Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

though these inclusions of submicron to few microns size usually cover less than
a few percent of the surface, they play the key role in the delamination of polymer
films from aluminum alloys because they are not covered by an insulating passive
oxide and thus reactions such as oxygen reduction can easily occur on their surface.
Cathodic water decomposition and especially oxygen reduction require
considerable overvoltages on Al and Al alloys. Usually, potentials below –400 mV
SHE
are required to activate a cathodic current. Thus, both water decomposition and
oxygen reduction occur together and both contribute to the overall current density.
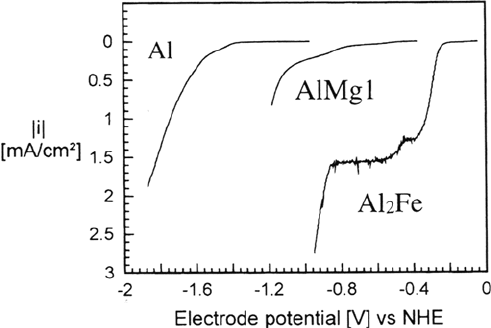
The effect of the inclusions on the current/potential curves can be seen in Figure 10,
where the curves for aluminum, Al
2
Fe, and AlMgl in an oxygen-purged 0.1 M
NaSO
4
electrolyte are shown. The scans start at the OCP with a velocity of 1mV/s,
while the samples are rotated at 200 rpm. Obviously, the inclusions in the AlMgl act
as active sites for the cathodic reactions.
Investigation of the inclusions in an AlMgl surface that has been polarized
to cathodic potentials for a short time typically shows a corrosive attack of the
Al matrix around the inclusion. Obviously, the inclusions act as local cathodes
and the matrix in their vicinity will dissolve anodically in the alkaline elec-
trolyte around the inclusion. The extent of the attack on the Al matrix surround-
ing the inclusion, i.e., the trenches around the inclusions, varies considerably.
This means the oxygen reduction occurs to a different extent on the different
inclusions, which can easily be explained by the concept of a microelectrode
array: at low rotation velocities the oxygen transport to the inclusions is limited
and diffusion cones issuing from the inclusions will evolve and overlap at a cer-
tain distance from the surface, from which a planar diffusion zone is effective.
Thus, the situation occurs that diffusion conesof inclusions active earlier than
others use up all the oxygen available and thus shield the other inclusions from
the oxygen. Figure 11 shows a scanning electron microscope (SEM) and an
Scanning Auger microscope (SAM) image of such an inclusion. Clearly visible
is the corrosion trench in the matrix surrounding the inclusion; also interesting is
the ringlike deposit of oxides around the trench. The surface of the inclusion
is mostly Fe
3
O
4
, whereas before its activation it showed a high content of
492 Rohwerder et al.
Figure 10 Current/potential curves for Al, AlMgl, and Al
2
Fe in oxygen-purged 0.1 M
Na
2
SO
4
electrolyte.
Copyright © 2002 Marcel Dekker, Inc.
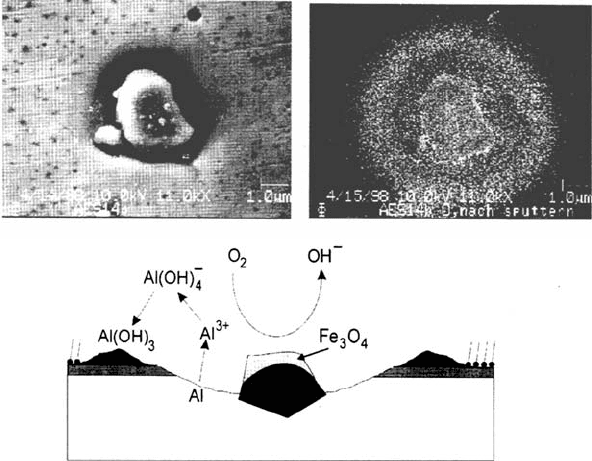
Al oxide. The lower part of Figure 11 depicts a schematic drawing of the
processes going on at the inclusions. Activation of the surface, i.e., the disso-
lution of the Al oxides from the surface of the inclusion as it is induced by an
increase in pH caused by oxygen reduction, results in an iron oxide–rich
surface, oxygen reduction can take place with high reaction rates, Al is dissolved
from the matrix surrounding the inclusion, and Al hydroxides precipitate in the
vicinity of the trenches.
A good test of the protective properties of self-assembled monolayer films is to
investigate to what extent these layers can inhibit the activation of the inclusions. This
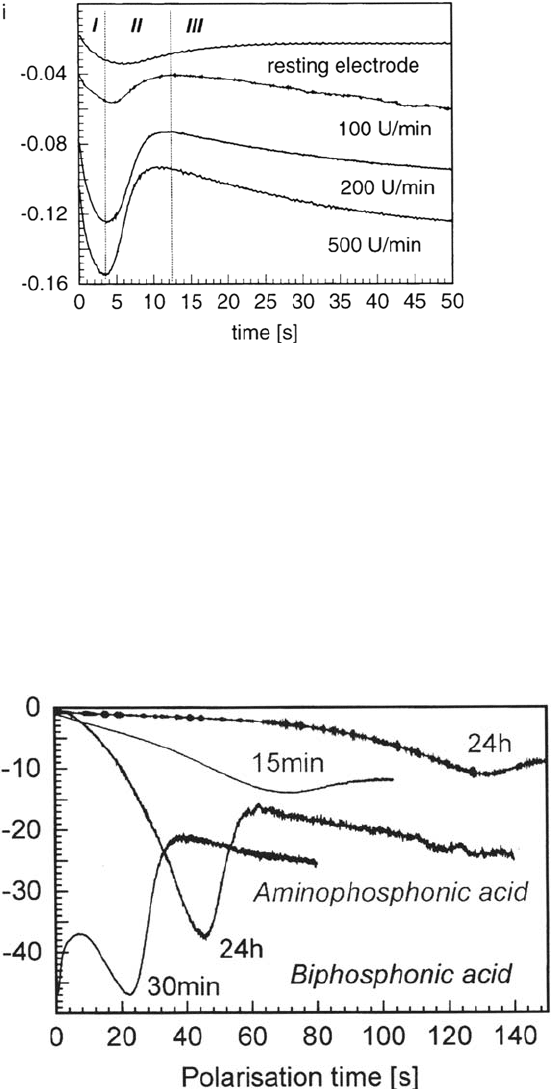
can be done by cathodic potential jumps and monitoring the current transients. Figure 12
shows such transients for an ummodified surface at different rotation velocities.
Three stages of the i(t) curves can be distinguished. For the first seconds an
onset of the cathodic currents can be seen. Here the activation of the inclusions
occurs. The decrease in the cathodic current in stage II is most likely due to the
onset of the anodic aluminum corrosion in the surrounding matrix. The behavior in
stage III is more complicated to explain; it is assumed that deposition of corrosion
products plays an important role here.
Figure 13 shows the transients for AlMgl samples modified with biphosphonic
or aminophosphonic acid; samples modified with octadecylphosphonic acid do not
show much difference from the unmodified samples. Especially the aminophosphonic
acids show a significant improvement in the corrosion behavior. Sputter profiles on the
matrix and inclusions show that similar films are formed on the Al matrix but that the
aminophosphonic acid forms several nanometer thick multilayer films on the inclusions,
much thicker films than the biphosphonic acid. In this way the inclusions are better
passivated in the case of modification with aminophosphonic acids.
Corrosion Prevention by Adsorbed Monolayers 493
Figure 11 (Top) SEM image and oxygen SAM mapping of an Al
2
Fe inclusion in AlMgl.
(Bottom) Reaction scheme for corrosion at the inclusions.
Copyright © 2002 Marcel Dekker, Inc.
At a first look the decrease of the peak current by a factor of 4–5 and the delay
of the activation peak by a factor of 20–40, from 3–4 s to 60–120 s, does not seem
to be sufficient for an effective improvement. In the case of an additional polymer
coating, a factor of 20 increase in activation time might extend the lifetime of
a product by several years. For this reason, aminophosphonate monolayers may
prove suitable as adhesion promoters.
Figure 14 shows the effect of the phosphonate monolayer (self-assembled
N-ethylaminophosphonate, NEAP) on the delamination of an amine-modified
494 Rohwerder et al.
Figure 12 Current transients for AlMgl samples after potential jumps from the OCP to
–800 mV for different rotation velocities.
Figure 13 Current transients for the resting samples after modification with aminophos-
phonic or biphosphonic acid, respectively.
Copyright © 2002 Marcel Dekker, Inc.
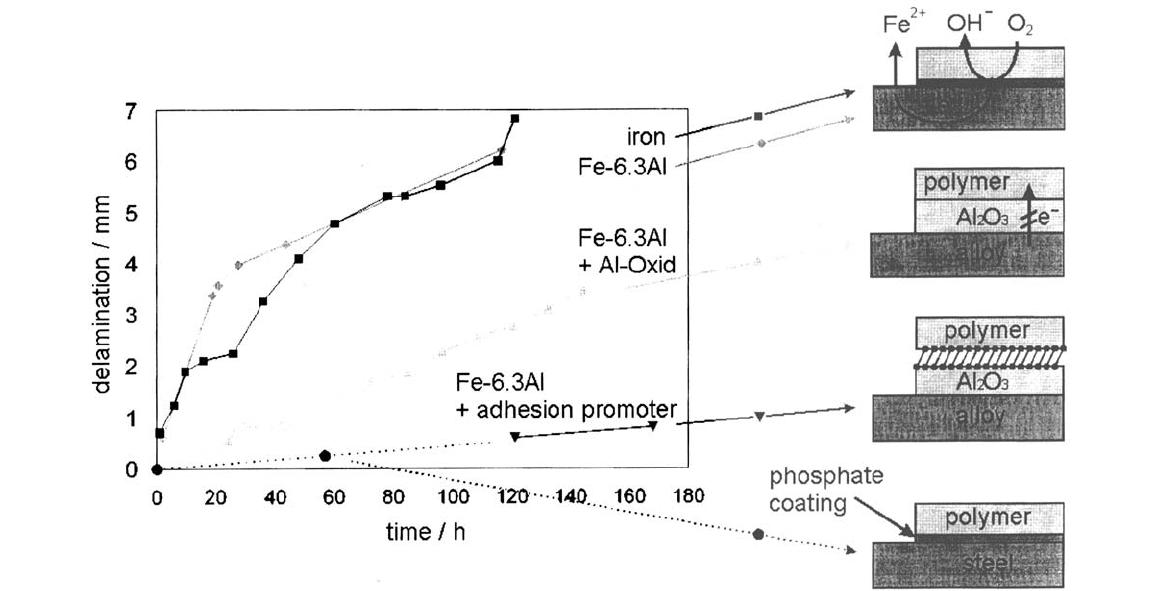
Corrosion Prevention by Adsorbed Monolayers 495
Figure 14 Delamination of an amino-modified epoxide ester from differently pretreated Fe-6.3Al-alloy surfaces and phosphated
steel as a reference.
Copyright © 2002 Marcel Dekker, Inc.
epoxide ester from Fe-6.3Al-alloy surfaces. This ester is a basis for a number of
industrial primers. As can be seen, the just polymer-coated alloy is delaminated
quite rapidly. Delamination is much slower if the alloy is thermally treated in such
a way that a thin insulating layer is formed by selective oxidation, and thus oxygen
reduction is inhibited. If an additional phosphonate layer is built into the interface,
the delamination is again significantly slowed down [64].
Adhesion Promotion by Phosphonate Films Besides inhibiting oxygen
reduction, the role of the self-assembled monolayer is to improve the adhesion of
the polymer film to the surface under mechanical strain. Usually, it is assumed that
it is the covalent bond of the head group of the molecules to the surface and the tail
group to the polymer that is responsible for the improved mechanical stability. To
protect microelectronic devices, especially microchips, from harmful environmental
influences, more than 90% of all semiconductor devices are encapsulated with
polymeric compounds. Often the polymers have to be briefly heated up to
temperatures around 200–250°C to improve their protective properties. In this case,
the explosive evaporation of adsorbed moisture can cause considerable mechanical
strain at the polymer/substrate interface, which in turn might result in breakage of
the polymer/substrate composite at the interface. More precisely, the failure occurs
mostly not directly at the interface but near the interface [65–72]. For this kind of
adhesive breakage near the interface, several nanometers of polymer coating are
usually still covering the substrate. It seems that the molecular forces between
substrate and polymer are not crucial for the failure behavior, but the transition zone
from the substrate into the polymer bulk plays the key role.
In order to investigate the effects of an aminophosphonate monolayer on the
adhesive properties, aluminum samples were modified with NEAP and then coated
with a polycyanurate film [a prepolymer of the dicyanate of bisphenol A (DCBA),
which consisted mostly of monomers and trimers of DCBA, and was dissolved in
tetrahydrofuran (THF) (25 mg/mL) and then the prepolymer solution spin coated on
the samples and exposed for 2h at 220°C in laboratory air to complete the
polymerization]. The triazine rings in the DCBA monomer are IR inactive when
parallel to the substrate surface and increasingly IR active with increasing normal
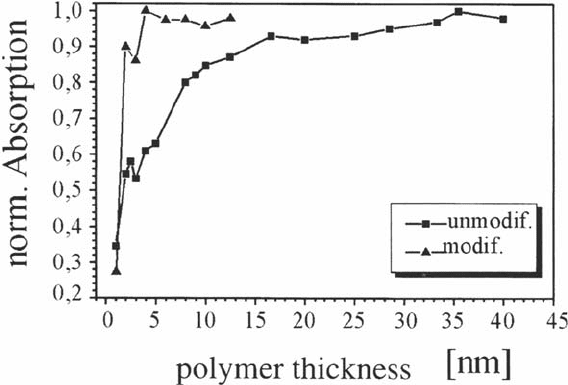
component. Figure 15 shows the normalized intensity of the corresponding IR signal
(at wave number 1380 cm
–1
) as a function of the thickness of the polymer film for
NEAP-modified and unmodified samples. The normalization of the signal was to
divide the signal obtained from thicker polymer films (> 40 nm) by their thickness as
obtained by ellipsometry and set this to one. It can be seen from Figure 15 that for the
unmodified samples the orientation of the triazine rings in the monomers near the
interface has, in comparison with the statistically oriented molecules in the polymer
bulk, a distinct preferred component parallel to the surface. This preferred orientation
extends up to about 10nm from the interface. For the modified sample this transition
zone extents only over the first 2 nm from the surface. The reason for this is that the
NEAP molecules are adsorbed on the surface as a oriented monolayer with the
secondary amino group of the NEAP molecules at the surface. This amino group can
react with the cyanate group of the polycyanurate prepolymer to an isourea bond,
forcing it to a more or less upright orientation. Thus the transition zone at the
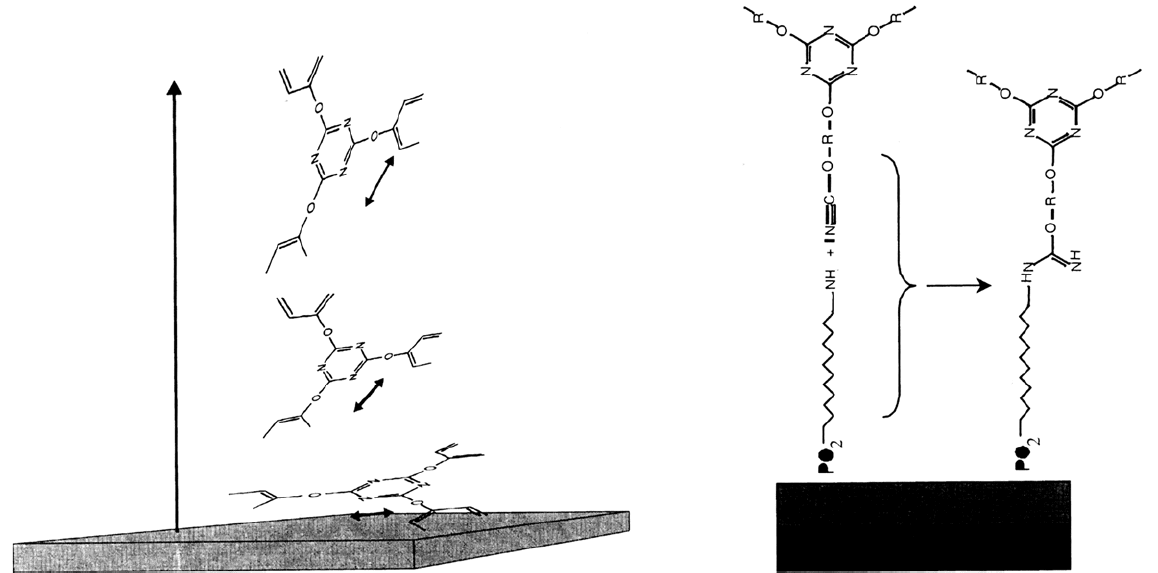
interface is reduced by a factor of 5. This is shown schematically in Figure 16.
496 Rohwerder et al.
Copyright © 2002 Marcel Dekker, Inc.
Technical butt joint tests on samples coated with 400-nm polycyanurate polymer
result for the samples not modified with NEAP in failures of the polymer/aluminum
bond for stresses between 2 and 6 MPa, depending on the relative humidity.
Interestingly, the failure occurred not directly at the interface but about 6–10 nm away
from it, as could be derived from SEM and XPS measurements. This clearly shows
that failure occurs within the transition zone. For samples modified with NEAP no
failure of the polycyanurate-polymer/NEAP/aluminum composite could be measured
because of cohesive breakage in the coupling glue (XD4600) used for the test at about
7 MPa, independent of humidity; i.e., much higher stability is to be expected.
Stability of Defective Monolayer Films
Defects at the interface most likely play an important role in the delamination
process. Unfortunately, the defects in self-assembled films are mostly nanoscopic
and can be studied only with atomic force microscopy (AFM) and STM, which
require very time-consuming preparation and limit the flexibility of the experiments.
Other operation modes such as scanning Kelvin probe force microscopy (SKPFM)
[73] will play an important role in future work [74].
Another way to study the role of defects is to prepare films with larger defects
with the Langmuir-Blodgett (LB) technique, which allows deposition of ordered
monolayers of suitable (amphipilic) organic molecules on a solid substrate.
Thiol LB Films
Thiols are classic SA molecules but it is possible to transfer thiol LB films onto
gold [75]. Lösch et al. [75] studied the LB transfer of thiol molecules onto gold under
electrochemical potential control. Because the gold electrode is charged as a conse-
quence of the polarization, there is an excess of counterions (Na
+
for a negatively
Corrosion Prevention by Adsorbed Monolayers 497
Figure 15 Normalized infrared adsorbance at the wave number 1380 cm
–1
for polycya-
nurate films of different thickness for the unmodified and the NEAP-modified sample.
Copyright © 2002 Marcel Dekker, Inc.
498 Rohwerder et al.
Figure 16 (Left) Supposed molecular structure within the polymer near the unmodified surface. The orientation of the molecules is parallel
to the surface. (Right) Molecular structure at the interface in the case of NEAP modification.
Copyright © 2002 Marcel Dekker, Inc.
charged surface and ClO
––
4
for a positively charged surface) in the electrolyte as part
of the electrochemical double layer. For any chemical interaction it is essential that
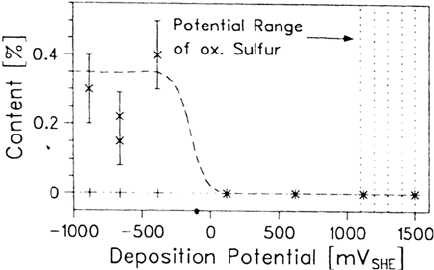
these counterions are replaced by the LB molecules. Figure 17 shows the amounts of
Na and Cl as detected by an XPS analysis of a gold electrode modified by octade-
cylmercaptan (ODM) at different electrode potentials. For potentials cathodic to the
potential of zero charge (PZC) Na
+
can be detected, but for potentials anodic to the
PZC no counterions are observed at all. Obviously, the sulfur of the ODM molecule,
which is partly negatively charged, is for electrostatic reasons not able to replace Na
+
at negative potentials. Indeed, the area of the sodium XPS signal is in the range
expected for the amount of Na
+
, which balances the charge of the metal surface. This
is in excellent agreement with the observed slowdown of the chemisorption during
self-assembly at cathodic potentials (as reported earlier). But whereas in this latter
case chemisorption finally occurs, the molecules transferred at negative potentials via
the LB technique are only physisorbed (see Fig. 18).
At potentials anodic of the PZC, the negatively charged sulfur atoms in the
ODM molecules balance the charge of the positively charged surface; no perchlorate
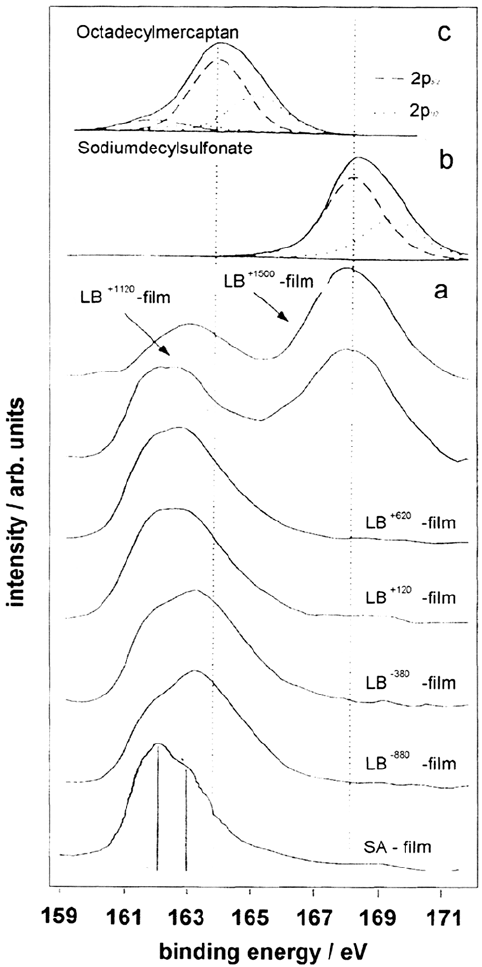
can be detected. This is also confirmed by Figure 18. Between –0.88 and +0.62 V
the binding energy is continuously shifted toward smaller values. This is direct
evidence for continous negative charge on the sulfur atom. At all potentials the
binding energy is smaller than the binding energy of S(2p) measured for bulk ODM.
This is attributed to the interaction with the metal surface. At +0.62 V the binding
energy (162 eV) is close to that of mercaptan molecules chemically attached to iron
substrates. Figure 18 shows that a chemical anchoring of the mercaptan is strongly
dependent on the electrode potential. A dramatic change in the high-resolution
spectra of sulfur is observed at high anodic potentials (+1120 mV and + 1500 mV).
A new peak shows up in the range 167 eV–169 eV. This band can be referred
to sulfur in the oxidation state + VI (R—SO
3
–
). Apparently, it is possible to oxidize
the sulfur at the mercaptan/substrate interface during the transfer process of the LB
film, while the ordering within the film remains intact.
The effect of the electrode potential during transfer on the film stability is
shown in Figure 19. Here cyclovoltammograms (CVs) of gold covered by one
monolayer of n-octadecylmercaptan are depicted. The CV of unmodified gold shows
Corrosion Prevention by Adsorbed Monolayers 499
Figure 17 The amount of Na
+
(×) and ClO
4
–
(+) detected by XPS on gold surfaces
modified by one monolayer of octadecylmercaptan at various deposition potentials [75].
Copyright © 2002 Marcel Dekker, Inc.
500 Rohwerder et al.
Figure 18 S(2p) XPS spectra of a gold surface modified by one monolayer of
octadecylmercaptan (ODM) at various electrode potentials (c). Reference spectra of
sodium decylsulfonate (b) and nonchemisorbed ODM (a) are included, as well as that of a
self-assembled film.
Copyright © 2002 Marcel Dekker, Inc.
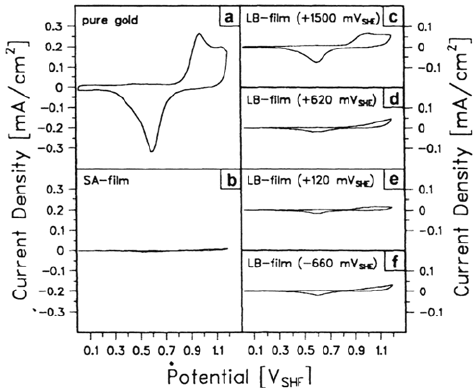
the typical peaks of gold oxidation and gold reduction, whereas these peaks are
diminished in size for the coated gold electrodes. The residual peaks are determined
by the part of the gold surface that is not blocked by chemisorbed thiol, and the
effect of the electrode potential on the stability is clearly visible.
Silanol LB Films
The LB technique can also be successfully used for transferring a monolayer of
silanol molecules onto a metal surface. Silanols are widely used for corrosion pro-
tection [76]. Now, in contrast to mercaptan, a dissociation of the head group takes
place on the water surface [77] followed by the formation of silanols and a poly-
merization. So a two-dimensional polymer results. The existence of the
Si—O—Si bonding is proved by infrared spectroscopy. Figure 20 shows the IR
spectrum measured in external reflection (RRAS) of an LB film prepared at
+500 mV in comparison with the KBr spectrum of an octadecyltrichlorosilane
(ODTCS) bulk polymer. In both cases the fingerprint region of the Si—O—Si
double peak (1112 and 21018 cm
–1
) is obvious. The weaker signals of the CH
2
stretching signals for the LB film are due to the selection rule of the IRRAS.
It should be noted that the two-dimensional polymer film formed on the water
aqueous subphase is rather stiff and viscous, so that during the transfer a cleavage of
the film occurs and an island structure of the monomolecular LB film results on
the substrate. This gives ideal samples for investigation of the role of defects
in delamination. An ideal tool for such investigations is the scanning Kelvin
probe (SKP).
Until recently it was impossible to analyze locally the integrity of the
metal/polymer bond under in situ reaction conditions. This is now possible using a
scanning Kelvin probe. This technique allows the measurement of localized
Corrosion Prevention by Adsorbed Monolayers 501
Figure 19 Cyclovoltammograms in deaerated borate buffer (pH 8.3; scan speed
100 mV/s) for a solvent-cleaned gold surface (a), gold modified by one monolayer of SA
film (b), and gold modified by one monolayer of LB films prepared at +1500 mV
SHE
(c),
+620 mV
SHE
(d), +120 mV
SHE
(e), and –660 MV
SHE
(f) [75].
Copyright © 2002 Marcel Dekker, Inc.
