environment and a given crevice configuration, the
repassivation potential becomes independent of the corrosion extent as soon
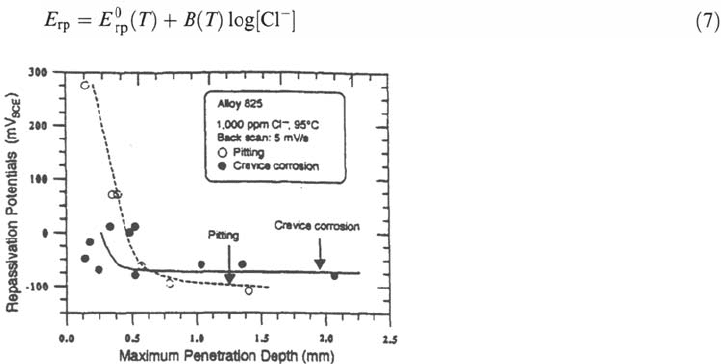
as some critical damage size is exceeded [8] (Fig. 28).
2. At least for high chloride concentrations, the repassivation potential exhibits
low values which are poorly dependent on the chloride content of the
environment [87] (Fig. 29). Dunn et al. expressed the average values of the
repassivation potential as follows:
376 Combrade
Figure 28 Influence of the corrosion damage on the repassivation potential of an alloy
825 in a 1000 ppm Cl
–
solution at 95°C [8].
Copyright © 2002 Marcel Dekker, Inc.
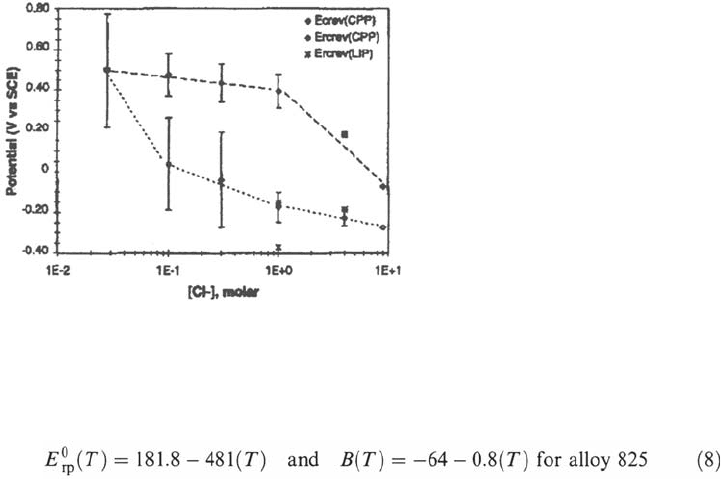
However, the Fig. 29 shows that the effect of chloride content is low on the
repassivation potential, particularly when looking at the lower bound of the scatter
b and of the measured value which is almost constant for chloride contents in excess
of 10
–1
M. This is consistent with the presence of a nearly saturated solution and/or
precipitated salt film in well developed crevice corrosion, and indeed such
environments should be almost independent on the bulk solution chemistry. Thus, a
repassivation potential which, according Pourbaix [36], is close to the local potential
of an actively corroding crevice should be poorly or no dependent on the bulk
environment and crevice geometry.
Nevertheless, this point still requires more work, particularly regarding the
exact meaning of the high values of the repassivation potentials measured in low
chloride and/or low temperature environments (left side of the Fig. 10, 11 and 29). In
this case, the local environment inside the crevice is likely to be controlled by mass
transport and thus the repassivation potential could be dependent on the crevice
geometry.
MODELING CREVICE CORROSION
In order to try to predict the behavior of passive materials in chloride environments
and particularly stainless alloys in seawater, many attempts have been made to
model the environment in a crevice, mostly for stainless Fe-Ni-Cr-Mo alloys. Two
kinds of model have been developed:
Steady state model [2,89–92], which try to calculate the environment in a
propagating crevice or, more frequently, a pit, and
Transient models [2,4,19,57,79,93–99], which intend to describe the environment
evolution in a crevice and, in most instances, to predict the passivity
breakdown by using a criterion usually based on a critical environment
resulting from experimental data.
Crevice Corrosion of Metallic Materials 377
Figure 29 Influence of the chloride content on the repassivation potential of an alloy
625 at 95°C [87].
with
Copyright © 2002 Marcel Dekker, Inc.
Modeling the environment or the environment in a crevice requires solving
a set of equations including:
The kinetics of surface reactions in the crevice, mainly the metal dissolution
because the cathodic reaction is generally assumed to occur only on the
outside surfaces
The kinetics of the reactions within the crevice solution, mainly the formation of
metal chlorides and the hydrolysis of cations, which is the fundamental
cause of pH drop within the crevice
The transport processes including electrolytic migration and chemical diffusion, as
most of the models assume no convection inside the crevice gap
The mass and electrical charges balance in the crevice solution, across the
metal-solution interfaces, and at the mouth of the crevice
Surface Reactions
With few exceptions [19], the passive anodic current of metal dissolution is usually
taken as a constant and potential independent during the initiation stage and/or it is a
parameter of the calculations.
During the propagation stage, different models are used, from the Tafel law
[100] to experimentally fitted laws [19]. To take into account the strong current
limitation due to the formation of a saturated salt layer on the dissolving surface,
Gartland [19] introduced a damping coefficient that increases exponentially as the
local concentration approaches saturation.
The possibility of water reduction inside the crevice gap is taken into account
by Gartland [19] as a possible situation when the local pH and potential become low
enough. Only one model [99] includes the cathodic reaction outside the crevice gap.
It is more or less implicitly assumed in most models that this reaction is not a
controlling process for the environment evolution inside the gap.
Solution Chemistry
The description of the crevice solution chemistry is probably the most difficult
step (at least from the corrosion or chemistry standpoint if not the computer
programming) as a result of the lack of reliable data. The main problems are:
The thermodynamic description of the solution inside the crevice, which cannot be
done by the dilute solution models, at least during the propagation stage
The nature of the complex ions formed in the crevice and their thermodynamic
properties, which are required to calculate the hydrolysis reactions governing
the environment evolution
Solution Models—Activity Coefficients
Most models use dilute solution assumptions and former models [93] assumed unit
values [2,19,93,94] of all activity coefficients (except in some cases for H
+
ions).
Bernhardsson et al. [4] reached a compromise by using two sets of equilibrium
constants, one for dilute solutions and the other for concentrated solutions.
378 Combrade
Copyright © 2002 Marcel Dekker, Inc.
Other authors used Debye-Hückel, the truncated Davies model [96,98], or the B-dot
Debye-Hückel model [98] to derive the activity coefficients in concentrated
solutions.
A particular case is the activity coefficient of H
+
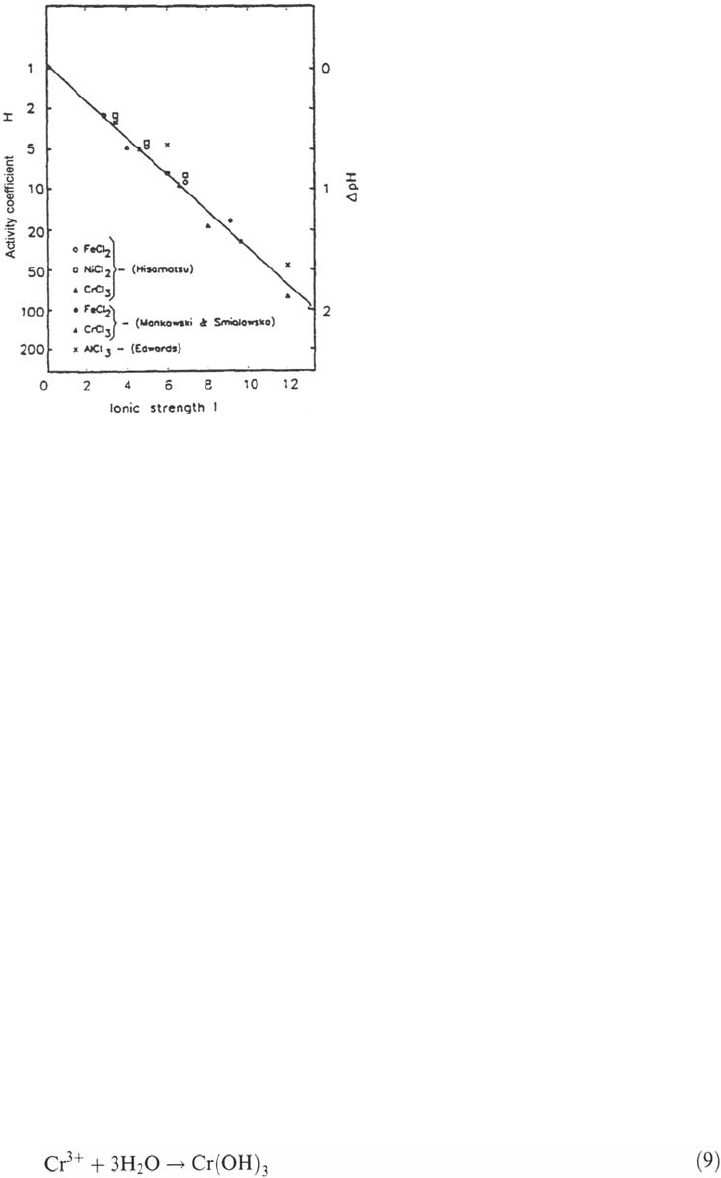
ions. It has been shown
experimentally (Fig. 30) that the presence of metal ions strongly decreases the pH of
a chloride solution. Many models [2,19,94,97] include a pH correction derived from
the results of Mankowski and Smialowska [33].
More recently, Brossia et al. [35] introduced an approach based on new
correlations for activity coefficients in concentrated solutions. This approach [101] is
based on the Helgeson-Kirkham-Flowers equation for standard-state properties with
a nonideal solution model based on the activity coefficient expression developed by
Bromley and Pitzer. Using specific software, Brossia et al. were able to predict the
dominant ionic species and the salt precipitation in Ni, Fe, Cr, and 308 stainless steels
crevice solutions. Fair agreement was observed with the in situ analyses of these
crevice solutions by Raman spectroscopy.
Hydrolysis and Complexation Products and Reactions
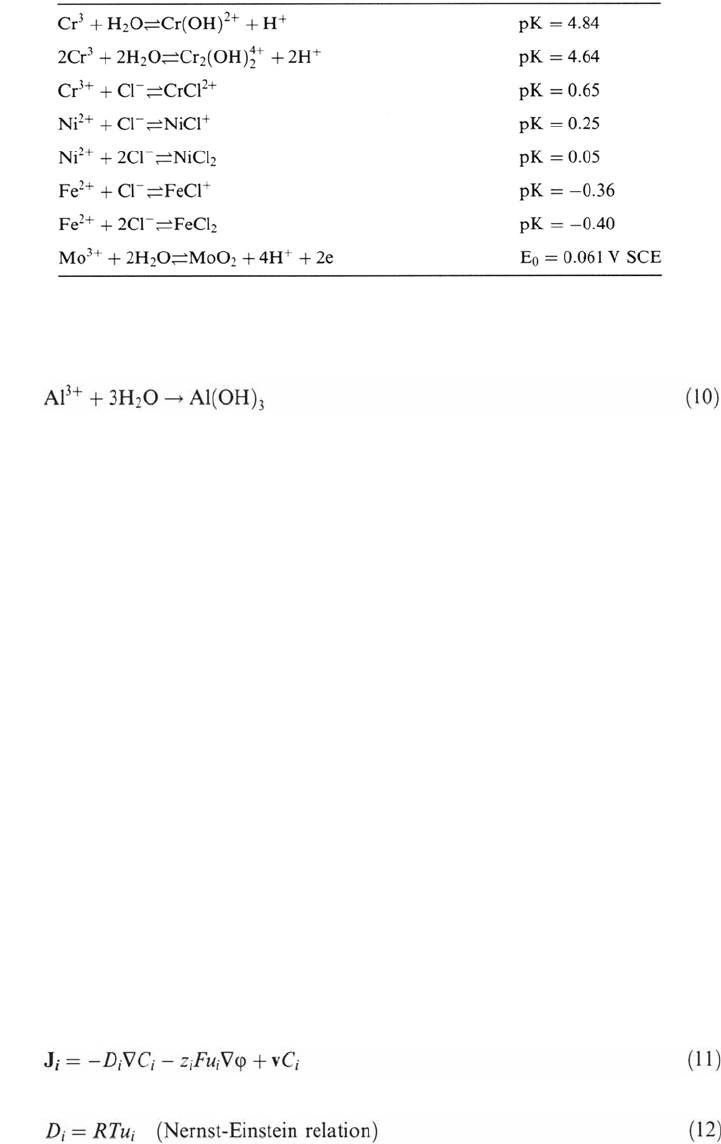
These species and reactions include the products of the metal cation hydrolysis
and the formation of metallic chlorides.
In one of the first models [93], the formation of metal chlorides was not
taken into account and the cation hydrolysis was assumed to produce solid
hydroxides. In the case of stainless steels, the pH drop was assumed to be driven
by the hydrolysis of chromium cations according to the following reaction:
Crevice Corrosion of Metallic Materials 379
Figure 30 Influence of the ionic strength on the H
+
activity in chloride solutions [19].
Copyright © 2002 Marcel Dekker, Inc.
In the case of aluminum alloys, the hydrolysis reaction was assumed to be
380 Combrade
Table 3 Solubility and Hydrolysis equilibria in the Model of Gartland [19]
Actually, the drop of pH is related to more complex reactions and species. Thus, in
more sophisticated models, several hydrolysis reactions and metal chloride formation
are taken into account but the selection of species and reactions is somewhat
different from model to model. Oldfield and Sutton [94] and Watson and
Postlethwaite [2] considered only hydroxides as the product of cation hydrolysis.
Sharland [96] introduced simple metallic chlorides. The most complete set of species
and reactions has been used by Bernhardsson et al. [4], which made available the
thermodynamic data of a large number of species, including several iron, nickel,
chromium, and molybdenum polycations as well as metal chlorides and
hydroxychlorides. Gartland [19] used a more limited set of species (Table 3) selected
among the Bernhardsson data. According to their experimental results, Hebert and
Alkire [95] included Al(OH)
3–n
n
as the hydrolysis product in their model of the crevice
corrosion of aluminum alloys.
A common feature of all the models is that all the chemical reactions of
complex formation and cation hydrolysis are fast enough for thermodynamic
equilibrium to be assumed. This may also be an incorrect assumption because we
have already mentioned that the pH of synthetic crevice solutions can evolve for
significant periods of time (up to several weeks) before reaching a stable value
[33,40].
Transport Processes
Most models use transport equations for dilute solutions including diffusion,
electrolytic migration, and convection such as:
with
Copyright © 2002 Marcel Dekker, Inc.
