Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

corrosion potentials without touching the surface under investigation [78–85]. The
Kelvin probe has a sharp vibrating needle that scans above the surface, the distance
between probe and substrate being approximately 10 μm. A capacitor is formed
between substrate and needle, and the capacitor is charged by a potential difference,
which is called the Volta potential difference, if both metals are connected by a
conducting wire. As the capacity of the capacitor is changed sinusoidally with the
vibration, an alternating current results between both metals. If an additional voltage
difference is imposed between the two metals, then the capacitor may be discharged
and the AC signal vanishes when the external voltage is equal to the Volta potential
difference.
In principle, therefore, the Volta potential of the metallic substrate is
measured with the Kelvin probe. For dry atmospheres, the Volta potential
difference (ΔΨ
Ref
sample
) is given by the difference between the work functions of the
substrate and the probe [86]. The work function of the sample, however, includes
the dipole potential of the metal:
502 Rohwerder et al.
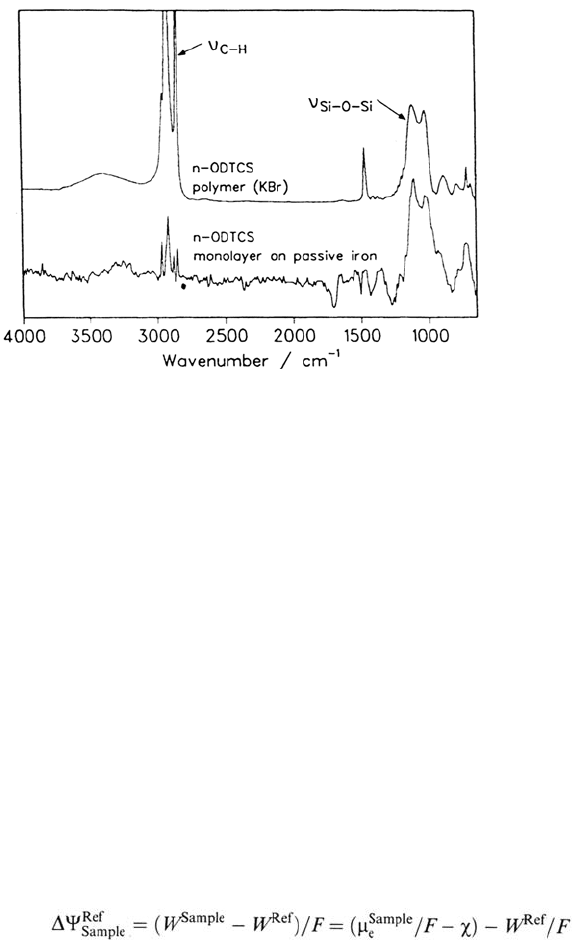
Figure 20 KBr spectrum of octadecyltrichlorosilane (ODTCS) after full polymerization
in H
2
O (top) and one monolayer of octadecyltrichlorosilane on iron after chemisorption at
+0.5 V in an LB balance (bottom).
where
W = work function of sample or reference metal
F = Faraday constant
μ
e
= electrochemical potential of the electrons in the sample (constant)
χ = dipole potential of the sample surface
The dipole potential of the surface will change in the presence of polar organic
molecules at the metal/air interface. This can be seen in Figure 21a, which shows
an SEM picture of an iron surface partly coated by one monolayer of octadecylsilanol.
In this situation, the individual molecules are ordered, as they have been deposited
by the Langmuir-Blodgett-Kuhn method. Laterally resolved Auger analysis shows
Copyright © 2002 Marcel Dekker, Inc.
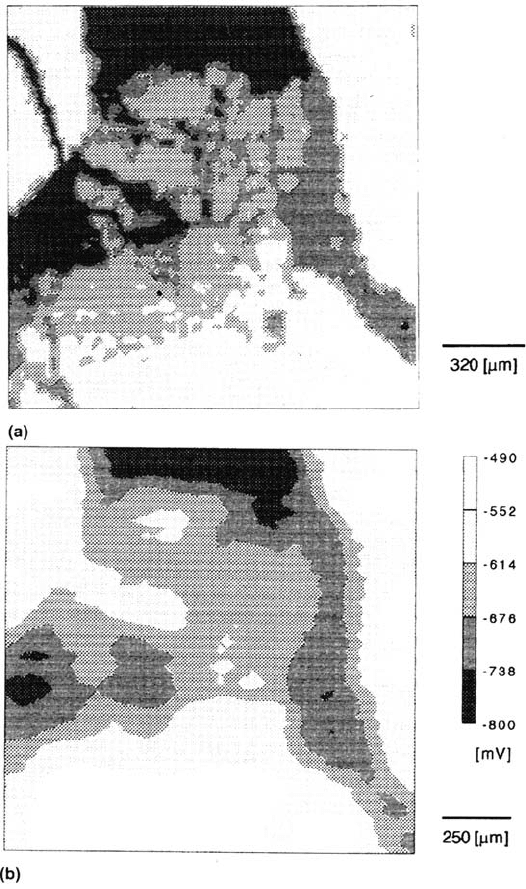
that the dark areas correspond to the noncoated substrate, whereas the bright
areas are coated by one monolayer of the organic molecule. It is interesting that
the same contract is obtained with a scanning Kelvin probe (Fig. 21b). This is
due to the fact that the covered part of the substrate has a different dipole potential
in comparison with the noncovered areas and this also results in a different Volta
potential [77].
Corrosion Prevention by Adsorbed Monolayers 503
Figure 21 (a) SEM map of iron modified by approximately 0.7 monolayer of
octadecylsilanol (ODS). Bright areas: surface covered by ODS; dark areas: surface not
covered. (b) Map of the Volta potential of the same surface as in (a) [79].
Copyright © 2002 Marcel Dekker, Inc.
In humid atmospheres, a thin electrolyte layer is formed on top of the
substrate surface. Under those circumstances the Volta potential of the surface of
the electrolyte layer is measured. It has been shown that this Volta potential is
mainly determined by the Galvani potential difference of the metal/electrolyte
interface and therefore by the electrode or corrosion potential [79,80,86]:
504 Rohwerder et al.
where
E
corr
=corrosion potential of the corroding surface
χ
El
Gas
=dipole potential of the electrolyte/gas interface
ε
ref
=half-cell potential of a standard reference electrode (e.g., SHE) needed to
relate the absolute potential scale to the electrochemical potential scale
Again, the Kelvin probe can be used to monitor the local corrosion potential of
a partly coated metal surface in a humid atmosphere. If, for example, the presence of
the organic molecule changes the kinetics of the metal dissolution reaction, then this
is reflected in a change of the corrosion potential: an acceleration of the metal
dissolution will shift the corrosion potential cathodically, a retardation will shift the
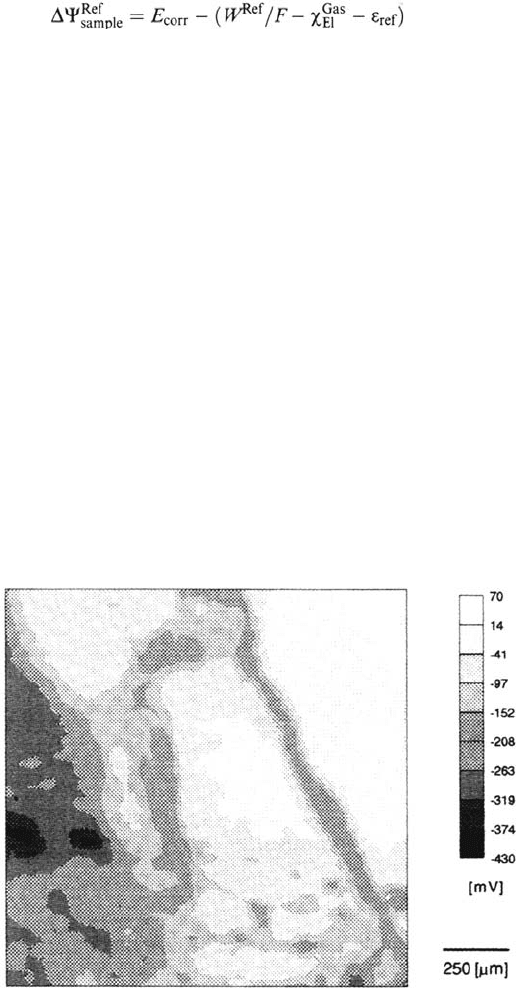
corrosion potential anodically. As an example, Figure 22 shows a map of the
corrosion potential of an iron surface that is partly coated by one monolayer of
octadecylsilanol as measured with the Kelvin probe in a humid atmosphere [87]. The
corrosion potential changes locally by several 100 mV due to the presence or absence
of the film and it has been proved that the anodic potentials correspond to the areas
that are coated by the polymer [53]. The potential plot of Figure 22 is therefore a
representation of the inhibition of the anodic metal dissolution in a humid atmosphere.
Figure 22 Map of the Volta potential of one monolayer of octadecylsilanol on iron after
exposure to 100% relative humidity for 343h. Area: 5 × 5mm [87].
Copyright © 2002 Marcel Dekker, Inc.
If the same specimen is exposed to a water-saturated atmosphere for longer
corrosion times, no significant change is observed in comparison with Figure 22:
the uncoated areas of the iron surface start to corrode (negative corrosion potentials),
whereas the areas that are covered by one monolayer of octadecylsilanol stay at
very anodic potentials. The scanning Kelvin probe is therefore an ideal tool for the
investigation of inhibiting properties of organic molecules and it also allows
analysis of the expansion of corrosion on partly inhibited surfaces.
A second example of the analysis of inhibitor-covered surface is shown in
Figure 23. Iron substrates have been immersed in a solution containing the inhibitor
ammonium benzoate at a concentration of 0.025 M [88]. After exposure, the samples
are removed from the electrolyte and the corrosion potential map is recorded with the
Kelvin probe. Large potential differences result for the surface immersed in the
solution with a low concentration of inhibitor. Again, anodic potentials correspond to
the inhibited area whereas negative potentials close to the free corrosion potential of
the active iron electrode are representative of the noninhibited surface. Obviously, at
a concentration of 0.025 M the inhibitor is not able to protect the iron surface
completely. If the inhibitor concentration is increased to 0.05 M, no active corrosion
is observed and the potential stays at very anodic values. Similar results have been
obtained by Schultze et al. [89,90] for the inhibitor toloylanaline on iron.
The scanning Kelvin probe is therefore an ideal tool for in situ analysis of the
delamination of ultrathin organic coatings on reactive metal substrates. Until now,
only the detection of intrinsic defects has been discussed. It is possible, however, to
prepare monolayer coatings nearly free of such defects. Suitable techniques are
self-assembly chemisorption and the Langmuir-Blodgett-Kuhn method, if the
monomers and preparation parameters are optimized. Then the Kelvin probe will
detect a homogeneous corrosion potential and the delamination may be studied only
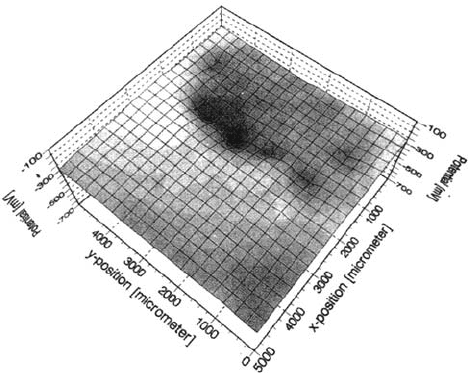
in the presence of artificial defects. As an example, Figure 24 shows a map
Corrosion Prevention by Adsorbed Monolayers 505
Figure 23 Map of the Volta potential of iron after immersion in a 0.025 M solution of
ammonium benzoate in 0.01 M Na
2
SO
4
for 24 h. Area: 5 × 5mm. Potential excess:
200 mV (bright area) to –500 mV (dark area) [88].
Copyright © 2002 Marcel Dekker, Inc.
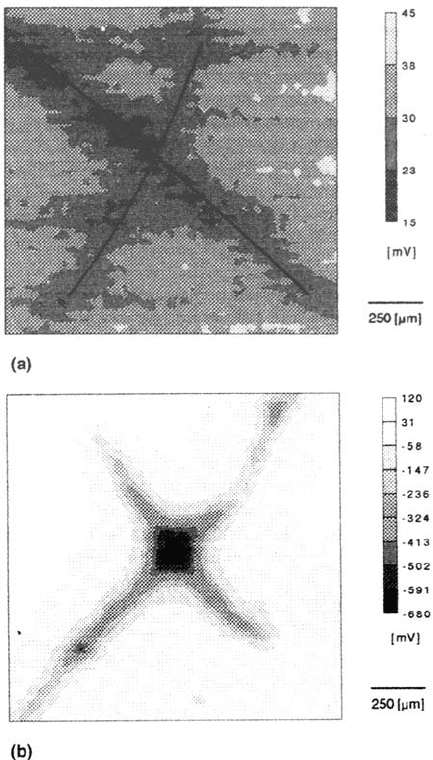
of the corrosion potential of an iron surface covered by one monolayer of
dimethylhexadecylsilanol [77,79]. The film has been destroyed by an artificial
defect in the form of a cross, which is clearly seen even in dry air (Fig. 24)
because of the change of the local dipole potential. The potential difference is
small in comparison with Figure 21, as the dipolar moment of dimethylhexadecylsilanol
is smaller than that of the monomer used in Figure 21. If such a
surface is exposed to humid air highly contaminated with SO
2
, the metal surface
is activated at the parts where the monolayer has been destroyed. This is seen in
rather negative local corrosion potentials (Fig. 24b). Even after prolonged
exposure, the active corrosion is limited to the same area and the width of the
506 Rohwerder et al.
Figure 24 (a) Map of the Volta potential of iron modified by one monolayer of
dimethylhexadecylsilanol. Surface has been scratched in the form of a cross. Area: 1 × 1 mm.
(b) map of the Volta potential of the same surface as (a) after adsorption of 0.5g/m
2
SO
2
and exposure to 100% relative humidity for 7 h. Area: 5 × 5 mm. (c) Same surface as in
(b) after exposure for 20h. at 100% relative humidity. Area: 5 × 5 mm [87].
Copyright © 2002 Marcel Dekker, Inc.
defect increase very slowly (Fig. 24c). The undermining of the monolayer close to
the defect is, however, much faster than the delamination of the intact film far from
the defect; in this respect the results are similar to those obtained with lacquers
and paints.
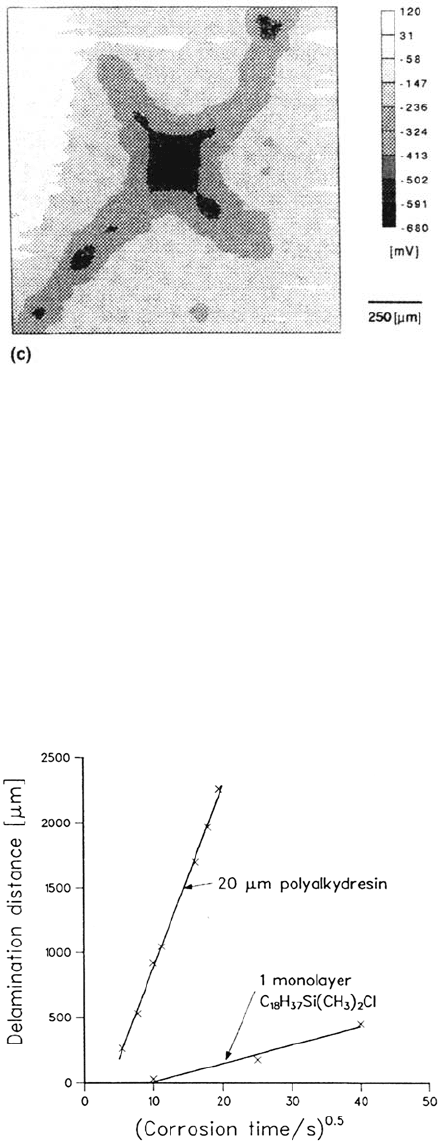
For the first time, the delamination of the monolayer coating can be evaluated
quantitatively and directly compared with the delamination of industrial paints. Such
a comparison is shown in Figure 25, and it is quite obvious that the delamination rate
is determined not by the thickness of the coating but by the bonds that prevail at the
interface. Using the scanning Kelvin probe in the future, the stability of other
Corrosion Prevention by Adsorbed Monolayers 507
Figure 24 (Continued).
Figure 25 Comparison of the delamination rate of iron coated by 20-μm polyalkyd resin
with iron coated by one monolayer of dimethylhexadecylsilanol. (Data from Ref. 32.)
Copyright © 2002 Marcel Dekker, Inc.
suitable metal/polymer bonds may be analyzed under corrosive conditions, and it
is very likely that coatings will be developed that are considerably superior to the
ones used up to now. It is essential, however, to understand the chemical or
electrochemical reactions that take place at the metal/polymer interface and that give
rise to the undermining of the defect as shown in Figure 24. Such studies are
performed preferentially on homogeneous electrode surfaces, as most spectroscopic
and electrochemical techniques do not provide spatially resolved information.
Exceptions are the scanning probe microscopy techniques such as scanning
tunneling microscopy (STM) or atomic force microscopy (AFM). However, as
already stated the application of these techniques for investigation of the corrosion
properties of chemically modified surfaces has only begun and few results have been
obtained so far.
PLASMA POLYMERS AS CORROSION-RESISTANT THIN FILMS ON METALS
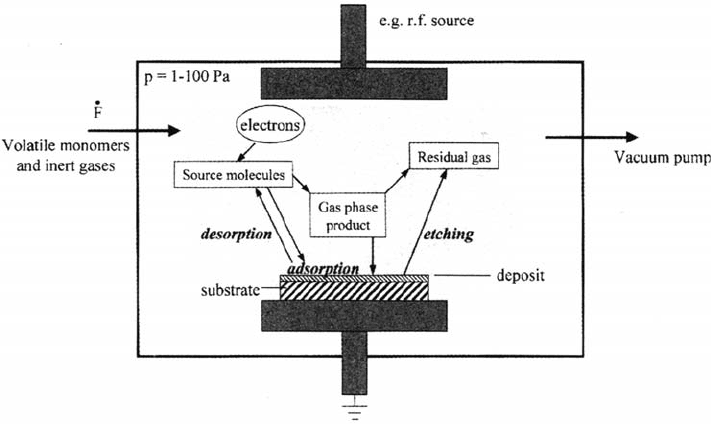
A typical reactor for plasma polymerization under reduced pressure is shown in
Figure 26 [47]. However, modern developments in plasma polymerization enable a
stable plasma even at atmospheric pressures. Details of the mechanisms of plasma
polymerization can be found in Ref. 45. Organic precursors.
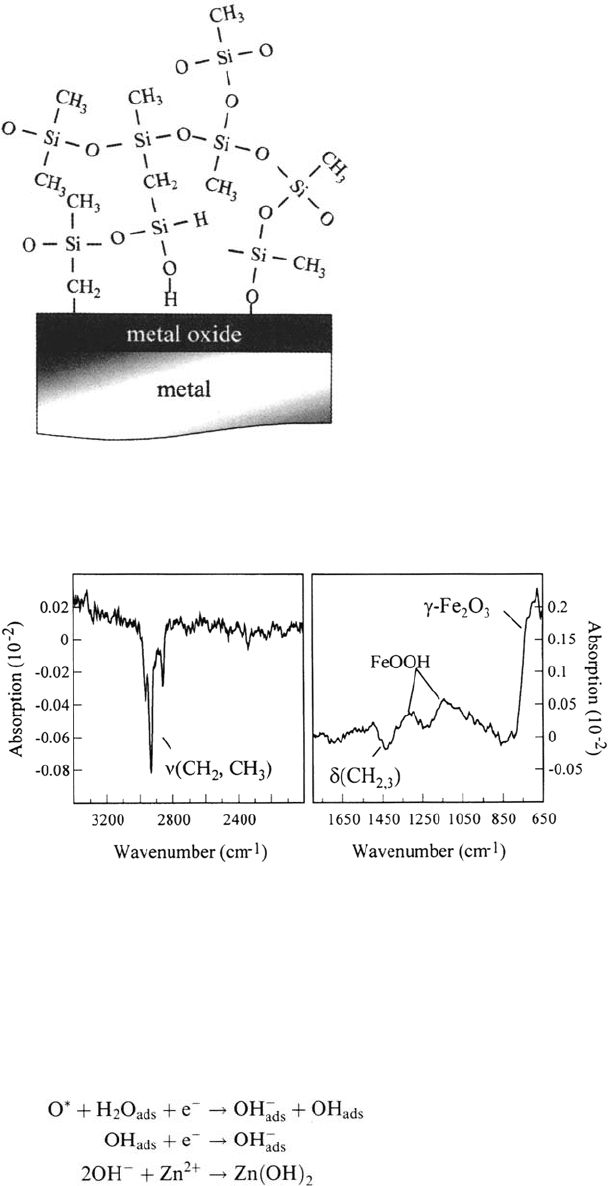
Plasma polymers are generally characterized by an extremely tight and
three-dimensional network. In Figure 27 a typical structure of an organosilicon
plasma polymer network is shown [47]. It has been shown that the water
permeability of a thin plasma polymer (thickness around 20 nm) can be two or three
orders of magnitudes lower than that of polyethylene [91]. Moreover, Yasuda et al.
showed that under special conditions of plasma polymerization even “atomic
interfacial mixing” (AIM) occurs providing a water-resistant interface [46].
Studies in the 1990s revealed the good corrosion protection properties of
silicon-based plasma polymers on steel substrates and the crucial influence of the
pretreatment process on the stability of the resulting interface [92–101]. The
pretreatments for trimethylsilane-based films may consist of an oxidative step
(O
2
-plasma) to remove organic contaminations from the substrate and a second
reductive step (Ar/H
2
-plasma) to remove the metal oxide layer. Although the
successive application of both steps provides the best corrosion protection of various
plasma treatments for steel in combination with a cathodic electrocoat, little is known
about the chemical structure of the interface. Yasuda et al. [101] and van Ooij and
Conners [97] in particular have shown that the deposition of plasma polymers on
steel and galvanized steel might even substitute the chromatation process.
Yasuda et al. [100,101] showed that the formation of carbides on the iron
surface prior to plasma polymer deposition led to excellent corrosion protection
properties in combination with a cathodic E-coat. In this work a special cathodic
plasma polymerization with magnetic enhancement was used. With this
experimental setup they were able to remove the oxide layer on steel almost
completely in an Ar/H
2
plasma and to form Fe—C groups. The formation of Fe—C
is the result of intensive ion bombardment of the surface.
In the following, plasma polymers are discussed as corrosion-resistant
thin layer with the focus on their morphology, chemical structure, and resistance
to corrosion.
508 Rohwerder et al.
Copyright © 2002 Marcel Dekker, Inc.
Plasma Cleaning of Metal Surfaces
Under atmospheric conditions, freshly prepared surfaces of reactive metals such as
iron or zinc are covered by an oxide with a thickness of about 2 to 3nm [102,103].
Moreover, organic molecules tend to adsorb strongly to the oxide surface, leading to
a lower surface energy compared with the uncovered oxide. For intimate adhesion
between the plasma polymer and the oxide, these residual organic contaminations
have to be removed. An oxygen plasma leads to oxidative etching of organic
molecules. It was also observed by ex situ XPS and AES sputter profiles [92,94] that
the thickness of the iron oxide increased during the oxygen plasma treatment.
Besides the oxygen plasma cleaning, argon and hydrogen plasmas can be
effectively used to clean metal surfaces and even partially reduce the oxide layer
[100,101,104].
As an example, Figure 28 shows the in situ infrared reflection absorption
spectrum (IRRAS) of the iron surface after the oxygen plasma treatment.
The spectrum measured right before the plasma treatment served as the reference
spectrum. The spectra were measured while the sample was still in the plasma
chamber at a pressure of 10
–4
mbar [105]. The negative peaks at 2900 and 1450 cm
–1
indicate the loss of amorphous organic contaminations, whereas the positive peaks at
about 1316, 1130 and 690 cm
–1
are assigned to the formation of iron oxyhydroxide
and iron oxide [106–108]. The peak at 690 cm
–1
can be assigned to γ-Fe
2
O
3
, while
the broad peaks between 900 and 1400 cm
–1
are in the region of oxyhydroxides and
vary in position and relative intensity as a function of the iron surface pretreatment.
The FeOOH peak cannot be assigned to any specific crystalline form of FeOOH.
Plasma oxidation of electrogalvanized steel also leads to the removal of organic
contaminations and the formation of an oxyhydroxide on the surface (see Fig. 29).
The latter shows a strong and broad absorption at 1150 cm
–1
[109]. Moreover, strong
Corrosion Prevention by Adsorbed Monolayers 509
Figure 26 Schematic of a plasma deposition reactor and the important plasma processes.
Copyright © 2002 Marcel Dekker, Inc.
negative peaks at 3250 and 1620 cm
–1
indicate the removal of associated H
2
O
molecules. It is likely that the electrolytic deposition of zinc leads to a surface with
adsorbed residual water layers. These water molecules could then take part in the
plasma oxidation process as electron acceptors at the oxide surface according to
510 Rohwerder et al.
Figure 27 Simplified schematic of an Si-organic plasma polymer (precursor:
hexamethyldisiloxane) as a surface layer on a metal substrate.
Figure 28 IRRA spectrum of an iron surface (Fe-coated quartz) that was exposed to an
oxygen plasma for 300s [110].
and would thereby lead to a highly protonated zinc oxyhydroxide.
Copyright © 2002 Marcel Dekker, Inc.
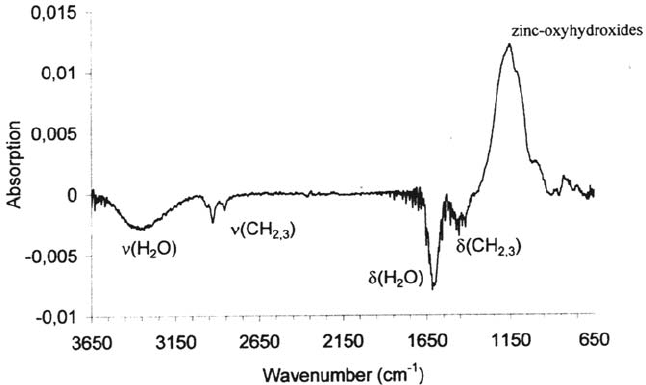
XPS sputter profiles, calibrated by sputtering of an SiO
2
layer of defined
thickness, confirmed the thickening of the oxide for both metals. For Fe, the sputter
profile shows 3nm for the native oxide in accordance with literature values and
6.5 nm after the plasma oxidation process [105,110]. For Zn, approximately 1.2
and 2.7-nm oxides were observed for the native oxide and plasma-oxidized surface
[105]. It is assumed that the thickness of the additional oxide produced during the
oxygen plasma treatment results from the high-field mechanism of oxide growth
initiated by the adsorption of oxide ions and hydroxides on top of the metal oxide.
The validity of the high-field growth mechanism for plasma oxidation of metals
was shown by Fromhold [111] for the oxidation of lead. Thus, in the case of iron
and zinc it can be assumed that Fe
2+
and Zn
2+
ions migrate from the metal/oxide to
the oxide/plasma interface.
Plasma Polymerization of Organosilanes
Chemical Structure of Organosilane Plasma Polymers
Organosilanes are the precursor molecules most often used for the deposition of
corrosion-resistant films. Details of organosilane plasma polymerization can be
found in Refs. 112–114. Figure 30 shows a comparison of the spectrum of the
hexamethyldisilane (HMDS) monomer in the gas phase and the IRRA spectrum of
a 13-nm-thick HMDS plasma polymer on an iron substrate [115]. The characteristic
features of the polymer spectrum revealing the cross-linking and termination are
assigned. The assignments of all bands are given in Table 1.
As indicated in the spectrum of the plasma polymer, siloxane (≡ Si—O—Si ≡),
dimethylsilyl(—Si(CH
3
)
2
—), and methylene (—CH
2
—) groups give rise to the
polymer cross-linking. In addition to this, hydrogen atoms bound to silicon
(≡ Si—H) are observed. The oxygen-containing residual gas in the vacuum
Corrosion Prevention by Adsorbed Monolayers 511
Figure 29 IRRA spectrum of a zinc surface (zinc-coated quartz) that was exposed to an
oxygen plasma for 600s [105].
Copyright © 2002 Marcel Dekker, Inc.
