Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

However, different attempts have been made to take into account the deviation
from ideality of the crevice solutions:
Walton et al. [98] wrote the transport equation (11) in a form using the activity
instead of the concentrations.
Crevice Corrosion of Metallic Materials 381
However, this formulation assumes the validity of the Nernst-Einstein equation for
any ionic strength.
Gartland [19] made an attempt to use corrected ionic mobility coefficients: he used
the diffusion coefficients collected by Bernhardsson [4] regardless of the
solution concentrations but, to calculate the ion mobility coefficient, he
introduced in the Nernst-Einstein equation an ionic strength–dependent
correction factor based on a limited set of experimental data.
Walton [102] also included a porosity tortuosity factor in the diffusion coefficient
to take into account the presence of porous solids and/or gas bubbles in the
crevice gap.
The ionic fluxes are generally used to derive the current flowing in the crevice:
One common assumption of most, if not all, the available models is that the
transport is purely unidimensional; i.e., the crevice is assumed to be narrow
enough for the transverse concentration and potential gradients to be negligible. In
fact, this assumption is required in order to simplify the numerical solutions.
Mass Balance
The mass balance equation:
includes the production of species by chemical reactions in the liquid phase R
i
. These
reactions are the complexation and hydrolysis reactions as well as dissolution
reactions on the crevice walls because models assume unidimensional transport fluxes.
Electrical Charge Balance
The electrical charge balance inside (and in one instance outside) the crevice is
modeled in two ways:
Many models assume the electrical neutrality in any point [4,93,95,96]:
The potential distribution is obtained by using the Ohm law applied to the
currents calculated in Eq. (14) and solution resistivity calculated from mobility
coefficients.
Copyright © 2002 Marcel Dekker, Inc.

Others [2,79,97,99] solve the Poisson equation, which also gives the potential
distribution along the crevice gap:
382 Combrade
The solution of the Poisson equation dramatically increases the complexity and
duration of the numerical simulations and requires numerical shortcuts. In addi-
tion, Watson and Postlethwaite [2] mentioned that the time required for electrical
neutrality to be reached appears to be less than 10
–10
s. This is very short com-
pared with the time required for chemical equilibrium, and it is not clear whether
the simple assumption of electrical neutrality could significantly change the
results of such models.
Crevice Initiation Criteria
All criteria rely on a critical environment for passivity breakdown:
Critical pH [93]
Critical crevice solution [2,19,46,94] taking into account the pH and the chloride
content but not the effect of metal cations
Critical dissolved Al content [95]
Despite the experimental observations, no attempt has been made to model crevice
initiation by local pitting.
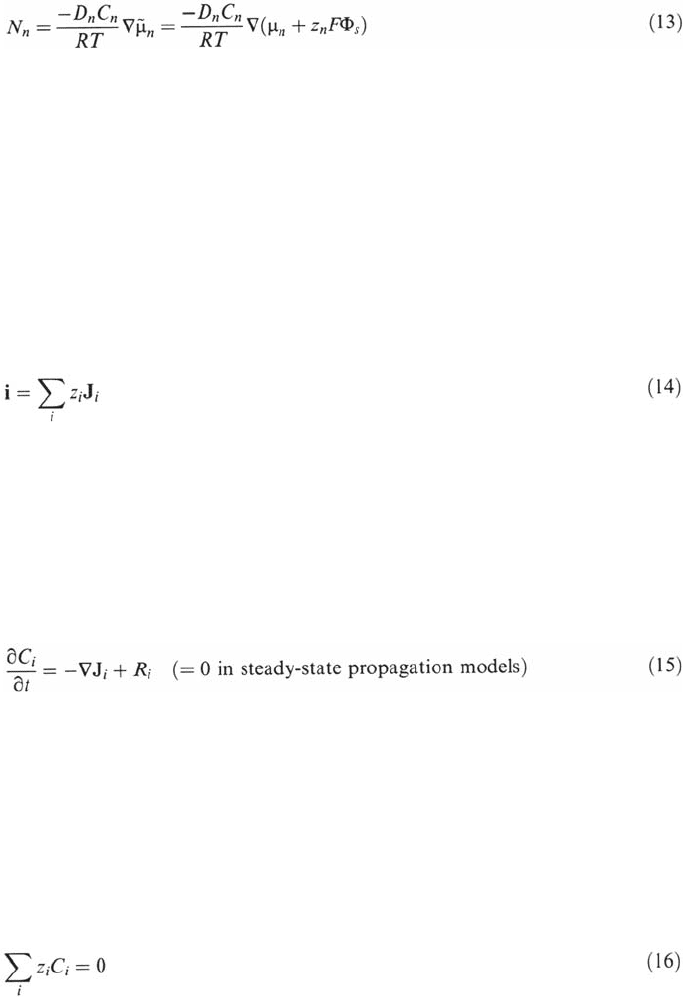
Results of the Models
As expected, the models predict the drop of local pH and the increase of chloride and
metal ion content in the crevice gap. The predictions of several models were
compared, with variable success, with the environment modifications observed in
artificial crevices. Figure 31 shows a typical example of results indicating that the
transport process is probably well enough modeled as indicated by the fair prediction
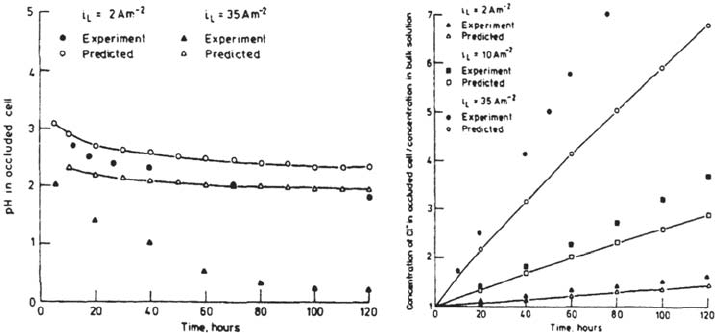
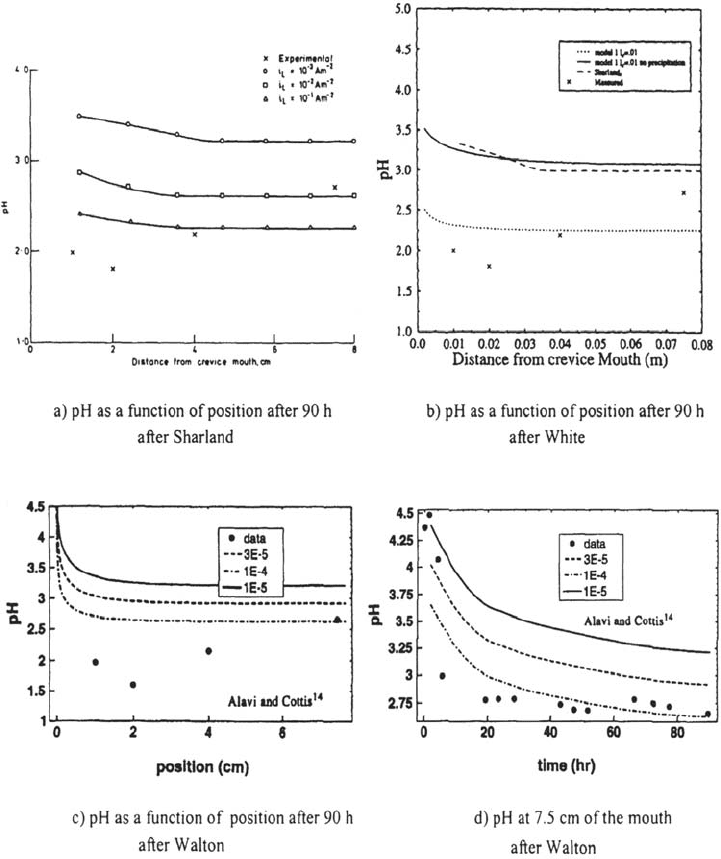
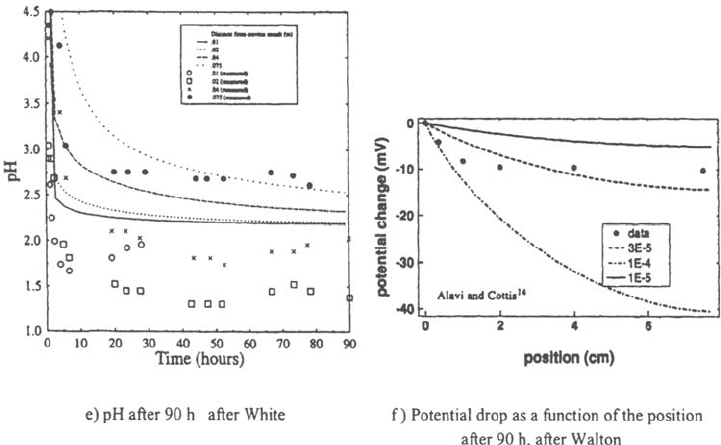
of the chloride content but that the pH calculations are far less accurate. In Figs. 32
and 33, the set of data of Alavi and Cottis [26] has been compared with several
models: the pH drops are generally underestimated (Fig. 32a–e), the potential
gradient inside the crevice is not well described (Fig. 32f), and no model is able to
predict the pH increase observed near the crevice tip (Fig. 3a–c).
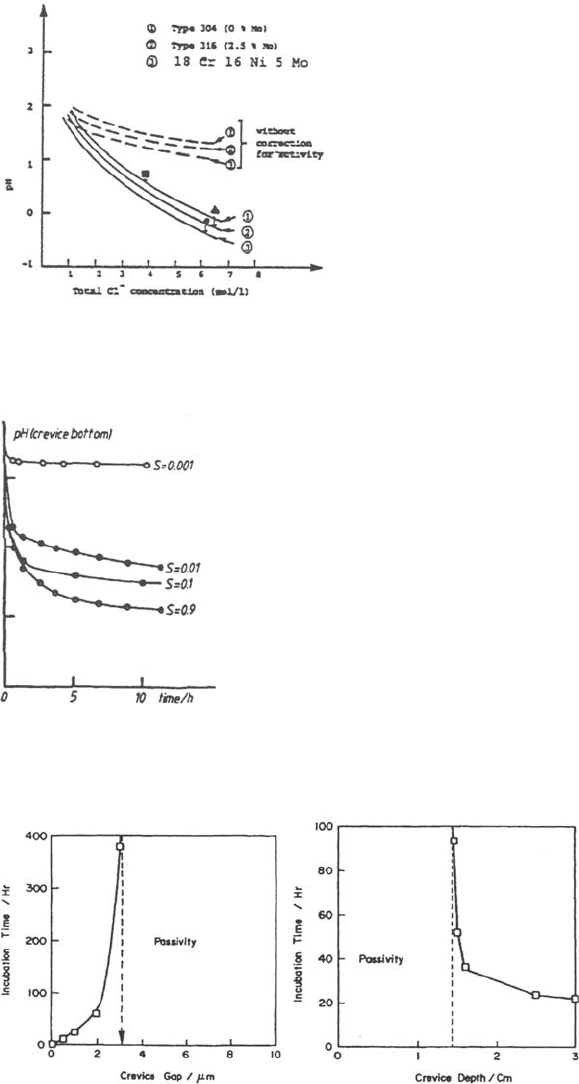
Gartland [19] showed (Fig. 33) that the use of a correction factor to evaluate the
H
+
activity is necessary to approach the actual pH, regardless of the fact that the
equilibria involving solubility and hydrolysis of metal cations are taken into account.
This may be one of the major causes of the underestimation of pH drop observed in
the preceeding models.
Beside the quantitative results, which are impaired by the lack of basic
knowledge of the crevice chemistry, the models provide interesting parametric results:
All models confirm that the crevice geometry is of major importance. As
previously mentioned, Bernhardsson [4] introduced an L
2
/h geometric
Copyright © 2002 Marcel Dekker, Inc.
factor (in fact, a severity factor S = i
a
L
2
/h, Fig. 34) that appears to control the
environment evolution. The trends shown in Figure 34 are one of the main
reasons to study in more detail the effect of crevice depth/width ratio on the
crevice repassivation (see Crevice Repassivation earlier).
More precisely, the results of the Watson model clearly indicate (Fig. 35) that
corrosion can start only if the crevice is tight and deep enough and support
the fact that a critical crevice size may exist. This is consistent with one
conclusion of White et al. [99] which indicates that their model is not able to
predict pH low enough for crevice initiation in gaps of the order of several
tens of micrometers. These results, as well as results of Gartland [19], also
show that efficient crevice gaps are of the order of micrometers or less, in
accordance with experimental results of Oldfield [3]. This supports the
assumption that microcrevices due to asperity may play a major role in
crevice initiation, as suggested by Sridhar and Dunn [60].
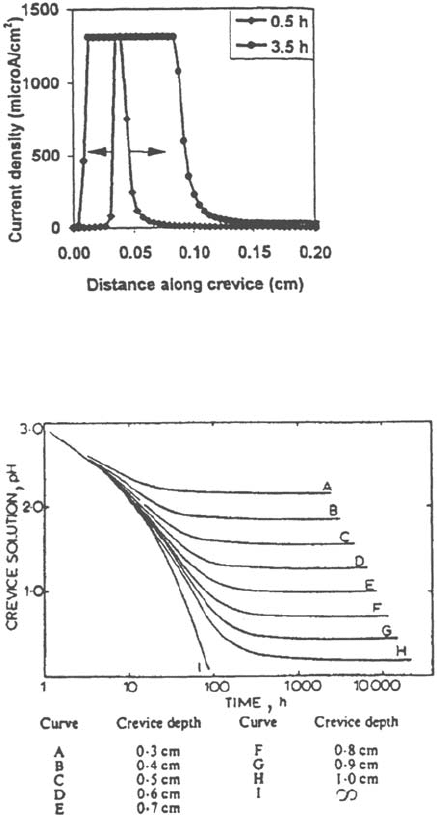
Gartland [19] also predicted, in agreement with observation (Fig. 26), that initial
crevice damages are mainly located near the crevice mouth (Fig. 36), where
the IR drop is low, which makes possible higher dissolution rates.
Finally, these models also show that the initial pH drop may be fast but that,
rapidly, the rate of evolution becomes very slow (Figs. 34 and 37).
In summary, these models supply interesting information but still rely on
experimental fitting to predict the initiation times correctly. Among their
weaknesses, there is lack of precise data on the local chemistry of concentrated
solutions and lack of prediction of the effect of the potential of the free surfaces. The
use of a unidimensional transport equation and the assumption of instantaneous
equilibrium of the hydrolysis and solubility reactions are also questionable.
Crevice Corrosion of Metallic Materials 383
Figure 31 Comparison of the results of Zuo et al. [37] with the predictions of the model
of Sharland [96]: note that the chloride content is fairly well predicted, whereas the pH
drop is underestimated.
Copyright © 2002 Marcel Dekker, Inc.
Finally, whatever their accuracy, the use of crevice initiation models will always
meet with some limitation because of the uncertainty in the actual crevice geometry.
However, a very useful model to be developed would be a propagation model,
particularly in an attempt to obtain theoretical support to describe the repassivation
potentials and, if possible, to predict self-arrest of crevice corrosion.
384 Combrade
Figure 32 Comparison of the experimental results of Alavi and Cottis [26] with the
predictions of the models of White et al. [99], Sharland [96], and Walton et al. [98] assuming
various current densities.
Copyright © 2002 Marcel Dekker, Inc.
EXPERIMENTAL CHARACTERIZATION OF THE ALLOYS VERSUS
CREVICE CORROSION
In order to select alloys for safe use in aerated chloride environments, a series of
tests and criteria has been designed. They can be divided in several classes:
Exposure tests in environments representative of the service environment
Exposure tests in conventional environments
Electrochemical tests, which can be divided into two classes: (a) those intended to
measure critical potentials for crevice initiation or protection and (b) those
used to derive initiation criteria from the behavior of materials in
conventional solutions supposed to simulate the crevice environment
For a more detailed description of crevice testing, the reader may consult the
papers of Oldfield [103] and Ijsseling [104]
Crevice Former Devices
All the exposure and electrochemical tests that involve the use of crevice former
devices encounter the problem of the geometry of the crevice. Many different designs
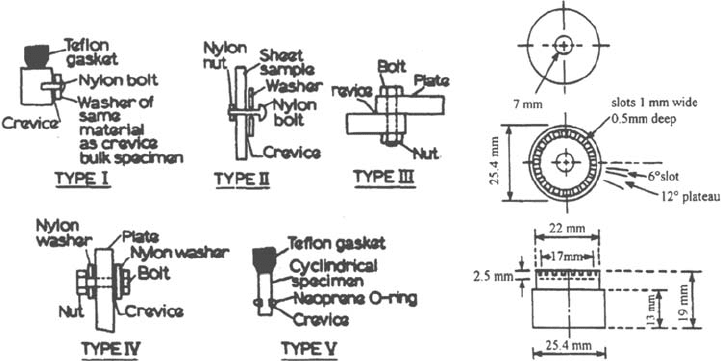
of crevice former have been used, some examples of which are given in Figure 38a.
The ASTM standard G78 defines a crevice former (Fig. 38b) that involves 20 crevice
zones and gives a series of recommendations, for example, for the setup of the
specimens in the test vessels. This device allows more reproducible results because
of the statistical effect of the large number of crevice zones. However, the standard
does not specify important points such as the condition of the contact surface of the
crevice former.
Crevice Corrosion of Metallic Materials 385
Figure 32
Copyright © 2002 Marcel Dekker, Inc.
386 Combrade
Figure 33 Effect of H
+
activity correction on the pH calculated by the Gartland model.
Figure 34 Effect of severity factor on pH drop calculated by the Bernhardsson model.
Figure 35 Effect of crevice geometry on crevice initiation time calculated by the Watson
model.
Copyright © 2002 Marcel Dekker, Inc.
Exposure Tests in Representative Environments
These tests are designed to rank the behavior of different alloys, to get orders of
magnitude of initiation times in service, and/or to select alloys that should guarantee
long-term resistance to crevice initiation. Thus, they can be very long (months or
even years) with carefully selected crevice former devices, materials, and
surface conditions. A problem is the test monitoring to detect crevice initiation
without any perturbation of the local processes.
Apart from the selection of the crevice former device, it is important to have in
mind that the severity of the test may depend on the availability of cathodic current.
Crevice Corrosion of Metallic Materials 387
Figure 36 Prediction of the initial corrosion damage near the crevice mouth by the
Gartland model (see Fig. 26 to compare with experimental data).
Figure 37 Drop of pH versus time as predicted by the Oldfield model.
Copyright © 2002 Marcel Dekker, Inc.
Thus, it is important to use specimens with large free surface area, particularly if
the environment has high electric conductivity that allows long-distance galvanic
coupling (seawater, for example).
There are different monitoring possibilities for exposure tests:
Periodic examination of the specimens is the simplest technique but it may
dramatically modify the incubation period if the crevice zone becomes more
or less dried during the examination. In particular, opening the crevice
former to observe the inner surfaces may modify completely the local
environment and cause the effective test time to go back to almost zero.
Monitoring of the corrosion potential allows one to detect any increase of the
corrosion rate due to the polarization of the cathodic reaction on the free
surfaces. Thus, the initiation of active corrosion in the crevice may be
detected. In addition, electrochemical noise (i.e., potential fluctuations)
indicating some dissolution transient may be interpreted as precursor events
of passivity breakdown or at least of a low degree of passivity. The analysis
of potential noise in terms of stochastic versus chaotic features has been
shown [106] to allow early detection of pit initiation. This type of analysis
should be checked for crevice corrosion.
Periodic measurements of polarization resistance may also be used to detect the
onset of active corrosion.
Exposure Tests in Conventional Environments:
The Ferric Chloride Test
This test is defined by the ASTM standard G48 and it is used for stainless steels
and nickel-base alloys. Coupons with a crevice former device are exposed to a 6%
FeCl
3
solution. Two criteria are used to characterize the tested materials:
The weight loss after a given time of exposure at a given temperature.
388 Combrade
Figure 38 Crevice former devices for crevice corrosion testing. (a) Different types of
crevice former (From Ref. 105). (b) ASTM G 78 crevice former device.
Copyright © 2002 Marcel Dekker, Inc.
A critical crevice temperature obtained by periodically increasing the temperature
by steps of 2.5 or 5°C until passivity breakdown occurs in the crevice.
However, because the passive film becomes more stable with increasing
exposure time, the critical temperature may depend to some extent on the
temperature selected to start the test.
An experimental correlation shows that this test can be used to rank the behavior of
stainless alloys in seawater. In practice, it is also used as a control test to guarantee
the constant quality of a product or to check the effect of fabrication parameters such
as thermal treatment, welding, and surface condition.
Electrochemical Tests
Determination of Critical Potentials for Initiation and Protection
These tests are the same as those used to determine the pitting resistance except that
a crevice former device is present on the test coupons. The measured parameters are:
The crevice potential and the repassivation potential obtained on polarization
curves (potentiokinetic technique) or, in some instances, by using potential
steps or potentiostatic tests
The critical temperature for the crevice at a given potential
Potentiokinetic Techniques Figure 7 of this chapter represents typical
polarization curves of a passivated alloy in a near-neutral chloride solution with and
without a crevice former device. The shapes of the two curves are identical except
that the crevice potential is definitely lower than the pitting potential. In addition, it
may depend on the crevice geometry if not properly measured.
As already discussed, the main difficulty of this technique, beside the problem
of the crevice former geometry and reproducibility, is that the results are strongly
dependent on the potential scan rate both because of the time-dependent stability of
the passive films and because of the time-dependent evolution of the environment
inside a crevice. In particular, the repassivation potential may be overestimated if
corrosion is not well developed in the crevice and it can be underestimated if the
potential backscan is too fast to allow the evolution of the local environment to be in
“quasi-steady” conditions. It is generally admitted that the scan rate has to be very
low, which causes the two critical potentials to become closer. But the appropriate
scan rate must be determined on each system because it may depend on the alloy and
on the environment.
Potentiostatic Techniques To determine the critical potential of crevice
initiation, coupons in a crevice former device are exposed for a fixed period of time
under potentiostatic control and monitoring of the anodic current is used to detect the
onset of active corrosion. Several experiments are performed at different potentials
and the crevice potential is the threshold potential that corresponds to an infinite
initiation time (see Fig. 8 and 9 at the beginning of this chapter).
To measure the repassivation potential, severe crevice corrosion must be
initiated on a “creviced” specimen and allowed to propagate for a fixed period of
time or, more usually, to a definite amount of anodic charge. Then the potential is
Crevice Corrosion of Metallic Materials 389
Copyright © 2002 Marcel Dekker, Inc.
stepped to a lower value and current monitoring is used to check the propagation or
repassivation. As shown in Figure 8, the repassivation time increases with increasing
applied potential and the repassivation potential is the potential corresponding to an
infinite repassivation time. Apparently, the repassivation potential may depend on the
development of corrosion before the potential is stepped down only if the amount of
accumulated charge is lower than a critical value.
The potentiostatic techniques should be preferred to the potentiokinetic ones,
but they require more specimens and longer experiments.
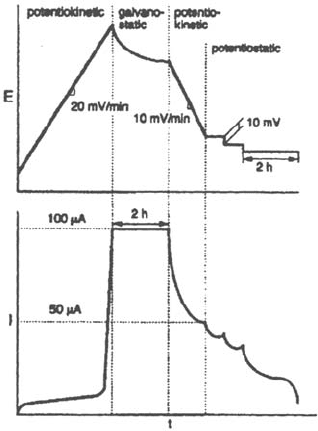
A more sophisticated repassivation test has been designed [7,12] to minimize
the number of specimens by using a stepwise backscan potential (Fig. 39). During
each step, the evolution of the current is analyzed and time is allowed to determine
the trend. If, after decreasing, the anodic current increases again, indicating that
propagation is not inhibited, the potential is stepped down again until repassivation
occurs.
Determination of the Material Behavior in Simulated Crevice
Environments
The tests that have been widely used in the past years are intended to determine the
conditions of passivity breakdown in the crevice. Alloy coupons with no crevice area
are tested in solutions that are supposed to simulate the local conditions in crevices.
This is usually done by using acidic solutions with an increased chloride content
compared with the bulk environment. For example, to perform tests to characterize
the behavior in seawater, solutions containing 30 to 150 g/L of NaCl were used, the
pH being lowered by HCl additions. Different measurements can be performed.
390 Combrade
Figure 39 Stepwise potential backscan and current response to determine the repassivation
potential [12].
Copyright © 2002 Marcel Dekker, Inc.
